Design and Application of a Flexible Blood Oxygen Sensing Array for Wearable Devices
Abstract
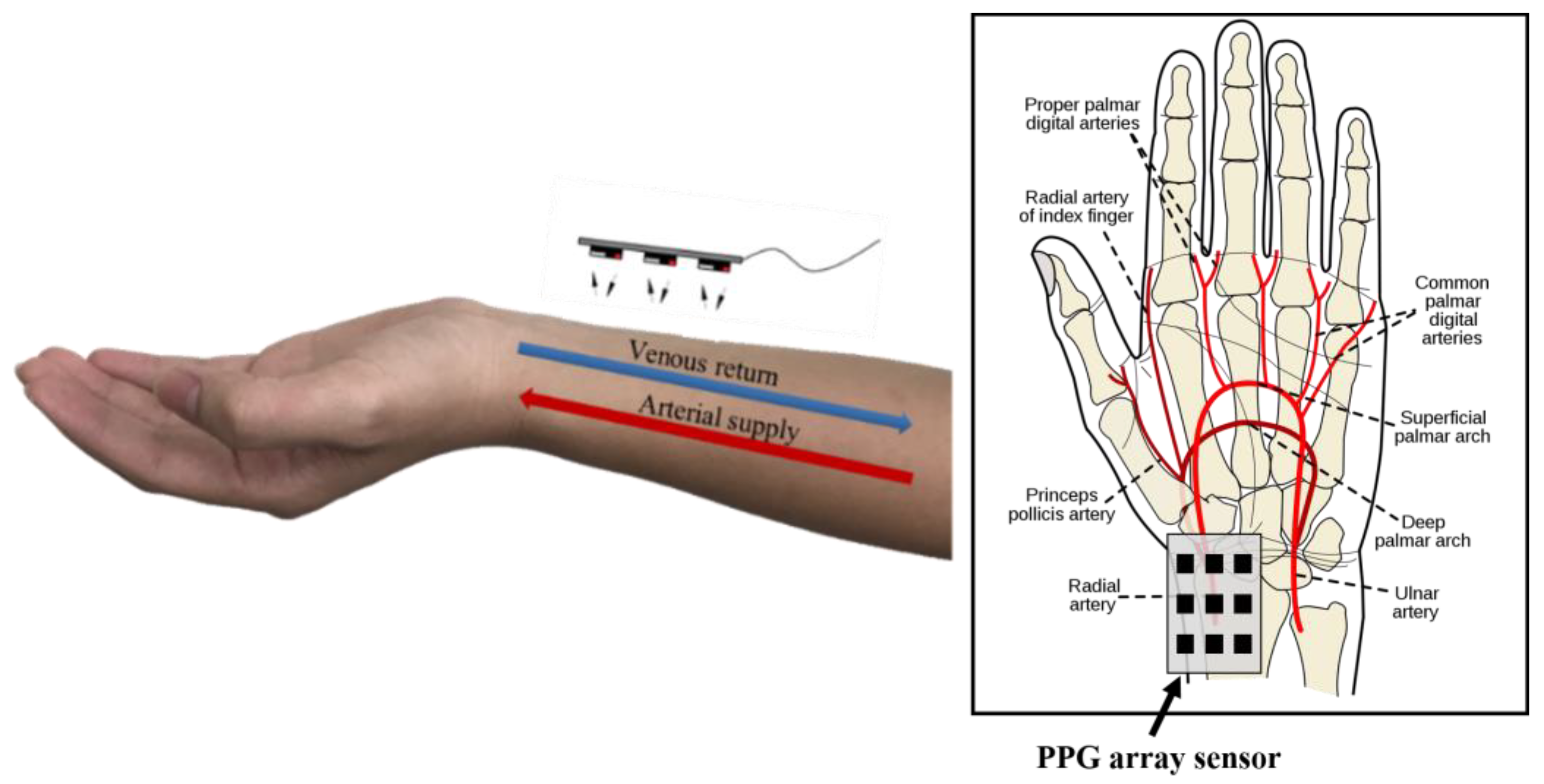
1. Introduction
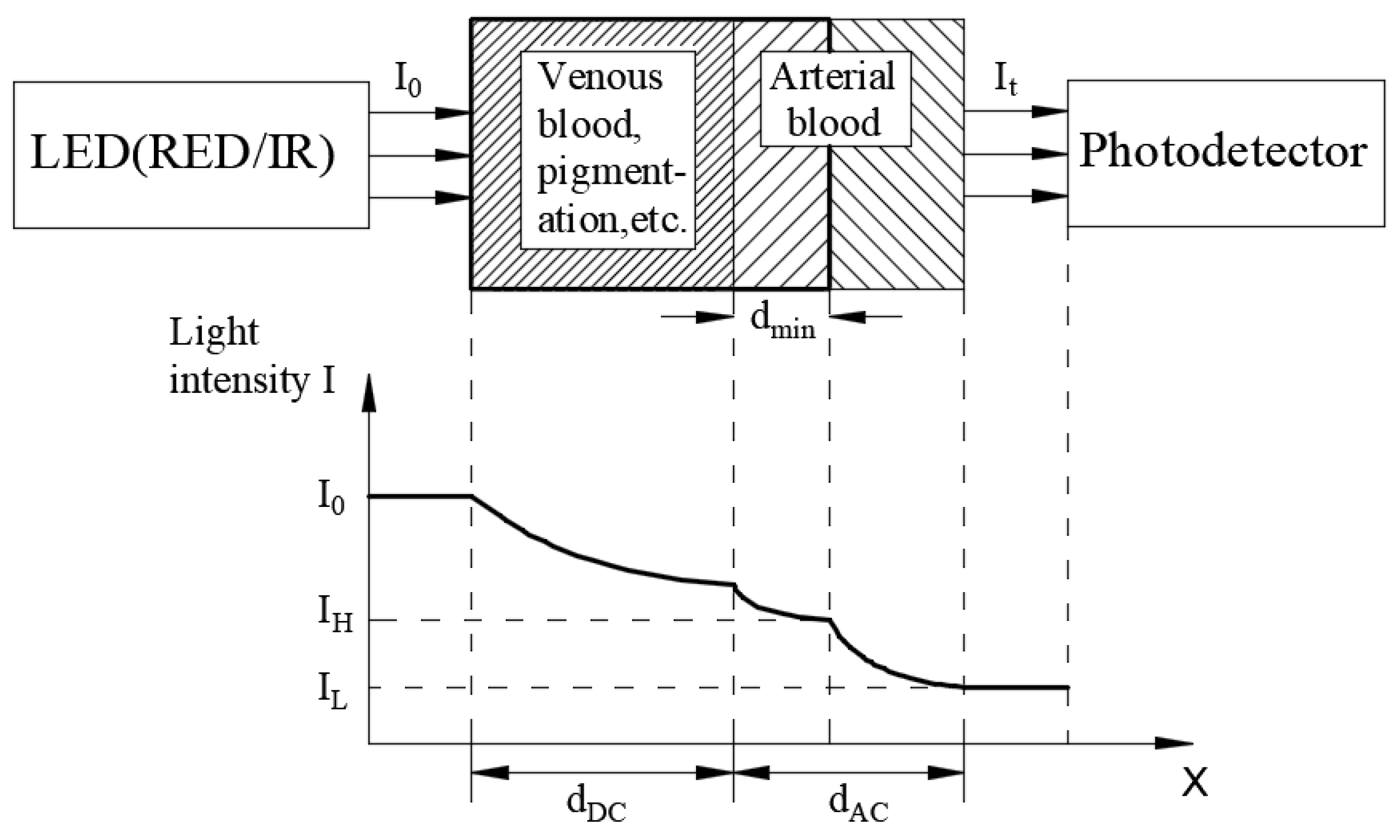
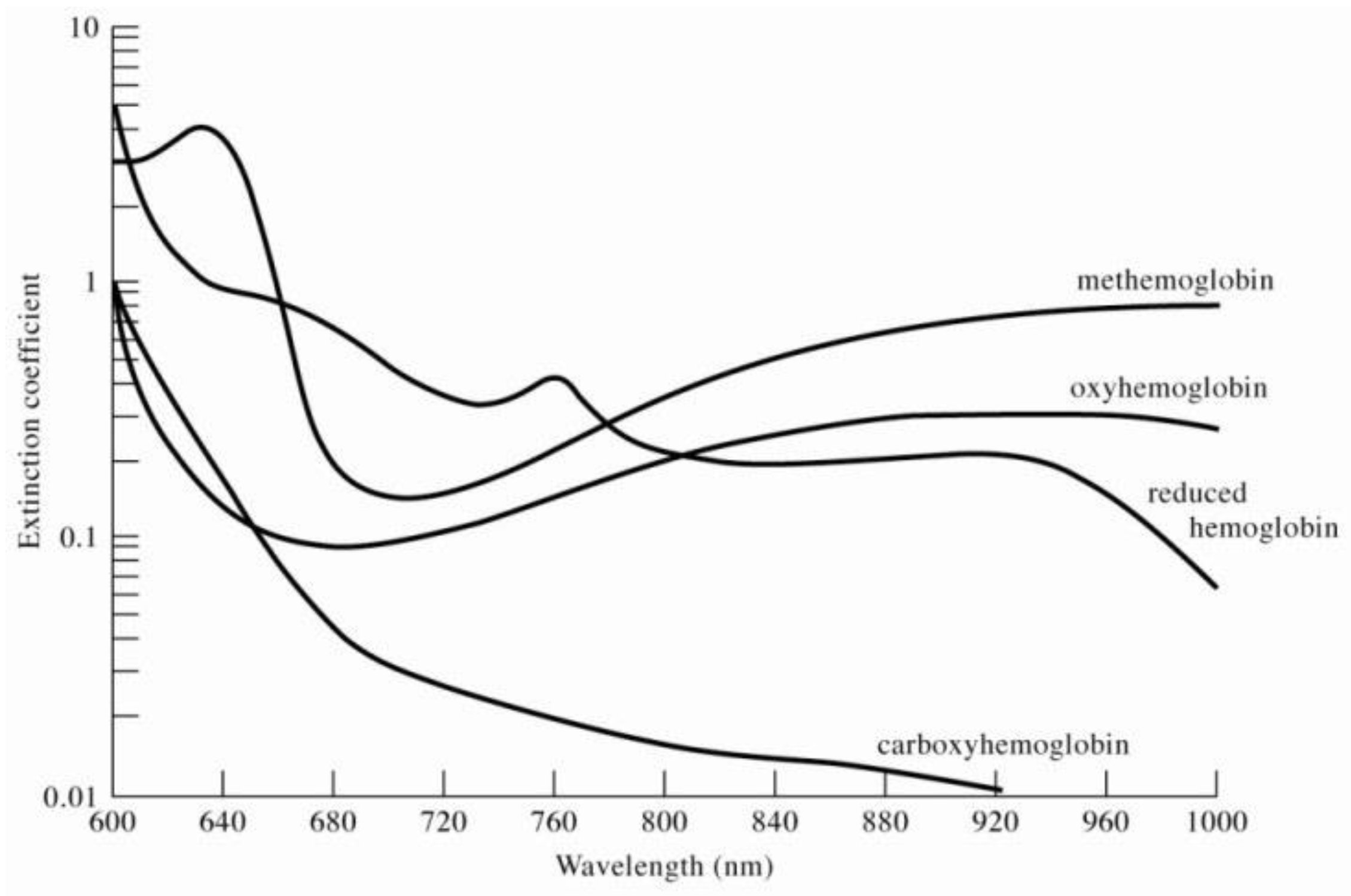
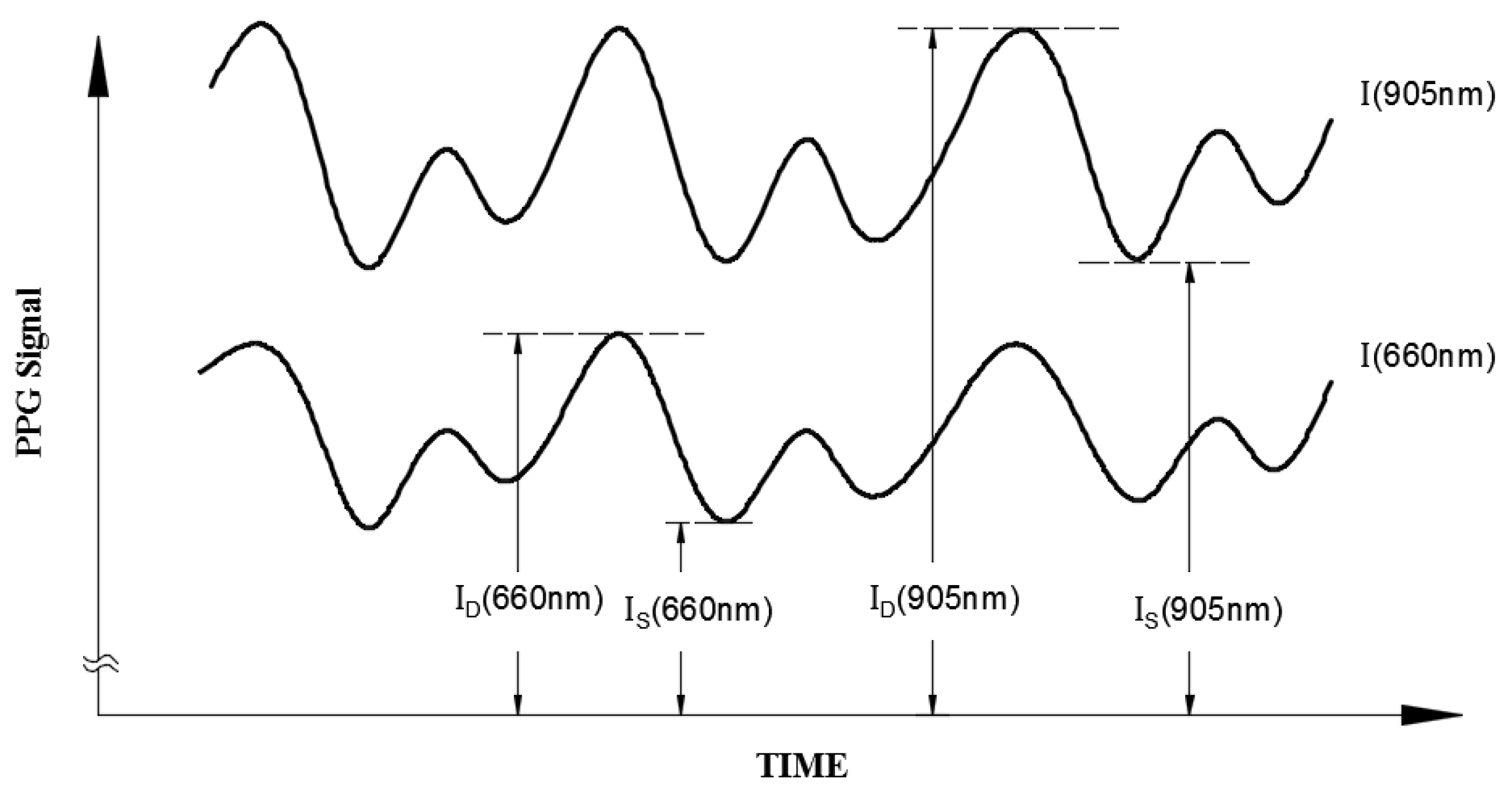
2. Research Method and Design
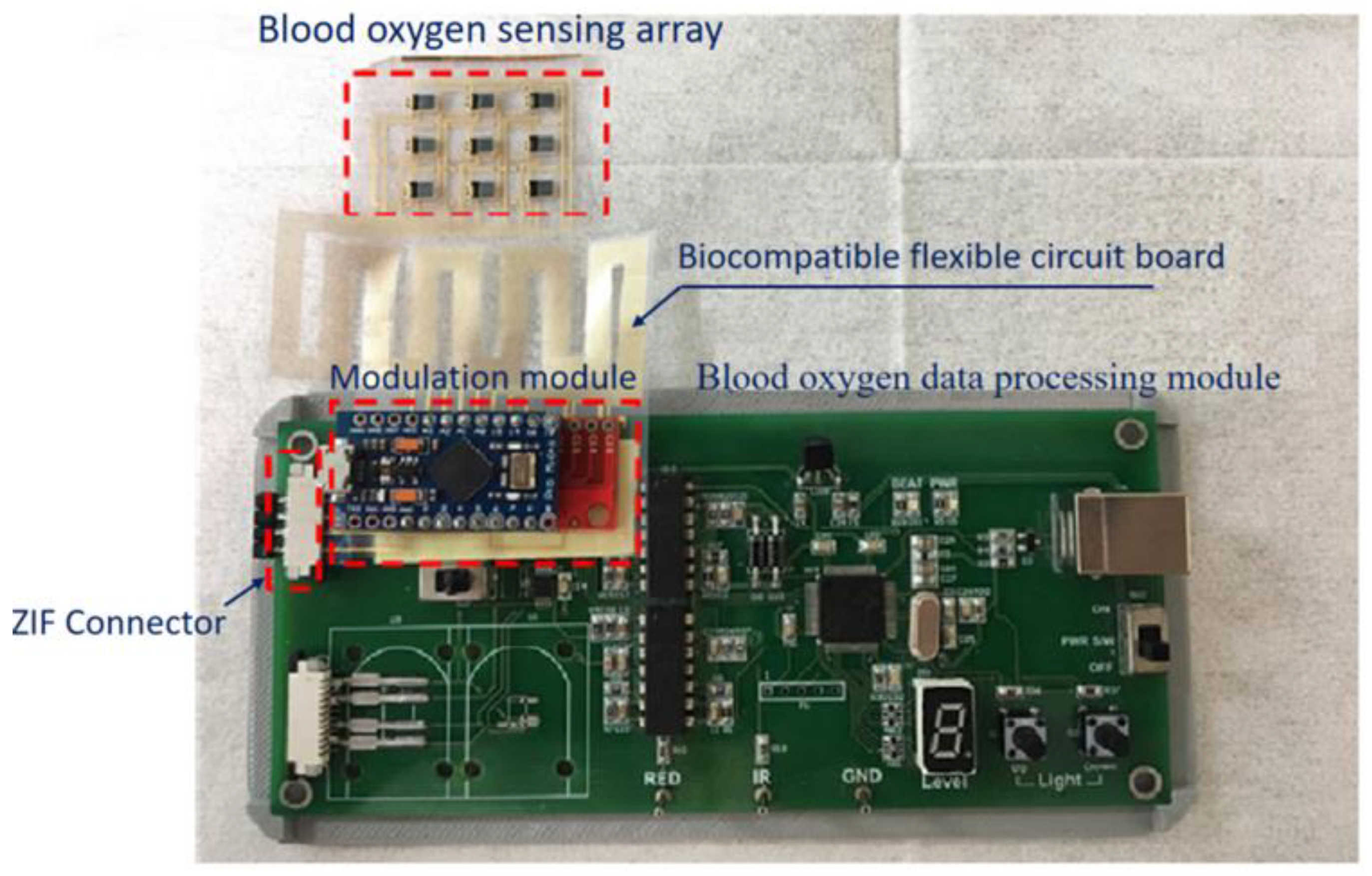
3. Fabrication
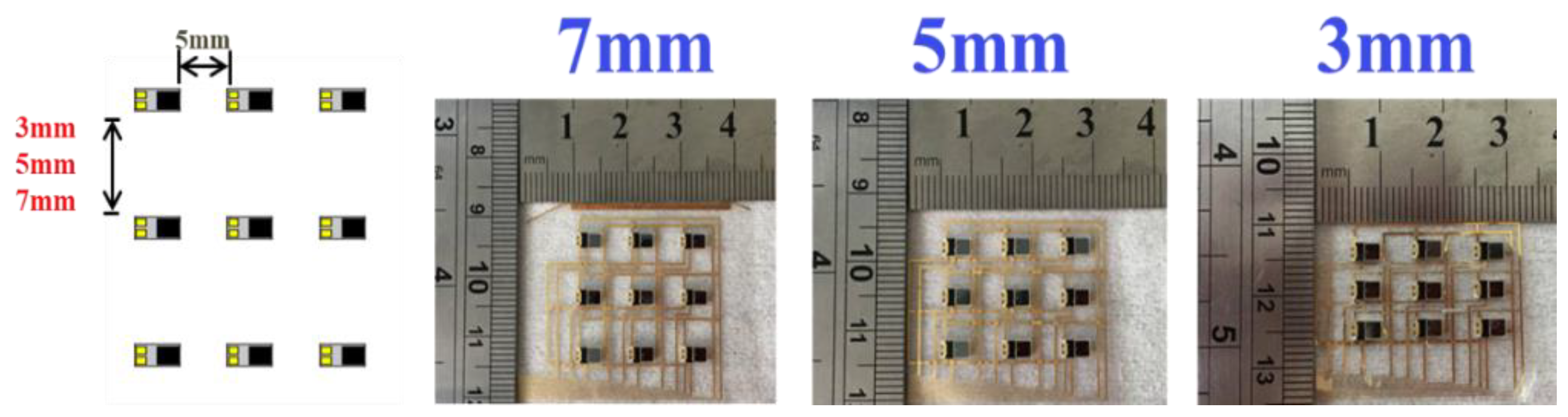
3.1. Blood Oxygen Sensing Array
3.2. Modulation Module
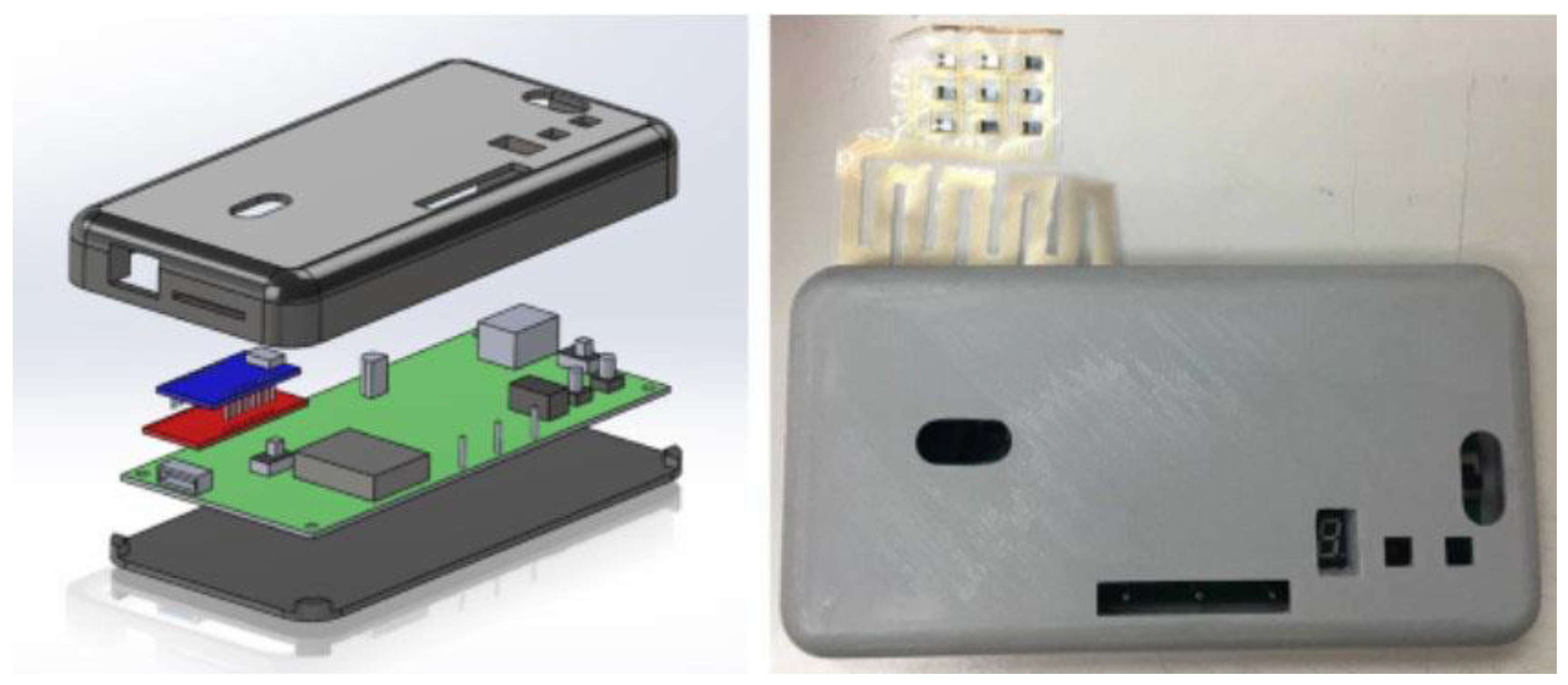
3.3. Blood Oxygen Data Processing Module
3.4. ZIF Connector
3.5. Biocompatible Flexible Circuit Board
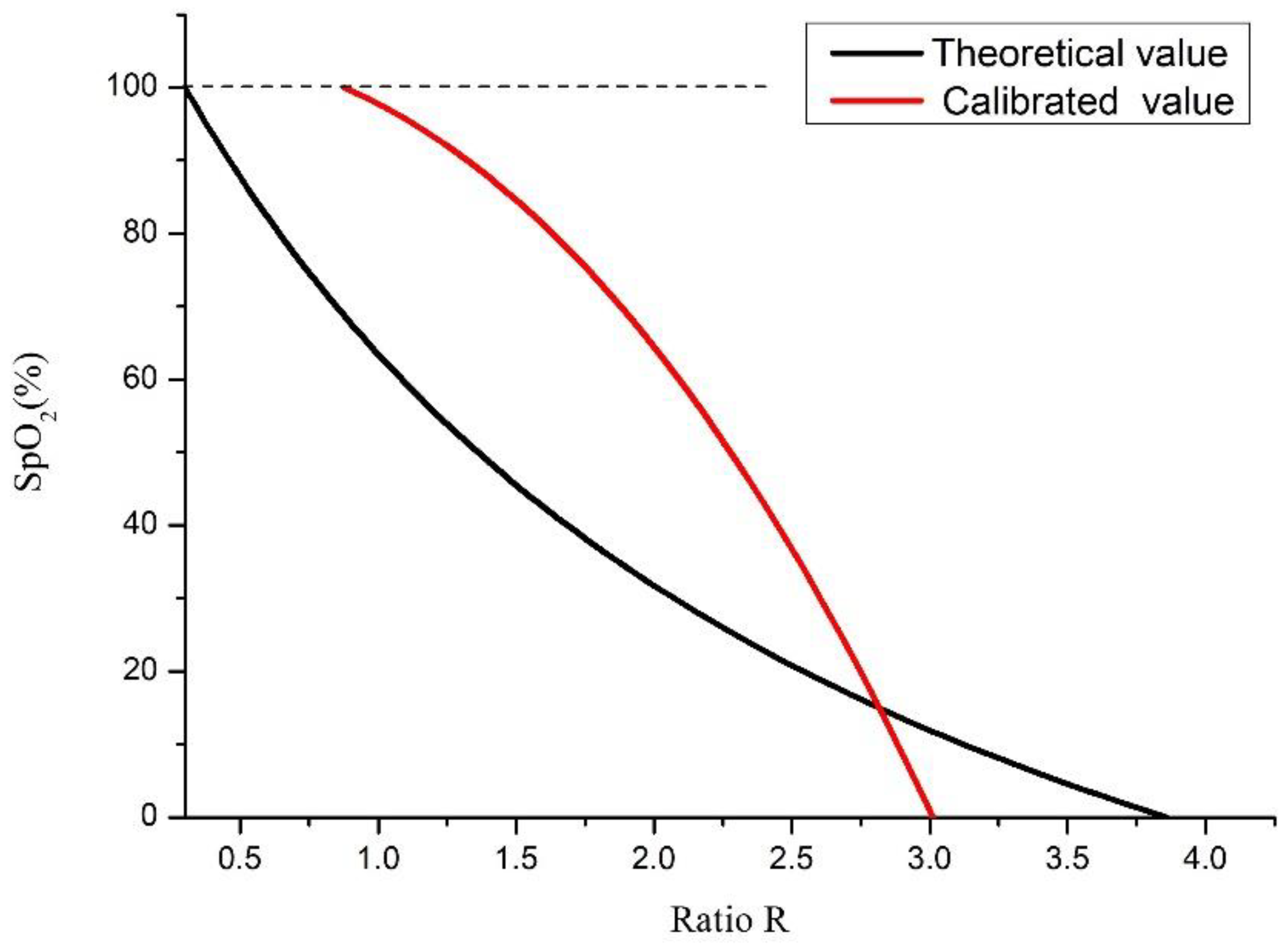
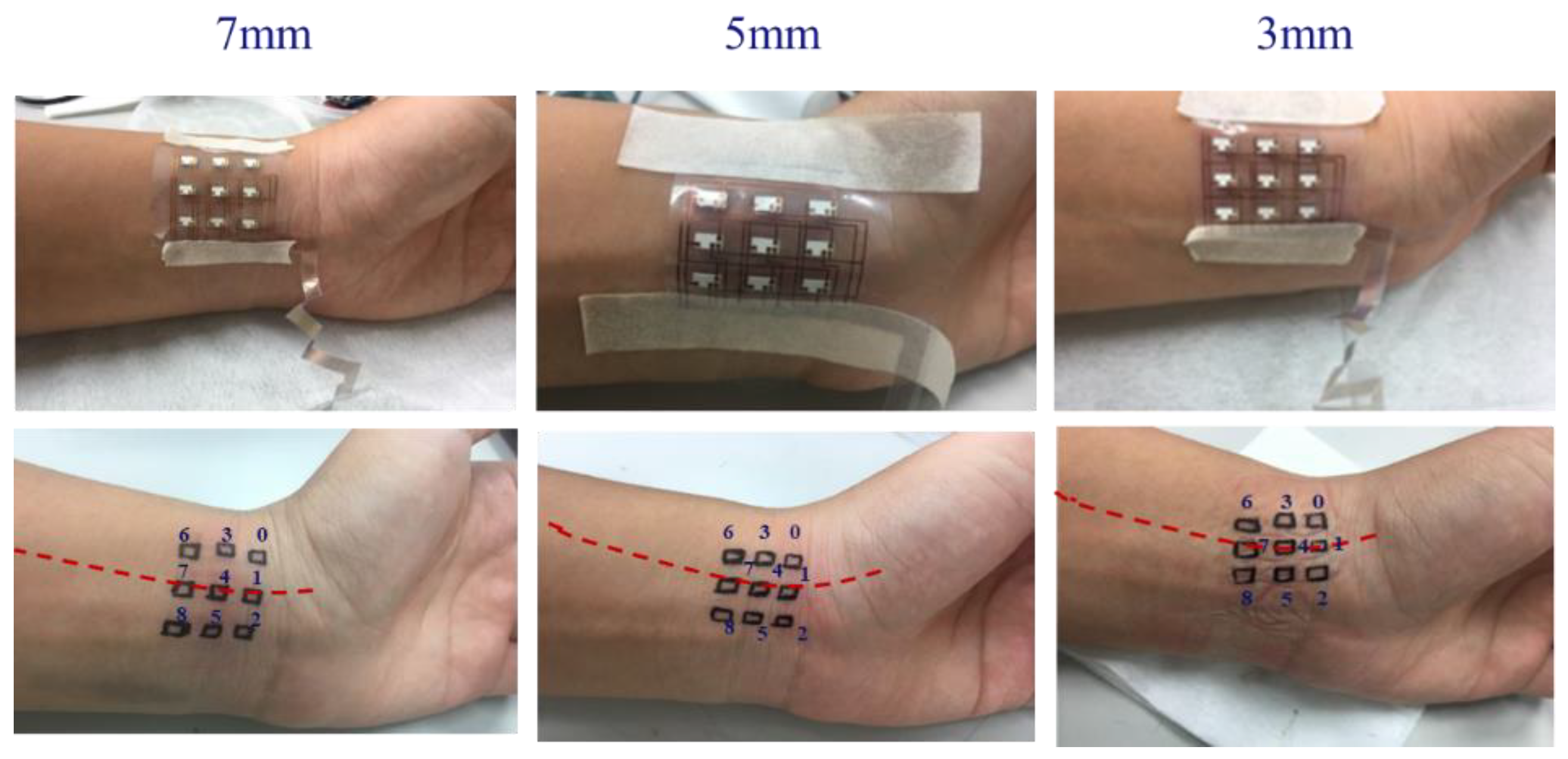
4. Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sugathan, A.; Roy, G.G.; Kirthyvijay, G.J.; Thomson, J. Application of arduino based platform for wearable health monitoring system. In Proceedings of the 2013 IEEE 1st International Conference on Condition Assessment Techniques in Electrical Systems (CATCON), Kolkata, India, 6–8 December 2013; pp. 1–5. [Google Scholar]
- Ji, S.; Zhang, Z.; Xia, Z.; Wen, H.; Zhu, J.; Zhao, K. RBHHM: A novel remote cardiac cycle detection model based on heartbeat harmonics. Biomed. Signal Process. Control 2022, 78, 103936. [Google Scholar] [CrossRef]
- Kamal, A.A.R.; Harness, J.B.; Irving, G.; Mearns, A.J. Skin photoplethysmography—A review. Comput. Methods Programs Biomed. 1989, 28, 257–269. [Google Scholar] [CrossRef]
- Alen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [PubMed]
- Hay, O.Y.; Cohen, M.; Nitzan, I.; Kasirer, Y.; Shahroor-karni, S.; Yitzhaky, Y.; Engelberg, S.; Nitzan, M. Pulse oximetry with Two infrared wavelengths without calibration in extracted arterial blood. Sensors 2018, 18, 3457. [Google Scholar] [CrossRef]
- Jensen, L.A.; Onyskiw, J.E.; Prasad, N.G.N.N. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung 1998, 27, 387–408. [Google Scholar] [CrossRef]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. The human ear canal: Investigation of its suitability for monitoring photoplethysmographs and arterial oxygen saturation. Physiol. Meas. 2014, 35, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Dresher, R. Wearable Forehead Pulse Oximetry: Minimization of Motion and Pressure Artifacts. Master’s Thesis, Department of Biomedical Engineering, Worcester Polytechnic Institute, Worcester, MA, USA, 2006. [Google Scholar]
- Lee, Y.; Shin, H.; Jo, J.; Lee, Y.K. Development of a wristwatch-type PPG array sensor module. In Proceedings of the 2011 IEEE International Conference on Consumer Electronic, Berlin Germany, 9–12 January 2011; pp. 168–171. [Google Scholar]
- Zijlstra, W.G.; Buursma, A.; Meeuwsen-Van der Roest, W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Engelberg, S. Three-wavelength technique for the measurement of oxygen saturation in arterial blood and in venous blood. J. Biomed. Opt. 2009, 14, 024046. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Peng, Q.; Feng, X.; Gao, B.; Zheng, Y. Single-wavelength blood oxygen saturation sensing with combined optical absorption and scattering. IEEE Sens. J. 2016, 16, 1943–1948. [Google Scholar] [CrossRef]
- Reichelt, S.; Fila, J.; Werber, A.; Foerster, K.; Heilmann, C.; Klemm, R.; Zappe, H. Development of an implantable pulse oximeter. IEEE Trans. Biomed. Eng. 2008, 55, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Zwart, A.; van Kampen, E.J.; Zijlstra, W.G. Results of routine determination of clinically significant hemoglobin derivatives by multicomponent analysis. Clin. Chem. 1986, 32, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Index 2 Pulse Oximeter Simulator. Available online: https://www.qrs-solutions.com/products/fluke-index-2-pulse-oximeter-simulator/ (accessed on 1 January 2022).
- Blitz, A.; Osterday, R.M.; Brodman, R.F. Harvesting the radial artery. Ann. Cardiothorac. Surg. 2013, 2, 533–542. [Google Scholar]
- Gutierrez, C.A.; Lee, C.; Kim, B.; Meng, E. Epoxy-less packaging methods for electrical contact to parylene-based flat flexible cables. In Proceedings of the 16th International Solid-State Sensors, Actuators and Microsystems Conference (Transducers 2011), Beijing, China, 5–9 June 2011; pp. 2299–2302. [Google Scholar]



| Wavelength (nm) | εHb | εHbO2 |
|---|---|---|
| 660 | 0.81 | 0.08 |
| 905 | 0.21 | 0.30 |
| SYMBOL | CHARACTERISCTIC | COMPONENTS | TEST CONDITION | MIN | TYP | MAX | UNITS |
|---|---|---|---|---|---|---|---|
| VF | Forward Voltage | LED1 | IF = 20 mA | - | 1.9 | 2.2 | V |
| LED2 | - | 1.3 | 1.5 | ||||
| PD | IF = 10 mA | 0.5 | - | 1.3 | |||
| IR | Revers Breakdown Current | LED1, LED2 | VR = 5 V | - | - | 10 | uA |
| PO | Output Power | LED1 | IF = 20 mA | - | 2 | - | mW |
| LED2 | - | 2 | - | ||||
| λ PEAK | Peak Wavelength | LED1 | IF = 20 mA | - | 660 | - | nm |
| LED2 | - | 905 | - | ||||
| PD | - | 940 | - | ||||
| Δλ | Half Wave Width | LED1 | IF = 20 mA | - | 30 | - | nm |
| Δλ V BR | Half Wave Width Reverse Breakdown Voltage | LED2 | IF = 20 mA IR = 100 Ua | - | 60 | - | nm V |
| PD | 35 | - | - | ||||
| I D | Reverse Dark Current | PD | V R = 10 V | - | - | 20 | nA |
| I L | Light Current | 1mW@940 nm | - | 21 | - | uA | |
| S | Spectral Response Range | - | 400 | - | 1050 | nm | |
| CJ | Junction Capacitance | VR = 3 V, f = 1 MHz | - | 20 | - | pF |
| Dimension | 35 mm × 18.5 mm × 4.5 mm |
|---|---|
| Microcontroller | ATmega32u4 |
| Operating Voltage | 5 V |
| Input Voltage (recommended) | 7–12 V |
| Digital I/O Pins | 20 |
| PWM Channels | 7 |
| Analog Input Channels | 12 |
| DC Current for 5V Pin | 40 mA |
| DC Current for 3.3V Pin | 50 mA |
| Flash Memory | 32 KB (ATmega32u4) of which 4 KB used by bootloader |
| SRAM | 2.5 KB |
| EEPROM | 1 KB |
| Clock Speed | 16 MHz |
| PARAMETER | SYMBOL | TEST CONDITIONS | VCC (V) | 25 °C | −40 °C TO 85 °C | −55 °C TO 125 °C | UNITS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VI (V) | VIS (V) | MIN | TYP | MAX | MIN | MAX | MIN | MAX | ||||
| HC TYPES | ||||||||||||
| High Level Input Voltage | VIH | - | - | 2 | 1.5 | - | - | 1.5 | - | 1.5 | - | V |
| 4.5 | 3.15 | - | - | 3.15 | - | 3.15 | - | V | ||||
| 6 | 4.2 | - | - | 4.2 | - | 4.2 | - | V | ||||
| Low Level Input Voltage | VIL | - | - | 2 | - | - | 0.5 | - | 0.5 | - | 0.5 | V |
| 4.5 | - | - | 1.35 | - | 1.35 | - | 1.35 | V | ||||
| 6 | - | - | 1.8 | - | 1.8 | - | 1.8 | V | ||||
| Maximum “ON” Resistance IO = 1 mA | RON | VCC or GND | VCC or GND | 4.5 | - | 70 | 160 | - | 200 | - | 240 | Ω |
| 6 | - | 60 | 140 | - | 175 | - | 210 | Ω | ||||
| VCC to GND | VCC to GND | 4.5 | - | 90 | 180 | - | 225 | - | 170 | Ω | ||
| 6 | - | 80 | 160 | - | 200 | - | 240 | Ω | ||||
| Maximum “ON” Resistance Between Any Two Switches | ΔRON | - | - | 4.5 | - | 10 | - | - | - | - | - | Ω |
| 6 | - | 8.5 | - | - | - | - | - | Ω | ||||
| Switch “Off” Leakage Current 16 Channels | IIZ | Ē = VCC | VCC or GND | 6 | - | - | ±0.8 | - | ±8 | - | ±8 | μA |
| Logic Input Leakage Current | II | VCC or GND | - | 6 | - | - | ±0.1 | - | ±1 | - | ±1 | μA |
| Quiescent Device Current IO = 0 mA | ICC | VCC or GND | - | 6 | - | - | 8 | - | 80 | - | 160 | μA |
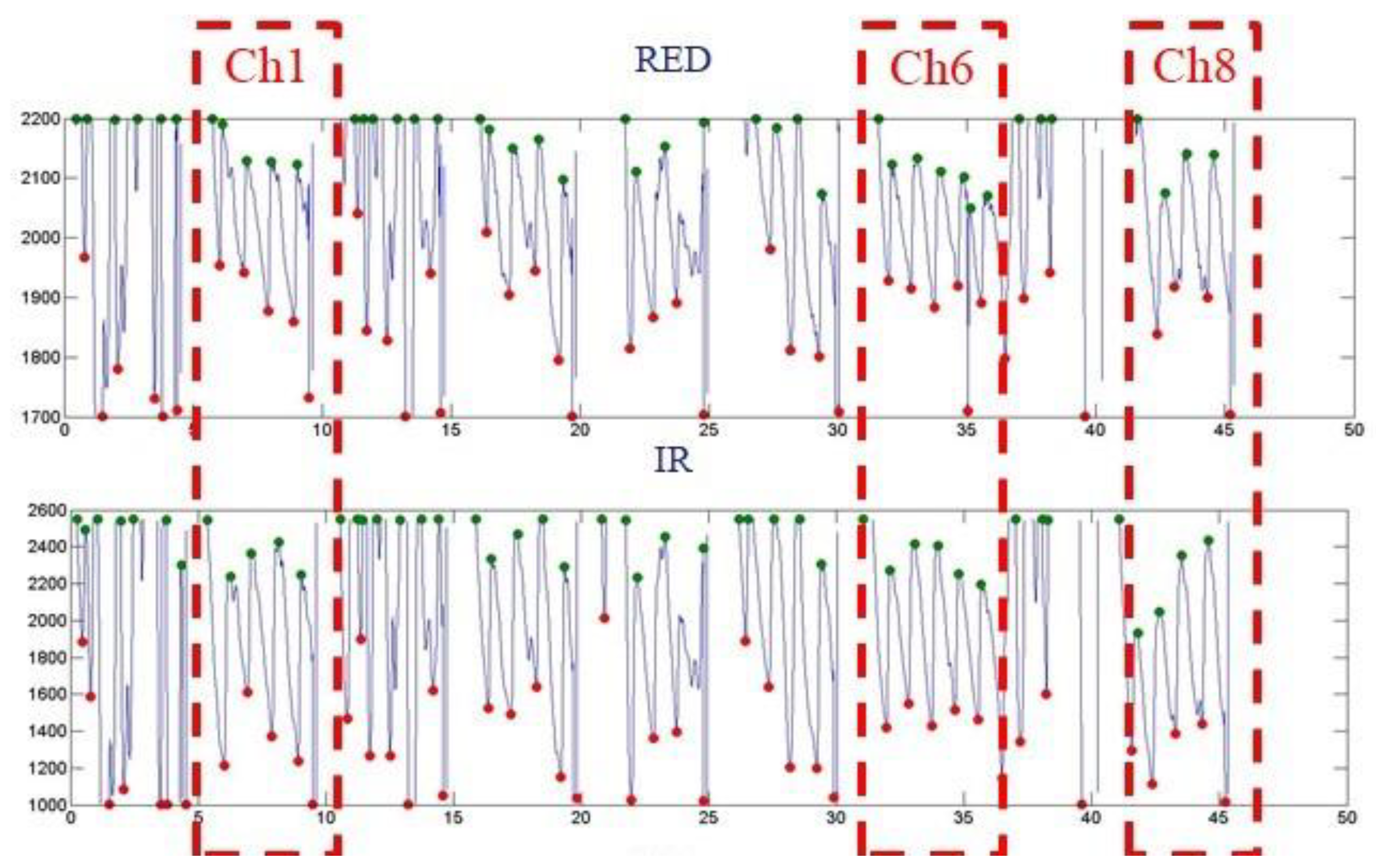
| Ch0 | Ch3 | Ch6 |
|---|---|---|
| Ave. SpO2 N.A | Ave. SpO2 N.A | Ave. SpO2 ~99 |
| Ch1 | Ch4 | Ch7 |
| Ave. SpO2 ~76 | Ave. SpO2 N.A | Ave. SpO2 N.A |
| Ch2 | Ch5 | Ch8 |
| Ave. SpO2 N.A | Ave. SpO2 N.A | Ave. SpO2 ~99 |
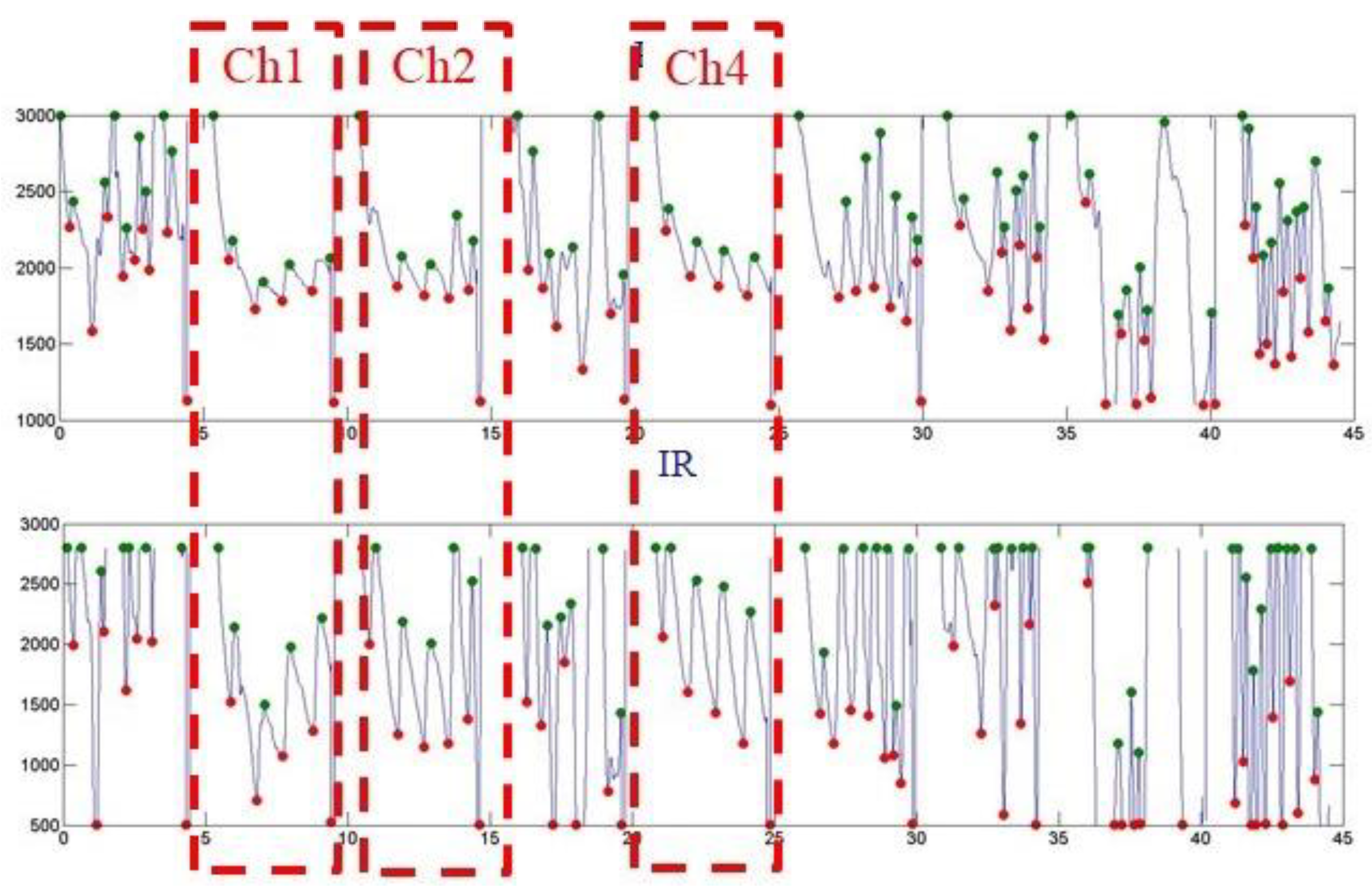
| Ch0 | Ch3 | Ch6 |
|---|---|---|
| Ave. SpO2 N.A | Ave. SpO2 N.A | Ave. SpO2 N.A |
| Ch1 | Ch4 | Ch7 |
| Ave. SpO2 ~99 | Ave. SpO2 ~98 | Ave. SpO2 N.A |
| Ch2 | Ch5 | Ch8 |
| Ave. SpO2 ~99 | Ave. SpO2 N.A | Ave. SpO2 N.A |
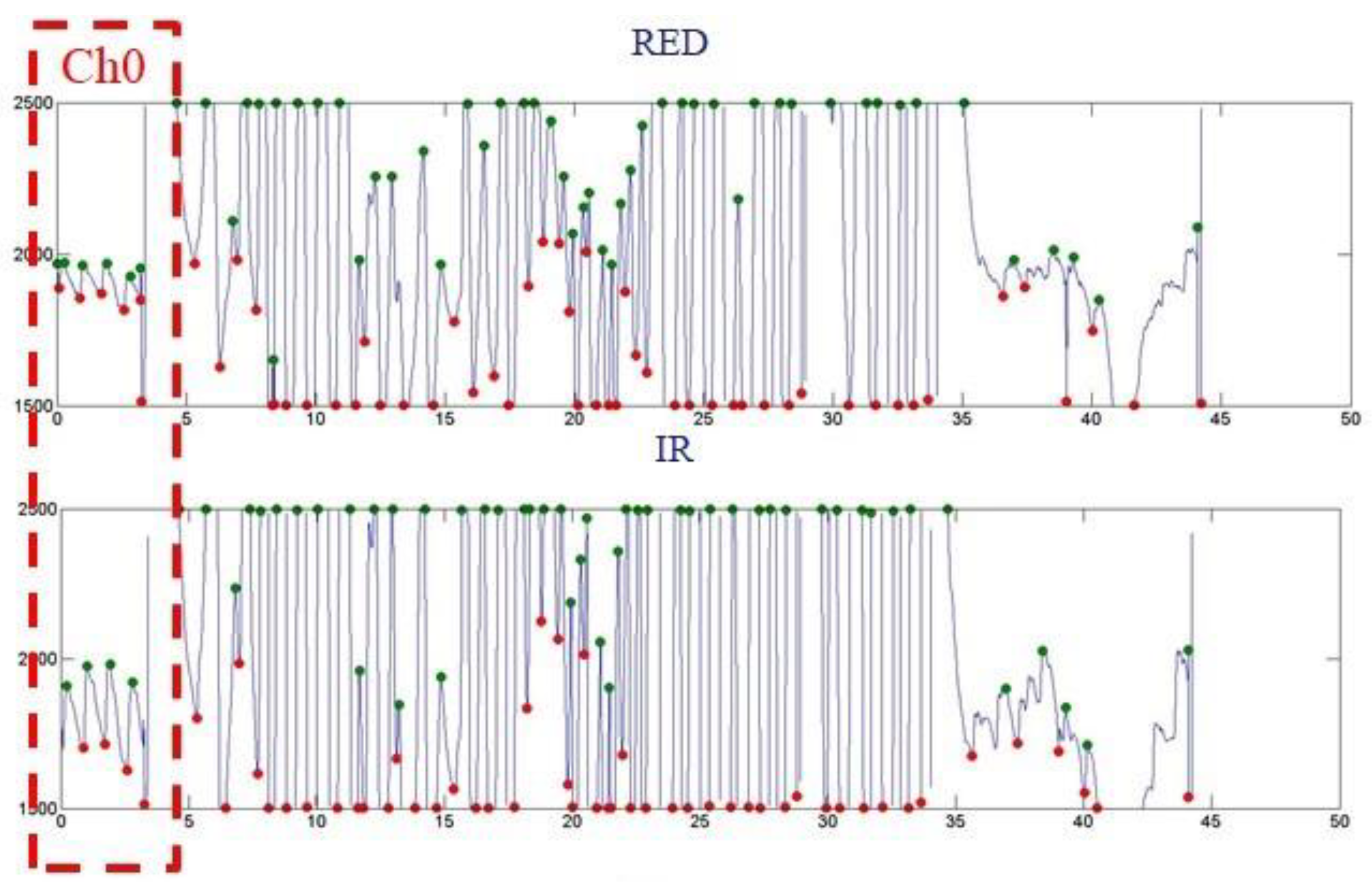
| Ch0 | Ch3 | Ch6 |
|---|---|---|
| Ave. SpO2 ~99 | Ave. SpO2 N.A | Ave. SpO2 N.A |
| Ch1 | Ch4 | Ch7 |
| Ave. SpO2 N.A | Ave. SpO2 N.A | Ave. SpO2 N.A |
| Ch2 | Ch5 | Ch8 |
| Ave. SpO2 N.A | Ave. SpO2 N.A | Ave. SpO2 N.A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, W.-C.; Wu, T.-C.; Wang, J.-S. Design and Application of a Flexible Blood Oxygen Sensing Array for Wearable Devices. Micromachines 2022, 13, 1742. https://doi.org/10.3390/mi13101742
Kuo W-C, Wu T-C, Wang J-S. Design and Application of a Flexible Blood Oxygen Sensing Array for Wearable Devices. Micromachines. 2022; 13(10):1742. https://doi.org/10.3390/mi13101742
Chicago/Turabian StyleKuo, Wen-Cheng, Tzu-Chien Wu, and Jun-Sheng Wang. 2022. "Design and Application of a Flexible Blood Oxygen Sensing Array for Wearable Devices" Micromachines 13, no. 10: 1742. https://doi.org/10.3390/mi13101742
APA StyleKuo, W.-C., Wu, T.-C., & Wang, J.-S. (2022). Design and Application of a Flexible Blood Oxygen Sensing Array for Wearable Devices. Micromachines, 13(10), 1742. https://doi.org/10.3390/mi13101742







