Simultaneous Determination of Dopamine and Uric Acid in Real Samples Using a Voltammetric Nanosensor Based on Co-MOF, Graphene Oxide, and 1-Methyl-3-butylimidazolium Bromide
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Apparatus
2.3. Synthesis of the CoMOF
2.4. Synthesis of GO and CoMOF-GO
2.5. Preparation of Modified Electrode
2.6. Preparation of the Real Samples
3. Results and Discussion
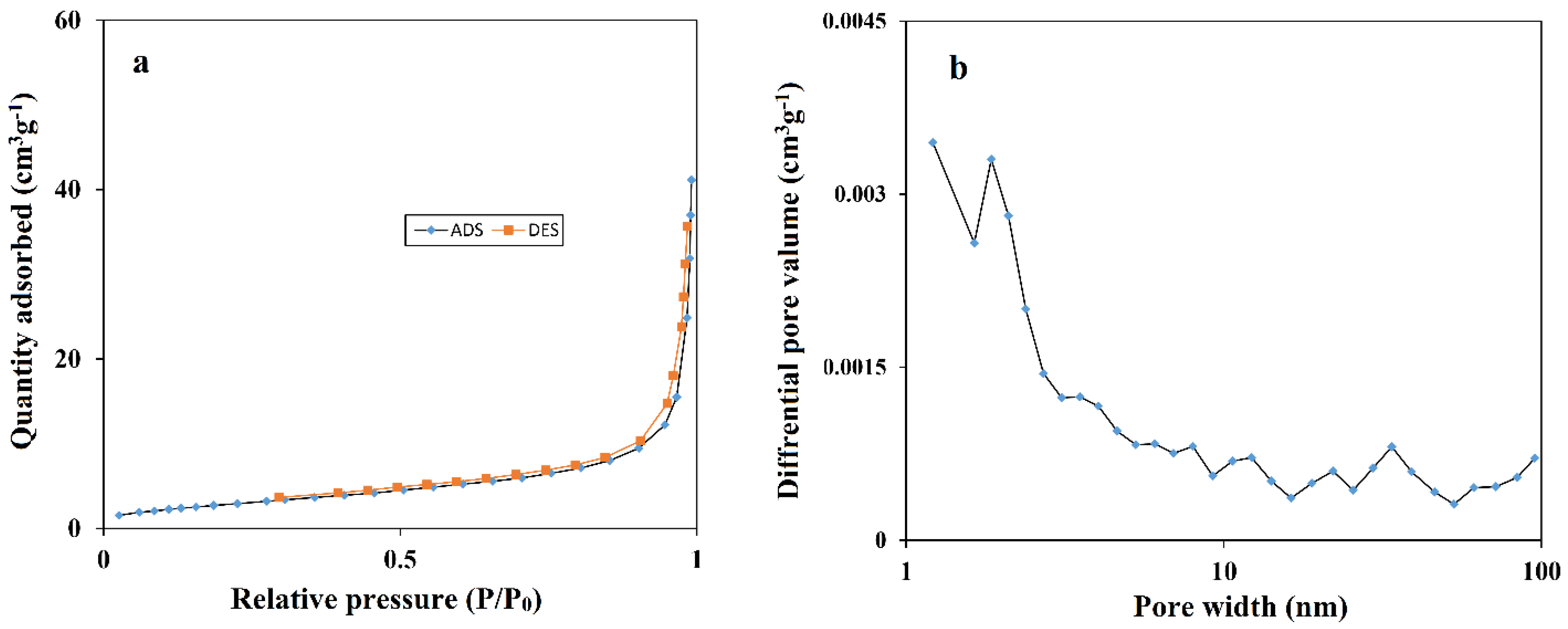
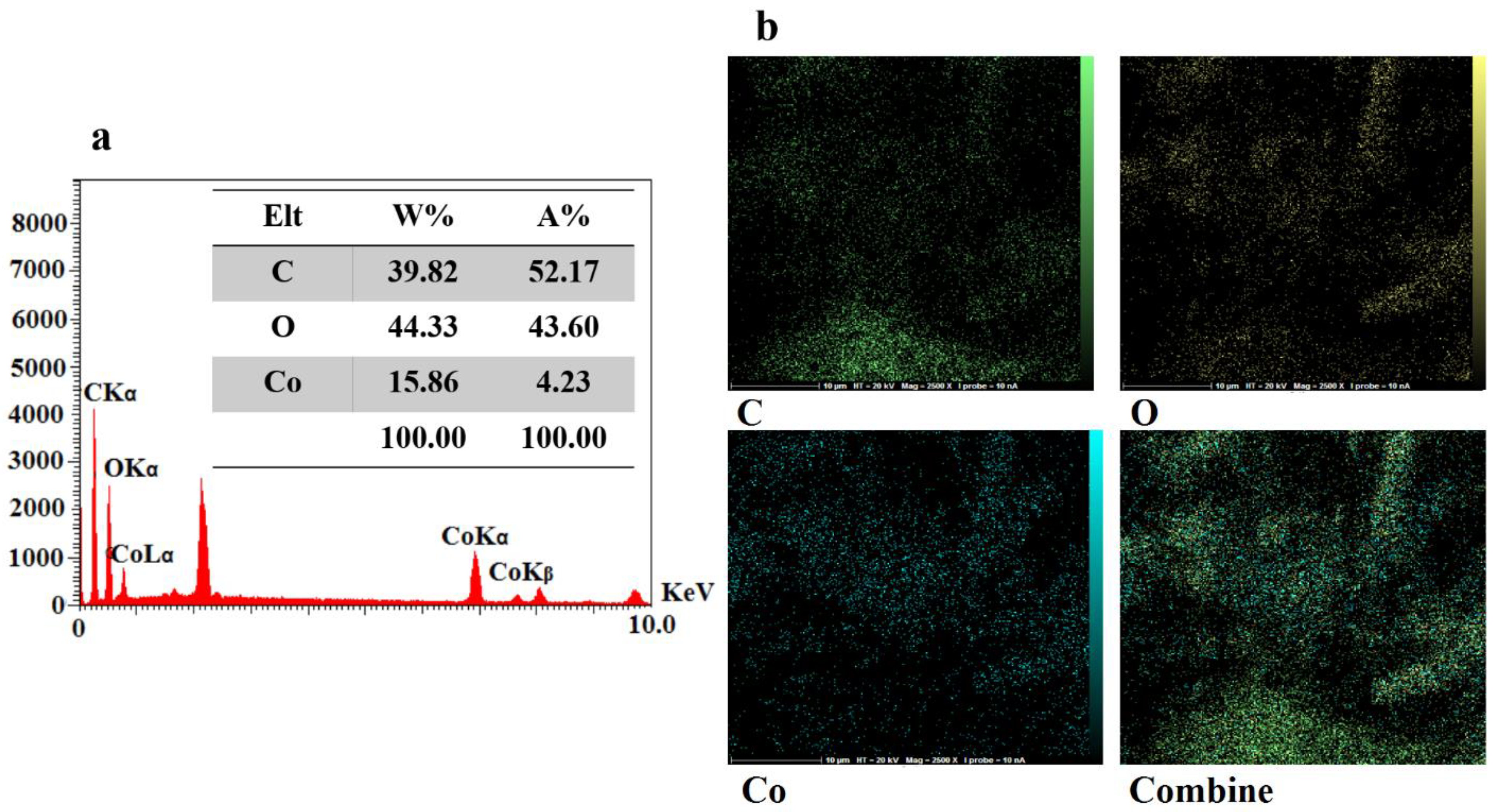
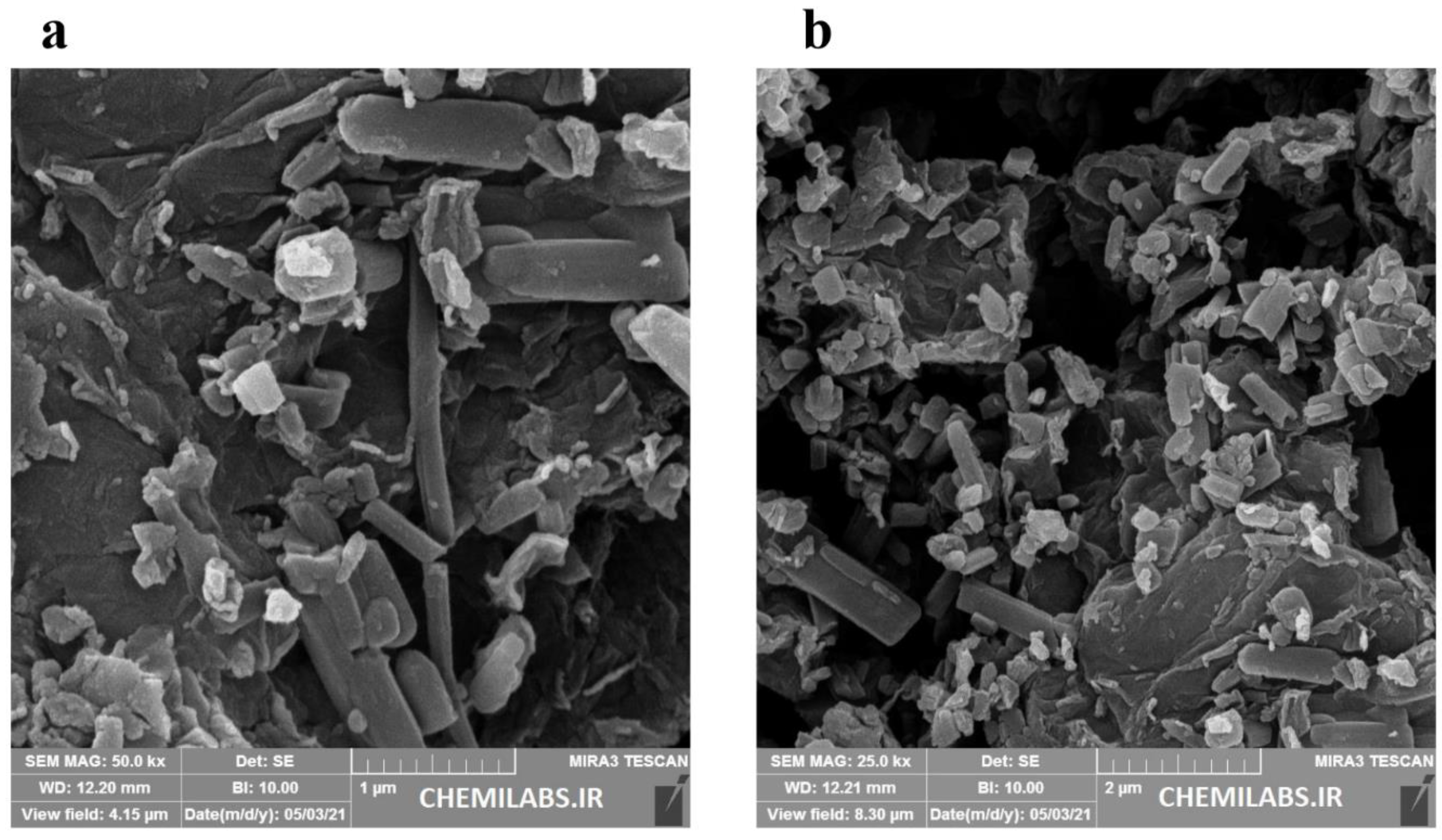
3.1. Characterization of the CoMOF-GO Nanocomposite
3.2. Electrochemical Behaviors of DA on the CoMOF-GO/1-M,3-BB/CPE
3.3. Effect of Scan Rate on the redox reaction of Dopamine
3.4. Chronoamperometric Analyses
3.5. Dynamic Range and Limit of Detection
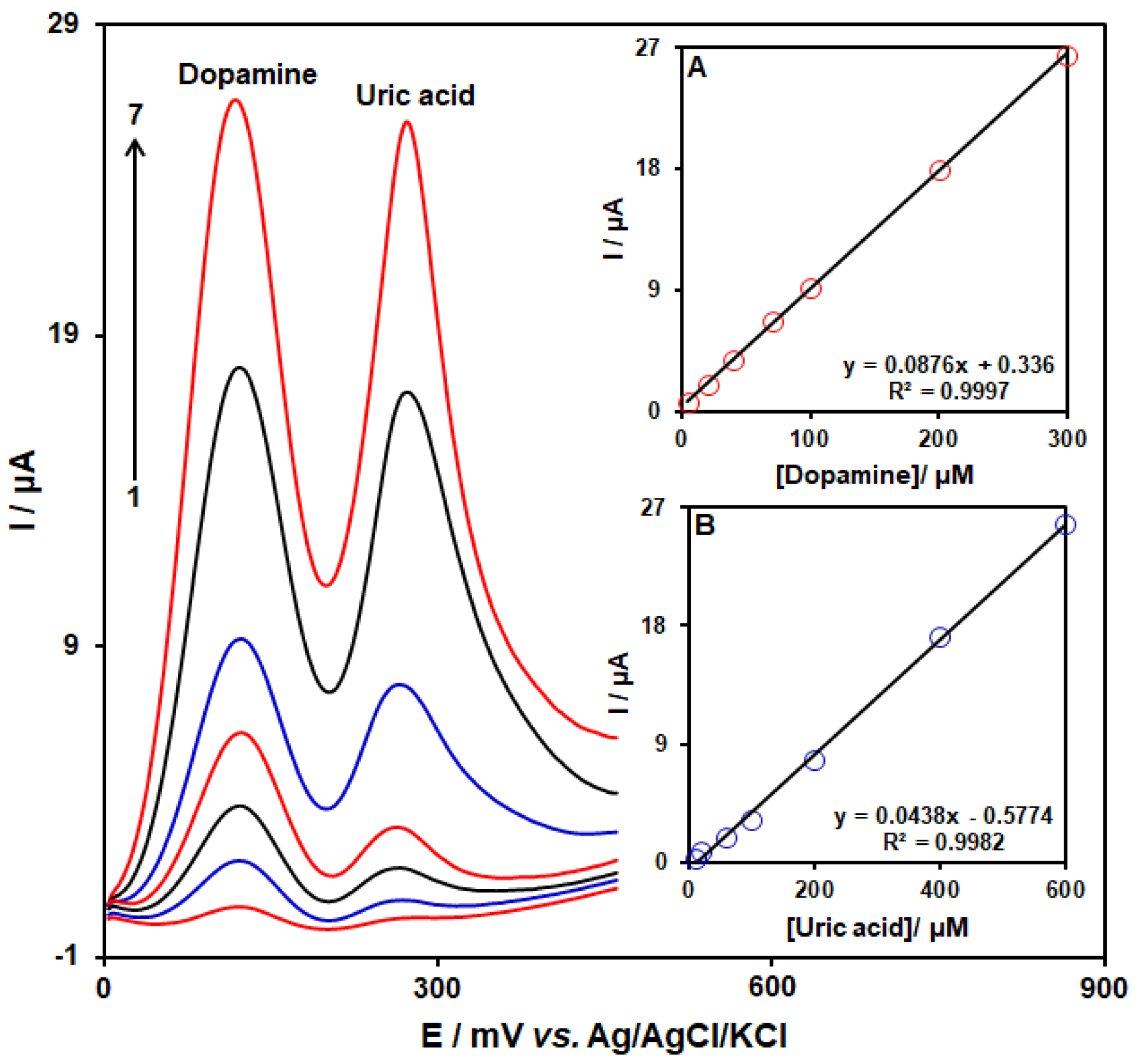
3.6. SWV Analysis for the Co-Detection of DA with UA
3.7. Stability and Repeatability of CoMOF-GO/1-M,3-BB/CPE
3.8. Analysis of Real Specimens
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Sun, L.; Li, G.; Hu, J.; He, Q. Ultrasensitive detection of dopamine via electrochemical route on spindle-like α-Fe2O3 Mesocrystals/rGO modified GCE. Mater. Res. Bull. 2021, 133, 111050. [Google Scholar] [CrossRef]
- Beytollahi, A. Biochemical Hormones and Financial Behaviours: A Mini Review on Dopamine and Oxytocin (2015–2019). Chem. Methodol. 2020, 5, 114–134. [Google Scholar]
- Keyan, A.K.; Yu, C.-L.; Rajakumaran, R.; Sakthinathan, S.; Wu, C.-F.; Vinothini, S.; Chen, S.M.; Chiu, T.-W. Highly sensitive and selective electrochemical detection of dopamine based on CuCrO2-TiO2 composite decorated screen-printed modified electrode. Microchem. J. 2021, 160, 105694. [Google Scholar] [CrossRef]
- Sankaranarayanan, P.; M Venkateswaran, S. Affordable voltammetric sensor based on anodized disposable pencil graphite electrodes for sensitive determination of dopamine and uric acid in presence of high concentration of ascorbic acid. J. Electrochem. Sci. Eng. 2020, 10, 263–279. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Hatami, M.; Moradi, R.; Khalilzadeh, M.A.; Amiri, S.; Sadeghifar, H. Synergic effect of Pt-Co nanoparticles and a dopamine derivative in a nanostructured electrochemical sensor for simultaneous determination of N-acetylcysteine, paracetamol and folic acid. Microchim. Acta 2016, 183, 2957–2964. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Kubendhiran, S.; Chen, S.M.; Manibalan, K.; Govindasamy, M.; Tamizhdurai, P.; Huang, S.T. Reduced Graphene Oxide Non—Covalent Functionalized with Zinc Tetra Phenyl Porphyrin Nanocomposite for Electrochemical Detection of Dopamine in Human Serum and Rat Brain Samples. Electroanalysis 2016, 28, 2126–2135. [Google Scholar] [CrossRef]
- Kumar, M.; Swamy, B.K.; Reddy, S.; Zhao, W.; Chetana, S.; Kumar, V.G. ZnO/functionalized MWCNT and Ag/functionalized MWCNT modified carbon paste electrodes for the determination of dopamine, paracetamol and folic acid. J. Electroanal. Chem. 2019, 835, 96–105. [Google Scholar] [CrossRef]
- Kong, D.; Zhuang, Q.; Han, Y.; Xu, L.; Wang, Z.; Jiang, L.; Chi, Y. Simultaneous voltammetry detection of dopamine and uric acid in human serum and urine with a poly(procaterol hydrochloride) modified glassy carbon electrode. Talanta 2018, 185, 203–212. [Google Scholar] [CrossRef]
- Lu, H.F.; Li, J.Y.; Zhang, M.M.; Wu, D.; Zhang, Q.L. A highly selective and sensitive colorimetric uric acid biosensor based on Cu (II)-catalyzed oxidation of 3, 3′, 5, 5′-tetramethylbenzidine. Sens. Actuators B Chem. 2017, 244, 77–83. [Google Scholar] [CrossRef]
- Li, H.; Zhou, K.; Cao, J.; Wei, Q.; Lin, C.T.; Pei, S.E.; Meng, L. A novel modification to boron-doped diamond electrode for enhanced, selective detection of dopamine in human serum. Carbon 2021, 171, 16–28. [Google Scholar] [CrossRef]
- Guan, J.F.; Zou, J.; Liu, Y.P.; Jiang, X.Y.; Yu, J.G. Hybrid carbon nanotubes modified glassy carbon electrode for selective, sensitive and simultaneous detection of dopamine and uric acid. Ecotoxicol. Environ. Saf. 2020, 201, 110872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, J. High-index {hk0} facets platinum concave nanocubes loaded on multiwall carbon nanotubes and graphene oxide nanocomposite for highly sensitive simultaneous detection of dopamine and uric acid. Talanta 2020, 207, 120296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, B.B.; Liang, E.W.; Gehrels, N.; Burrows, D.N.; Mészáros, P. Making a short gamma-ray burst from a long one: Implications for the nature of GRB 060614. Astrophys. J. 2007, 655, L25. [Google Scholar] [CrossRef] [Green Version]
- Peitzsch, M.; Prejbisz, A.; Kroiß, M.; Beuschlein, F.; Arlt, W.; Januszewicz, A.; Eisenhofer, G. Analysis of plasma 3-methoxytyramine, normetanephrine and metanephrine by ultraperformance liquid chromatographytandem mass spectrometry: Utility for diagnosis of dopamine-producing metastatic phaeochromocytoma. Ann. Clin. Biochem. 2013, 50, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klegeris, A.; Korkina, L.G.; Greenfield, S.A. Autoxidation of dopamine: A comparison of luminescent and spectrophotometric detection in basic solutions. Free Radic. Biol. Med. 1995, 18, 215–222. [Google Scholar] [CrossRef]
- Lin, V.S.Y.; Lai, C.Y.; Huang, J.; Song, S.A.; Xu, S. Molecular recognition inside of multifunctionalized mesoporous silicas: Toward selective fluorescence detection of dopamine and glucosamine. J. Am. Chem. Soc. 2001, 123, 11510–11511. [Google Scholar] [CrossRef]
- Kang, J.; Yin, X.B.; Yang, X.; Wang, E. Electrochemiluminescence quenching as an indirect method for detection of dopamine and epinephrine with capillary electrophoresis. Electrophoresis 2005, 26, 1732–1736. [Google Scholar] [CrossRef]
- Sasa, S.; Blank, C.L. Determination of serotonin and dopamine in mouse brain tissue by high performance liquid chromatography with electrochemical detection. Anal. Chem. 1997, 49, 354–359. [Google Scholar] [CrossRef]
- Liu, S.; Yan, J.; He, G.; Zhong, D.; Chen, J.; Shi, L.; Jiang, H. Layer-by-layer assembled multilayer films of reduced graphene oxide/gold nanoparticles for the electrochemical detection of dopamine. J. Electroanal. Chem. 2012, 672, 40–44. [Google Scholar] [CrossRef]
- Qu, Z.; Muhammad, Y.; He, W.; Li, J.; Gao, Z.; Fu, J.; Zhao, Z. Designing C-Fe-O bonded MIL-88B (Fe)/jasmine petal-derived-carbon composite biosensor for the simultaneous detection of dopamine and uric acid. Chem. Eng. J. 2021, 404, 126570. [Google Scholar] [CrossRef]
- Yue, H.Y.; Huang, S.; Chang, J.; Heo, C.; Yao, F.; Adhikari, S.; Lee, Y.H. ZnO nanowire arrays on 3D hierachical graphene foam: Biomarker detection of Parkinson’s disease. ACS Nano 2014, 8, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Salaria, S.; Beitollahi, H. Sensitive Simultaneous Detection of Methyldopa and Hydrochlorothiazide on Ce3+-NiO Hexagonal Nanoparticles-Modified Electrode. Chem. Methodol. 2021, 5, 407–415. [Google Scholar]
- Karimi-Maleh, H.; Karimi, F.; Orooji, Y.; Mansouri, G.; Razmjou, A.; Aygun, A.; Sen, F. A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; A highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci. Rep. 2021, 10, 11699. [Google Scholar] [CrossRef] [PubMed]
- Ameri Akhtiar Abadi, M.; Masrournia, M.; Abedi, M. Simultaneous Extraction and Preconcentration of Benzene, Toluene, Ethylbenzene and Xylenes from Aqueous Solutions Using Magnetite–Graphene Oxide Composites. Chem. Methodol. 2021, 5, 11–20. [Google Scholar]
- Liu, X.; Hu, M.; Wang, M.; Song, Y.; Zhou, N.; He, L.; Zhang, Z. Novel nanoarchitecture of Co-MOF-on-TPN-COF hybrid: Ultralowly sensitive bioplatform of electrochemical aptasensor toward ampicillin. Biosens. Bioelectron. 2019, 123, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Darabi, R.; Shabani-Nooshabadi, M.; Karimi-Maleh, H.; Gholami, A. The potential of electrochemistry for one-pot and sensitive analysis of patent blue V, tartrazine, acid violet 7 and ponceau 4R in foodstuffs using IL/Cu-BTC MOF modified sensor. Food Chem. 2022, 368, 130811. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Sheikhshoaie, I.; Nugraha, A.S.; Jang, H.W.; Shokouhimehr, M. Performance of metal–organic frameworks in the electrochemical sensing of environmental pollutants. J. Mater. Chem. A 2021, 9, 8195–8220. [Google Scholar] [CrossRef]
- Hira, S.A.; Annas, D.; Nagappan, S.; Kumar, Y.A.; Song, S.; Kim, H.J.; Park, K.H. Electrochemical sensor based on nitrogen-enriched metal–organic framework for selective and sensitive detection of hydrazine and hydrogen peroxide. J. Environ. Chem. Eng. 2021, 9, 105182. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M.; Abdi, J. In situ deposition of Ag/AgCl on the surface of magnetic metal-organic framework nanocomposite and its application for the visible-light photocatalytic degradation of Rhodamine dye. J. Hazard. Mater. 2019, 378, 120741. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Yang, W.; Shen, W.; Xue, H.; Pang, H. MOF-derived metal oxide composites for advanced electrochemical energy storage. Small 2018, 14, 1704435. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, X.M.; Ma, J.P.; Liu, Q.K.; Wang, P.; Dong, Y.B. Cu (I)-MOF: Naked-eye colorimetric sensor for humidity and formaldehyde in single-crystal-to-single-crystal fashion. Chem. Commun. 2014, 50, 1444–1446. [Google Scholar] [CrossRef] [Green Version]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Llabrés i Xamena, F.X.; Garcia, H. Semiconductor behavior of a metal-organic framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Liu, S.; Xu, M. The applications of metal− organic frameworks in electrochemical sensors. Chem. Electro. Chem. 2018, 5, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Alezi, D.; Belmabkhout, Y.; Suyetin, M.; Bhatt, P.M.; Weseliński, Ł.J.; Solovyeva, V.; Eddaoudi, M. MOF crystal chemistry paving the way to gas storage needs: Aluminum-based soc-MOF for CH4, O2, and CO2 storage. J. Am. Chem. Soc. 2015, 137, 13308–13318. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, C.; Ye, W.; Liu, W. An electrochemical sensor for H2O2 based on a new Co-metal-organic framework modified electrode. Sens. Actuators B Chem. 2015, 215, 489–496. [Google Scholar] [CrossRef]
- Nabi Bidhendi, G.; Mehrdadi, N.; Firouzbakhsh, M. Removal of Lead from Wastewater by Iron–Benzenetricarboxylate Metal-Organic Frameworks. Chem. Methodol. 2021, 5, 271–284. [Google Scholar]
- Umesh, N.M.; Jesila, J.A.; Wang, S.F.; Vishnu, N.; Yang, Y.J. Novel voltammetric detection of norfloxacin in urine and blood serum using a flexible Ni foam based Ni-Co-MOF ultrathin nanosheets derived from Ni-Co-LDH. Microchem. J. 2021, 160, 105747. [Google Scholar] [CrossRef]
- Li, H.K.; Ye, H.L.; Zhao, X.X.; Sun, X.L.; Zhu, Q.Q.; Han, Z.Y.; He, H. Artful union of a zirconium-porphyrin MOF/GO composite for fabricating an aptamer-based electrochemical sensor with superb detecting performance. Chin. Chem. Lett. 2021, 32, 2851–2855. [Google Scholar] [CrossRef]
- Beyranvand, H.S.; Sarlak, H. Effects of graphene oxide (GO) nanoparticle and SDS on storage capacity of CO2 in hydrate. Eurasian Chem. Commun. 2021, 3, 233–243. [Google Scholar]
- Zarandi, M.P.; Beitollahi, H. Design of electrochemical sensor based on N-doped reduced graphene oxide/copper oxide nanocomposite and ionic liquid for the simultaneous determination of 4-aminophenol and acetaminophen. Microchem. J. 2022, 181, 107726. [Google Scholar] [CrossRef]
- Silva, L.M.; Goncalves, B.S.; Braga, J.D.O.; de Souza, T.C.; de Castro, V.G.; Silva, G.G.; Nunes, E.H. Preparation of titania-reduced graphene oxide composite coatings with electro-and photosensitive properties. Appl. Surf. Sci. 2021, 538, 148029. [Google Scholar] [CrossRef]
- Nemati, F.; Rezaie, M.; Tabesh, H.; Eid, K.; Xu, G.; Ganjali, M.R.; Karimi-Maleh, H. Cerium functionalized graphene nano-structures and their applications; A review. Environ. Res. 2022, 208, 112685. [Google Scholar] [CrossRef]
- Jahani, P.M.; Beitollahi, H.; Tajik, S. Surface amplification of graphite screen printed electrode using reduced graphene oxide/polypyrrole nanotubes nanocomposite; A powerful electrochemical strategy for determination of sulfite in food samples. Food Chem. Toxicol. 2022, 167, 113274. [Google Scholar] [CrossRef]
- Tian, J.; Shan, Q.; Yin, X.; Wu, W. A facile preparation of graphene/reduced graphene oxide/Ni(OH)2 two dimension nanocomposites for high performance supercapacitors. Adv. Powder Technol. 2019, 30, 3118–3126. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Q.; Liu, J.; Zhang, H.; Li, Z.; Li, R.; Wang, J. Interfacial growth of a metal–organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium (VI). J. Mater. Chem. A 2017, 5, 17933–17942. [Google Scholar] [CrossRef]
- Hernández-Vargas, S.G.; Cevallos-Morillo, C.A.; Aguilar-Cordero, J.C. Effect of Ionic Liquid Structure on the Electrochemical Response of Dopamine at Room Temperature Ionic Liquid-modified Carbon Paste Electrodes (IL–CPE). Electroanalysis 2020, 32, 1938–1948. [Google Scholar] [CrossRef]
- Sengiz, C.; Congur, G.; Erdem, A. Development of ionic liquid modified disposable graphite electrodes for label-free electrochemical detection of DNA hybridization related to Microcystis spp. Sensors 2015, 15, 22737–22749. [Google Scholar] [CrossRef] [Green Version]
- Abd El Salam, H.M.; Nassar, H.N.; Khidr, A.S.; Zaki, T. Antimicrobial activities of green synthesized Ag nanoparticles@ Ni-MOF nanosheets. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2791–2798. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Cho, H.Y.; Yang, D.A.; Kim, J.; Jeong, S.Y.; Ahn, W.S. CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating. Catal. Today 2012, 185, 35–40. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Kim, Y.R.; Bong, S.; Kang, Y.J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Li, C.X.; Ding, X.T.; Yang, Q.; Qi, Y.M.; Zhang, H.M.; Qu, L.T. Detection of dopamine at graphene-ZIF-8 nanocomposite modified electrode. Chin. Chem. Lett. 2017, 28, 1473–1478. [Google Scholar] [CrossRef]
- Kumar, M.; Fu, Y.; Wang, M.; Swamy, B.K.; Jayaprakash, G.K.; Zhao, W. Influence of cationic surfactant cetyltrimethylammonium bromide for electrochemical detection of guanine, uric acid and dopamine. J. Mol. Liq. 2021, 321, 114893. [Google Scholar] [CrossRef]
- Shin, J.W.; Kim, K.J.; Yoon, J.; Jo, J.; El-Said, W.A.; Choi, J.W. Silver nanoparticle modified electrode covered by graphene oxide for the enhanced electrochemical detection of dopamine. Sensors 2017, 17, 2771. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Ouyang, X.; Ding, Y.; Luo, L.; Xu, D.; Ning, Y. Electrochemical preparation of nickel and copper oxides-decorated graphene composite for simultaneous determination of dopamine, acetaminophen and tryptophan. Talanta 2016, 146, 114–121. [Google Scholar] [CrossRef]
- Zhou, K.; Shen, D.; Li, X.; Chen, Y.; Hou, L.; Zhang, Y.; Sha, J. Molybdenum oxide-based metal-organic framework/polypyrrole nanocomposites for enhancing electrochemical detection of dopamine. Talanta 2020, 209, 120507. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, Y.; Feng, C.; Wang, S.; Wu, H.; Zhang, G. Synthesis of CuO/g-C3N4 composites, and their application to voltammetric sensing of glucose and dopamine. Microchim. Acta 2019, 186, 10. [Google Scholar] [CrossRef]







| Electrode | Anodic Peak Current (μA) | Anodic Peak Potential (mV) | Cathodic Peak Current (μA) | Cathodic Peak Potential (mV) |
|---|---|---|---|---|
| CPE | 3.4 | 380 | −1.6 | 160 |
| CoMOF/CPE | 4.3 | 380 | −1.9 | 160 |
| 1-M,3-BB/CPE | 5.3 | 370 | −1.7 | 235 |
| GO/CPE | 6.7 | 205 | −4.2 | 0 |
| CoMOF-GO/CPE | 9.3 | 170 | −4.2 | 0 |
| CoMOF-GO/1-M,3-BB/CPE | 14 | 130 | −4.9 | 0 |
| Modifier | Linear Range (µM) | LOD (nM) | References |
|---|---|---|---|
| AuNBP/MWCNTs | 0.05–2700 | 15 | [10] |
| Graphene | 4–100 | 2640 | [53] |
| Graphene-ZIF-8 | 3–1000 | 1000 | [54] |
| Cationic surfactant Cetyltrimethylammonium Bromide | 1–70 | 200 | [55] |
| Ag/GO/ITO | 0.1–100 | 200 | [56] |
| NiO-CuO/GR | 0.5–20 | 167 | [57] |
| CuTRZMoO4@PPy-n | 1–100 | 80 | [58] |
| CuO/g-C3N4 | 0.2–16 | 60 | [59] |
| CoMOF-GO/1-M,3-BB | 0.01–300 | 40 | This work |
| Sample | Spiked | Found | Recovery (%) | R.S.D. (%) | ||||
|---|---|---|---|---|---|---|---|---|
| DA | UA | DA | UA | DA | UA | DA | UA | |
| DA injection | 0 | 0 | 4.0 | - | - | - | 2.9 | - |
| 1.0 | 5.0 | 4.9 | 5.1 | 98.0 | 102.0 | 3.1 | 1.7 | |
| 2.0 | 7.5 | 6.1 | 7.3 | 101.7 | 97.3 | 1.9 | 2.3 | |
| 3.0 | 10.0 | 6.9 | 10.1 | 98.6 | 101.0 | 2.4 | 2.2 | |
| 4.0 | 12.5 | 8.2 | 12.4 | 102.5 | 99.2 | 2.7 | 3.5 | |
| Urine | 0 | 0 | - | 3.0 | - | - | - | - |
| 5.0 | 1.0 | 5.1 | 3.9 | 102.0 | 97.5 | 1.8 | 3.0 | |
| 7.0 | 3.0 | 6.8 | 6.1 | 97.1 | 101.7 | 2.6 | 2.0 | |
| 9.0 | 5.0 | 8.8 | 7.9 | 97.8 | 98.7 | 3.2 | 2.7 | |
| 11.0 | 7.0 | 11.1 | 10.4 | 100.9 | 104.0 | 2.6 | 2.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roostaee, M.; Beitollahi, H.; Sheikhshoaie, I. Simultaneous Determination of Dopamine and Uric Acid in Real Samples Using a Voltammetric Nanosensor Based on Co-MOF, Graphene Oxide, and 1-Methyl-3-butylimidazolium Bromide. Micromachines 2022, 13, 1834. https://doi.org/10.3390/mi13111834
Roostaee M, Beitollahi H, Sheikhshoaie I. Simultaneous Determination of Dopamine and Uric Acid in Real Samples Using a Voltammetric Nanosensor Based on Co-MOF, Graphene Oxide, and 1-Methyl-3-butylimidazolium Bromide. Micromachines. 2022; 13(11):1834. https://doi.org/10.3390/mi13111834
Chicago/Turabian StyleRoostaee, Maryam, Hadi Beitollahi, and Iran Sheikhshoaie. 2022. "Simultaneous Determination of Dopamine and Uric Acid in Real Samples Using a Voltammetric Nanosensor Based on Co-MOF, Graphene Oxide, and 1-Methyl-3-butylimidazolium Bromide" Micromachines 13, no. 11: 1834. https://doi.org/10.3390/mi13111834
APA StyleRoostaee, M., Beitollahi, H., & Sheikhshoaie, I. (2022). Simultaneous Determination of Dopamine and Uric Acid in Real Samples Using a Voltammetric Nanosensor Based on Co-MOF, Graphene Oxide, and 1-Methyl-3-butylimidazolium Bromide. Micromachines, 13(11), 1834. https://doi.org/10.3390/mi13111834







