Study of the Role of Titanium and Iron Cathodic Cages on Plasma-Nitrided AISI 430 Ferritic Stainless Steel
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
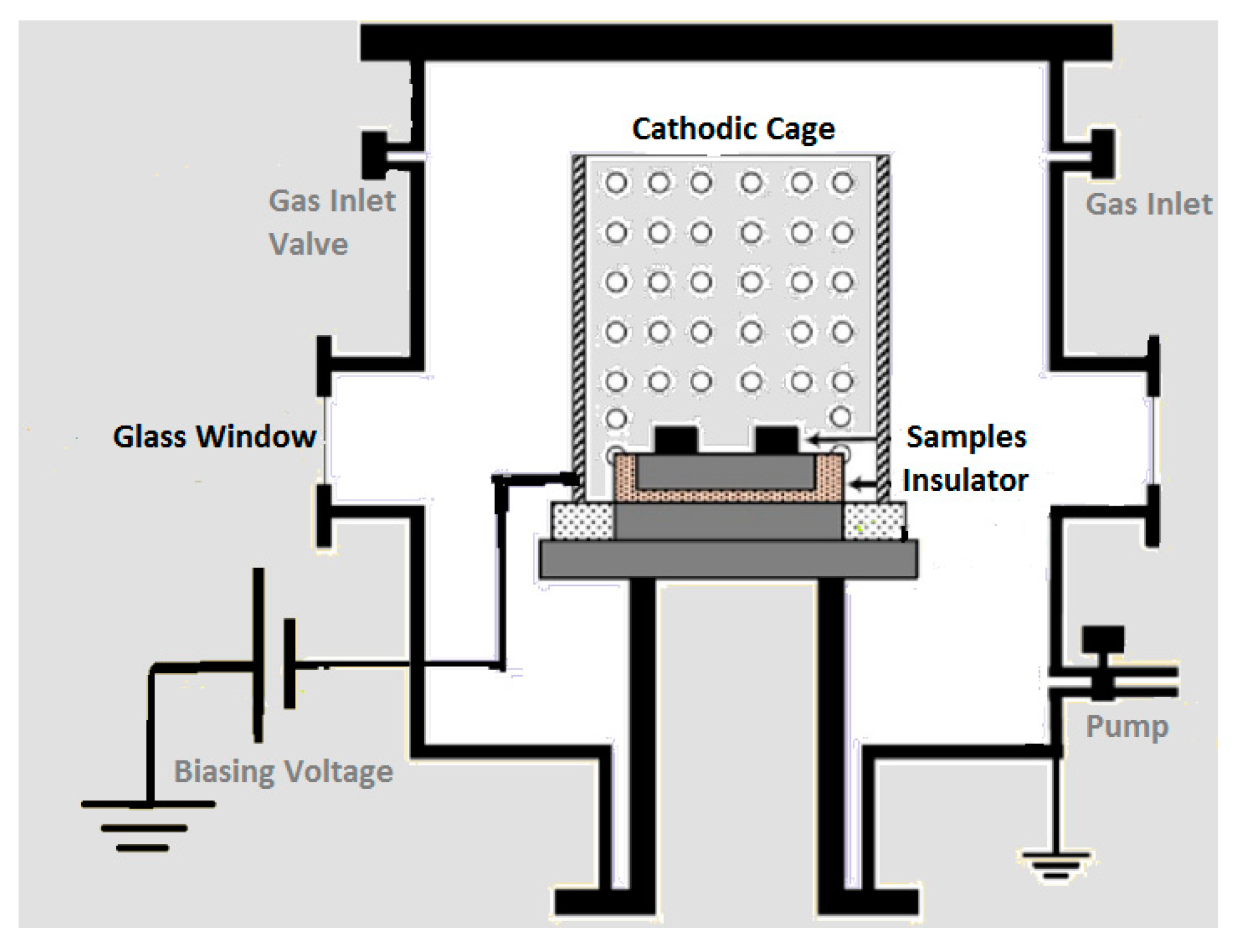
2.2. CCPN System
2.3. Deposition of Coatings and Plasma Parameters
3. Structural and Mechanical Characteristics
4. Results and Discussion
4.1. Surface and Cross-Sectional Morphology
4.2. Microhardness with Indentational Toughness Analysis
4.3. Phase Analysis
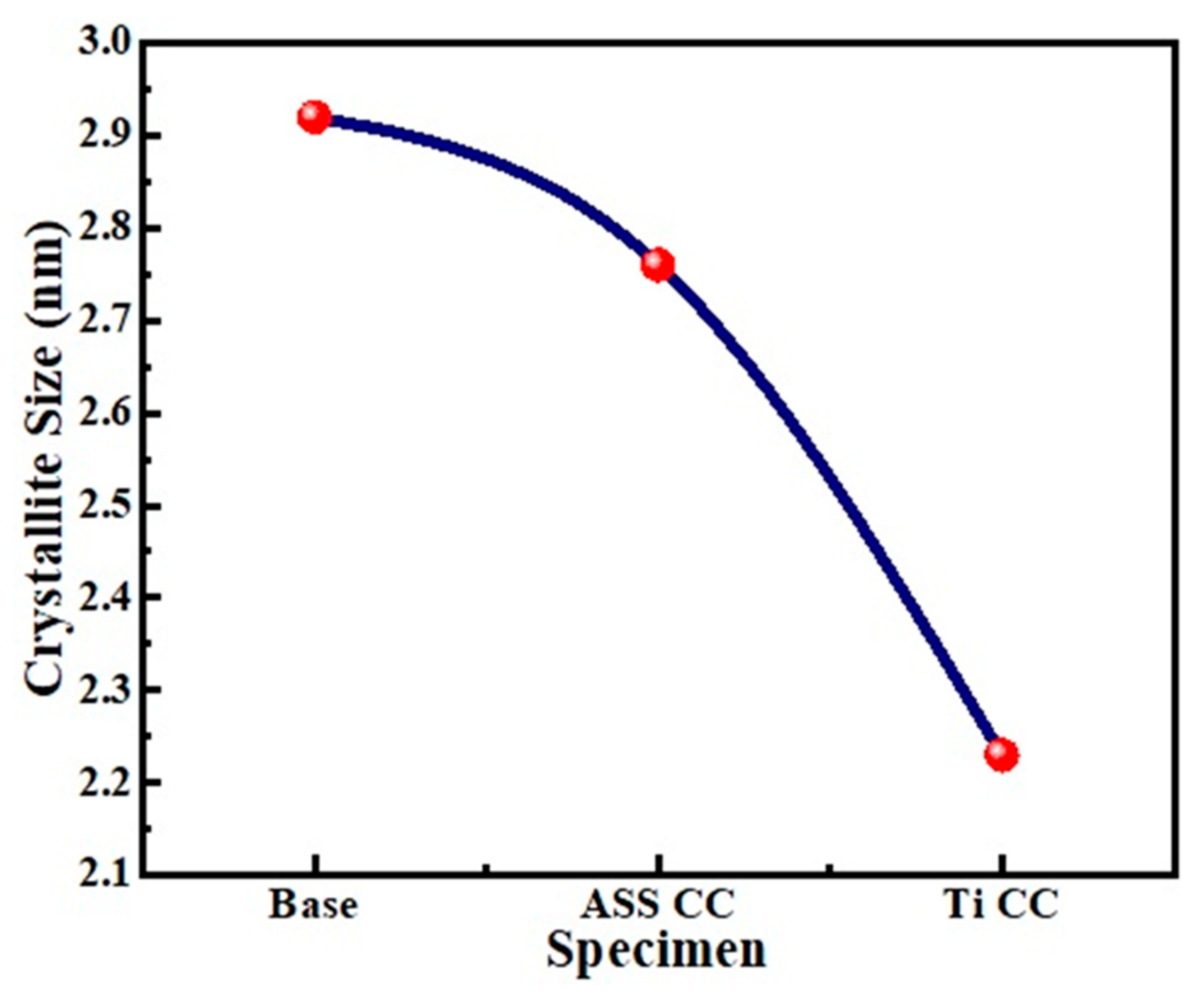
4.4. Crystallite Size and Micro-Strain
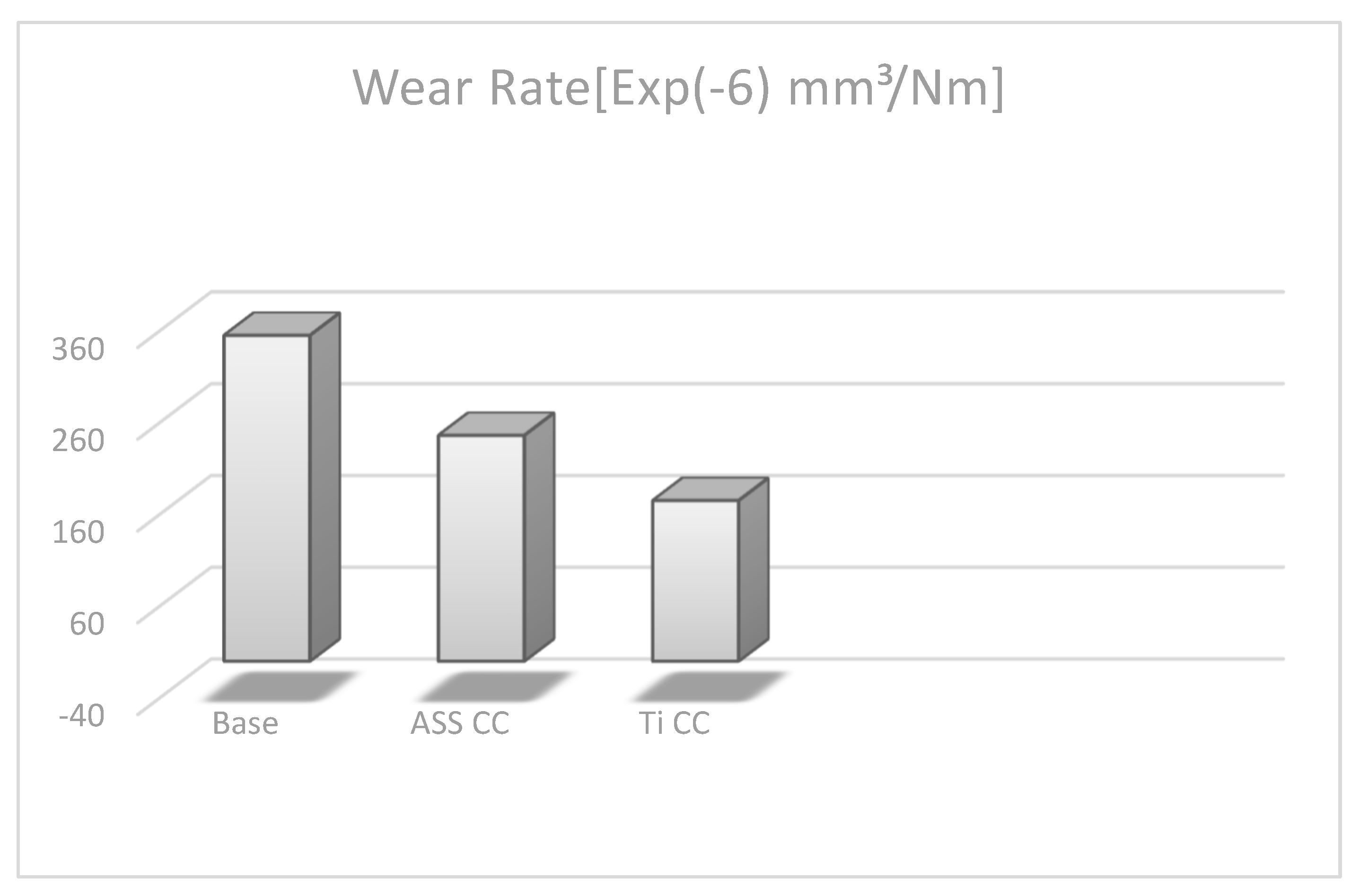
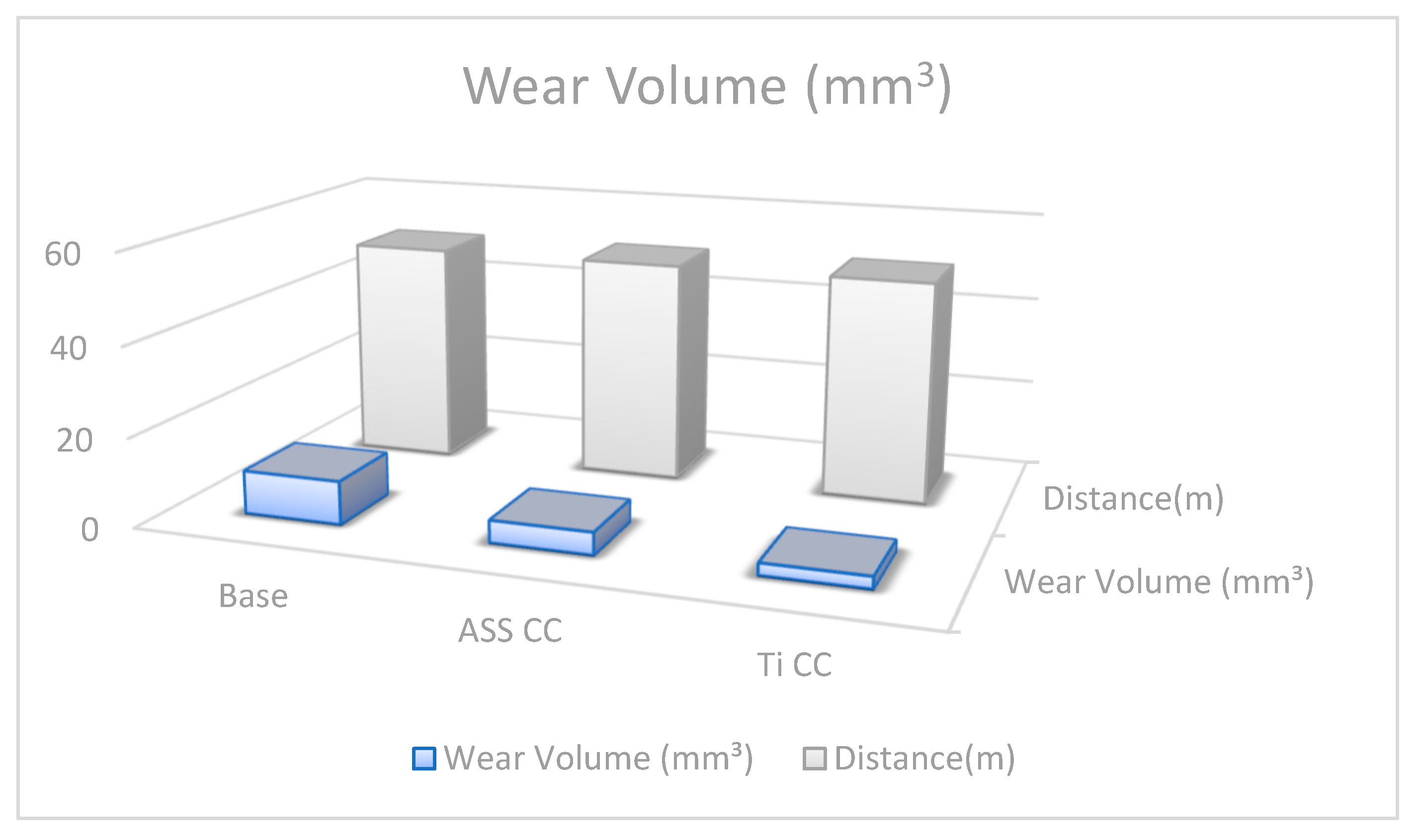
4.5. Wear Volume and Wear Rate
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuzucu, V.; Aksoy, V.M.; Korkut, M.H. The effect of strong carbide forming elements such as Mo, Ti, V and Nb on the microstructure of ferritic stainless steel. Mater. Process. Technol. 1998, 82, 165–171. [Google Scholar] [CrossRef]
- Moustafa, I.M.; Moustafa, M.A.; Nofal, A.A. Carbide formation mechanism during solidification and annealing of 17% Cr-ferritic steel. Mater. Lett. 2000, 42, 371–379. [Google Scholar] [CrossRef]
- Xia, Y.; Hu, J.; Zhou, F.; Lin, Y.; Qiao, Y.; Xu, T. Friction and wear behavior of plasma nitrided 1Cr18Ni9Ti austenitic stainless steel under lubrication condition. Mater. Sci. Eng. A 2005, 402, 135–141. [Google Scholar] [CrossRef]
- Nascimento, F.C.; Lepienski, C.M.; Foerster, C.E.; Assmann, A.; da Silva, S.L.R.; de Siqueira, M.C.J.; Chinelatto, A.L. Structural, mechanical and tribological properties of AISI 304 and AISI 316L steels submitted to nitrogen–carbon glow discharge. Mater. Sci. 2009, 44, 1045–1053. [Google Scholar] [CrossRef]
- Li, C.X.; Bell, T. A comparative study of low temperature plasma nitriding, carburising and nitrocarburizing of AISI 410 martensitic stainless steel. Mater. Sci. Technol. 2007, 23, 355–361. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, D.; Han, D. Improvement of corrosion and wear resistances of AISI 420 martensitic stainless steel using plasma nitriding at low temperature. Surf. Coat. Technol. 2008, 202, 2577–2583. [Google Scholar] [CrossRef]
- Aydin, H.; Bayram, A.; Topçu, S. Friction characteristics of nitrided layers on AISI 430 ferritic stainless steel obtained by various nitriding processes. Mater. Sci. 2013, 19, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Larisch, B.; Brusky, U.; Spies, H.J. Plasma nitriding of stainless steel at low temperatures. Surf. Coat. Technol. 1999, 116, 205–211. [Google Scholar] [CrossRef]
- Maia de Oliveira, A.; Castelleti, L.; Riofano, R. Study of plasma nitriding and nitrocarburising of AISI 430F stainless steel for high hardness and corrosion resistance. In Proceedings of the 59th ABM Annual Conference, ABM, San Pablo, Brazil, 21–25 July 2004; pp. 4015–4023. [Google Scholar]
- Tuckart, W.; Gregorio, M.; Iurman, L. Sliding wear of plasma nitrided AISI 405 ferritic stainless steel. Surf. Eng. 2010, 26, 185–190. [Google Scholar] [CrossRef]
- Gontijo, L.C.; Machado, R.; Casteletti, L.; Kuri, S.E.; Nascente, P.A.P. X-ray diffraction characterisation of expanded austenite and ferrite in plasma nitrided stainless steels. Surface Eng. 2010, 26, 265–270. [Google Scholar] [CrossRef]
- Olaya, J.J.; Rodil, S.S.; Muhl, S. Comparative study of niobium nitride coatings deposited by unbalanced and balanced magnetron sputtering. Thin Solid Film 2008, 516, 8319–8326. [Google Scholar] [CrossRef]
- Arslan, E. Structural, mechanical and corrosion properties of NbN films deposited using dc and pulsed dc reactive magnetron sputtering. Surf. Eng. 2010, 26, 615–619. [Google Scholar] [CrossRef]
- Cansever, N. Properties of niobium nitride coatings deposited by cathodic arc physical vapor deposition. Thin Solid Film 2007, 515, 3670–3674. [Google Scholar] [CrossRef]
- Gotoh, Y.; Nagao, M.; Ura, T.; Tsuji, H.; Ishikawa, J. Ion beam assisted deposition of niobium nitride thin films for vacuum microelectronics devices. Nucl. Instr. Meth. Phys. Res. B 1999, 148, 925–929. [Google Scholar] [CrossRef]
- Mercier, F.; Coindeau, S.; Lay, S.; Crisci, A.; Benz, M.; Encinas, T.; Boichot, R.; Mantoux, A.; Jimenez, C.; Weiss, F.; et al. Niobium nitride thin films deposited by high temperature chemical vapor deposition. Surf. Coat. Technol. 2014, 260, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Madanipour, H.; Soltanieh, M.; Nayebpashaee, N. Investigation of the formation of Al, Fe, N intermetallic phases during Al pack cementation followed by plasma nitriding on plain carbon steel. Mater. Des. 2013, 51, 43–50. [Google Scholar] [CrossRef]
- Hakami, F.; Pramanik, A.; Basak, A.K. Duplex surface treatment of steels by nitriding and chromizing. Aust. J. Mech. Eng. 2017, 15, 55–72. [Google Scholar] [CrossRef]
- Sanjari, S.; Heydarzadeh Sohi, M.; Habibolahzadeh, A. Duplex surface treatment of AISI 1045 steel via plasma nitriding of Titanized layer. Int. J. Eng. 2015, 28, 1193–1198. [Google Scholar]
- Dadfar, M.; Salehi, M.; Golozar, M.A.; Trasatti, S. Surface modification of 304 stainless steels to improve corrosion behavior and interfacial contact resistance of bipolar plates. Int. J. Hydrogen Energy 2016, 41, 21375–21384. [Google Scholar] [CrossRef]
- Hakami, F.; Heydarzadeh Sohi, M.; Rasizadeh Ghani, J. Duplex surface treatment of AISI 1045 steel via plasma nitriding of chromized layer. Thin Solid Film 2011, 519, 6792–6796. [Google Scholar] [CrossRef]
- Kheyrodin, M.; Habibolahzadeh, A.; Mousavi, B. Wear and corrosion behaviors of duplex surface treated 316L austenitic stainless steel via combination of boriding and chromizing. Prot. Met. Phys. Chem. Surf. 2017, 53, 105–111. [Google Scholar] [CrossRef]
- Chen, X.; Hou, P.Y.; Jacobson, C.P.; Visko, S.J.; De Jonghe, L.C. Protective coating on stainless steel interconnect for SOFCs: Oxidation kinetics and electrical properties. Solid State Ion. 2005, 176, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Xia, G.; Simner, S.P.; Stevenson, J.W. Thermal Growth and Performance of Manganese Cobaltite Spinel Protection Layers on Ferritic Stainless Steel SOFC Interconnects. J. Electrochem. Soc. 2005, 152, 1896–1904. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, G.; Li, X.; Stevenson, J.W. (Mn,Co)3O4 spinel coatings on ferritic stainless steels for SOFC interconnect applications. Int. J. Hydrogen Energy 2007, 32, 3648–3654. [Google Scholar] [CrossRef]
- Bateni, M.R.; Wei, P.; Deng, X.; Petric, A. Spinel coatings for UNS 430 stainless steel Interconnects. Surf. Coat. Technol. 2007, 201, 4677–4684. [Google Scholar] [CrossRef]
- Wei, P.; Deng, X.; Bateni, M.R.; Petric, A. Oxidation and Electrical Conductivity Behavior of Spinel Coatings for Metallic Interconnects of Solid Oxide Fuel Cells. Corrosion 2007, 63, 529. [Google Scholar] [CrossRef]
- Deng, X.; Wei, P.; Bateni, M.R.; Petric, A. Cobalt plating of high temperature stainless steel interconnects. J. Power Source 2006, 160, 1225–1229. [Google Scholar] [CrossRef]
- Chou, Y.S.; Stevenson, J.W.; Singh, P. Effect of aluminizing of Cr-containing ferritic alloys on the seal strength of a novel high-temperature solid oxide fuel cell sealing glass. J. Power Source 2008, 185, 1001–1008. [Google Scholar] [CrossRef]
- Mevrel, R.; Duret, C.; Pichoir, R. State of the art on high-temperature corrosion-resistant coatings. Mater. Sci. Technol. 1986, 2, 194–205. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Rose, S.R.; Datta, P.K. Pack Cementation Coatings for High-Temperature Oxidation Resistance of AISI 304. Stainl. Steel Mater. Sci. Technol. 2002, 18, 1479. [Google Scholar]
- Xiang, Z.D.; Datta, P.K. Relationship between pack chemistry and aluminide coating formation for low-temperature aluminisation of alloy steels. Acta Mater. 2006, 54, 4453–4463. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Banerjee, S.; Kulwant, S.; Sharma, I.G.; Grover, A.K.; Suri, A.K. Studies on the development of protective coating on TZM alloy and its subsequent characterization. J. Mater. Process. Technol. 2008, 207, 2408. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, N.; Tong, W.; Lu, J.; Lu, K. Diffusion of chromium in nanocrystalline iron produced by means of surface mechanical attrition treatment. Acta Mater. 2003, 51, 4319. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, J.; Lu, K. Chromizing behaviors of a low carbon steel processed by means of surface mechanical attrition treatment. Acta Mater. 2005, 53, 2081–2089. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, J.; Lu, K. Wear and corrosion properties of a low carbon steel processed by means of SMAT followed by lower temperature chromizing treatment. Surf. Coat. Technol. 2006, 201, 2796–2801. [Google Scholar] [CrossRef]
- Pistofidis, N.; Vourlias, G.; Chaliampalias, D.; Pavlidou, E.; Chrissafis, K.; Stergioudis, G.; Polychroniadis, E.; Tsipas, D. DSC study of the deposition reactions of zinc pack coatings up to 550 °C. Therm. Anal. Calorim. 2006, 84, 191–194. [Google Scholar] [CrossRef]
- Naeem, M.; Awan, S.; Shafiq, M.; Raza, H.A.; Iqbal, J.; Díaz-Guillén, J.C.; Sousa, R.R.M.; Jelani, M.; Abrar, M. Wear and corrosion studies of duplex surface-treated AISI-304 steel by a combination of cathodic cage plasma nitriding and PVD-TiN coating. Ceram. Int. 2022, 48, 21514–21523. [Google Scholar] [CrossRef]
- Babur, M.Z.; Iqbal, Z.; Shafiq, M.; Naz, M.Y.; Makhlouf, M.M. Comparative study of PVD titanium nitride coating with cathodic cage plasma nitriding of austenitic 201 stainless steel for enhanced tribological properties. Appl. Phys. A 2021, 127, 954. [Google Scholar] [CrossRef]
- Naeem, M.; Shafiq, M.; Zaka-ul-Islam, M.; Bashir, M.; DíazGuillén, J.; Lopez-Badillo, C.; Zakaullah, M. Novel duplex cathodic cage plasma nitriding of non-alloyed steel using aluminum and austenite steel cathodic cages. J. Alloys Compd. 2017, 721, 307–311. [Google Scholar] [CrossRef]
- Lee, I.; Jeon, K.H. Plasma post oxidation of plasma nitrocarburized, SKD 61 steel. Mater. Sci. Technol. 2008, 24, 136–138. [Google Scholar]
- Vaz, F.; Cerqueira, P.; Rebouta, L.; Nascimento, S.; Alves, E.; Goudeau, P.; Riviera, J.; Pischow, K.; de Rijk, J. Structural, optical and mechanical properties of coloured TiN thin flms. Thin Solid Film 2004, 447, 449–454. [Google Scholar] [CrossRef]
- She, D.; Yue, W.; Fu, Z.; Gu, Y.; Wang, C.; Liu, J. The effect of nitriding temperature on hardness and microstructure of die steel pre-treated by ultrasonic cold forging technology. Mater. Des. 2013, 49, 392–399. [Google Scholar] [CrossRef]
- Ochoa, E.; Figueroa, C.; Alvarez, F. The influence of the ion current density on plasma nitriding process. Surf. Coat. Technol. 2005, 200, 2165–2169. [Google Scholar] [CrossRef]
- Panjan, P.; Cekada, M.; Panjan, M.; Kek-Merl, D. Growth Defects in PVD hard Coatings. Vacuum 2009, 84, 209–214. [Google Scholar] [CrossRef]
- Kao, W.H.; Su, Y.L.; Horng, J.H.; Hsieh, Y.T. Improved tribological properties, electrochemical resistance and biocompatibility of AISI 316L stainless steel through duplex plasma nitriding and TiN coating treatment. J. Biomater. Appl. 2017, 32, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Stagno, E.; Pinasco, M.R.; Ienco, M.G.; Palombarini, G.; Bocchini, G.F. Behaviour of sintered 410 low carbon steels towards ion nitriding. J. Alloys Compd. 1997, 247, 172–179. [Google Scholar] [CrossRef]
- Karakan, M.; Alsaran, A.; Celik, A. Effect of process time on structural and tribological properties offerritic nitrocarburizing AISI 4140 steel. Mater. Des. 2004, 25, 349–353. [Google Scholar] [CrossRef]
- Kumar, M.K.; Saravanan, R.; Sellamuthu, R.; Narayanan, V. Microstructure, hardness and wear rate of heat treated titanium surface alloyed AISI304 stainless steel. Mater. Today Proc. 2018, 5, 7571–7576. [Google Scholar] [CrossRef]
- Anandana, S.; Pityanab, S.; Majumdar, J.D. Structure–property-correlation in laser surface alloyed AISI 304 stainless steel with WC + Ni + NiCr. Mater. Sci. Eng. A 2012, 536, 159–169. [Google Scholar] [CrossRef]
- Jiang, D.E.; Carter, E.A. Carbon dissolution and diffusion in ferrite and austenite from first principles. Phy. Rev. B 2003, 67, 2141031–2141042. [Google Scholar] [CrossRef] [Green Version]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 3, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Hall, E.O. Variation of hardness of metals with grain size. Nature 1954, 173, 948–949. [Google Scholar] [CrossRef]
- Liu, M.Y.; Shi, B.; Wang, C.; Ji, S.K.; Cai, X.; Song, H.W. Normal Hall–Petch behavior of mild steel with submicron grains. Mater. Lett. 2003, 57, 2798–2802. [Google Scholar] [CrossRef]
- Nakata, K.; Yamauchi, W.; Akamatsu, K.; Ushio, M. Plasma nitriding behavior of low carbon binary alloy steels. Surf. Coat. Technol. 2003, 174, 1206–1210. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, M.; Sun, Z.; Wang, Y.; You, Y.; Bai, B.; Chen, L.; Long, Z.; Li, R. Optimizing the mechanical properties of M50NiL steel by plasma nitrocarburizing. Appl. Surf. Sci. 2014, 315, 28–35. [Google Scholar] [CrossRef]
- Haftlang, F.; Habibolahzadeh, A.; Sohi, M.H. Comparative tribological studies of duplex surface treated AISI 1045 steels fabricated by combinations of plasma nitriding and aluminizing. Mater. Des. 2014, 60, 580–586. [Google Scholar] [CrossRef]



| Elements | C | Cr | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|---|---|
| Composition (Wt%) | 0.10~0.12 | 16.2~17.1 | 0.37~0.5 | 0.92~1.17 | 0.02 | 0.32 | Balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babur, M.Z.; Noori, A.S.; Iqbal, Z.; Shafiq, M.; Asghar, M.; Alghtani, A.H.; Tirth, V.; Algahtani, A.; Zaman, A. Study of the Role of Titanium and Iron Cathodic Cages on Plasma-Nitrided AISI 430 Ferritic Stainless Steel. Micromachines 2022, 13, 1739. https://doi.org/10.3390/mi13101739
Babur MZ, Noori AS, Iqbal Z, Shafiq M, Asghar M, Alghtani AH, Tirth V, Algahtani A, Zaman A. Study of the Role of Titanium and Iron Cathodic Cages on Plasma-Nitrided AISI 430 Ferritic Stainless Steel. Micromachines. 2022; 13(10):1739. https://doi.org/10.3390/mi13101739
Chicago/Turabian StyleBabur, Mirza Z., Aiyah S. Noori, Zafar Iqbal, Muhammad Shafiq, Muhammad Asghar, Abdulaziz H. Alghtani, Vineet Tirth, Ali Algahtani, and Abid Zaman. 2022. "Study of the Role of Titanium and Iron Cathodic Cages on Plasma-Nitrided AISI 430 Ferritic Stainless Steel" Micromachines 13, no. 10: 1739. https://doi.org/10.3390/mi13101739
APA StyleBabur, M. Z., Noori, A. S., Iqbal, Z., Shafiq, M., Asghar, M., Alghtani, A. H., Tirth, V., Algahtani, A., & Zaman, A. (2022). Study of the Role of Titanium and Iron Cathodic Cages on Plasma-Nitrided AISI 430 Ferritic Stainless Steel. Micromachines, 13(10), 1739. https://doi.org/10.3390/mi13101739






