Enhanced Performance of Perovskite Light-Emitting Diodes via Phenylmethylamine Passivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Device Characterization
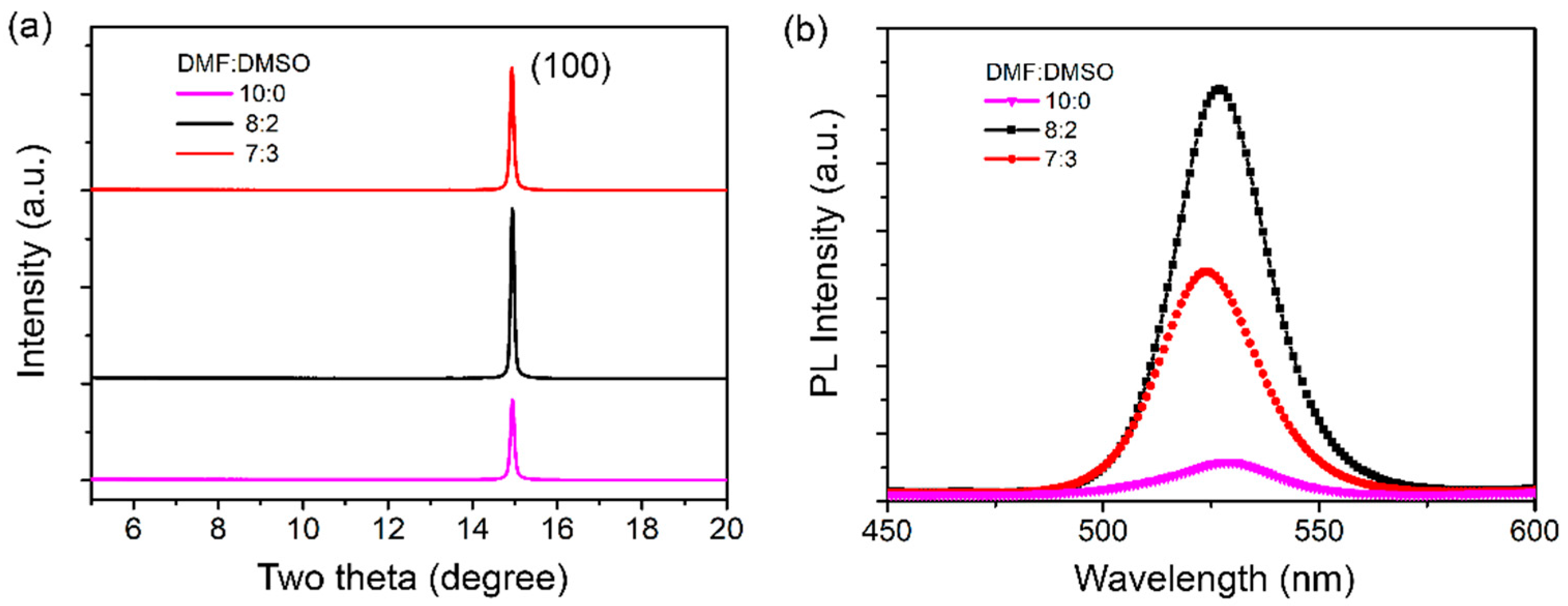
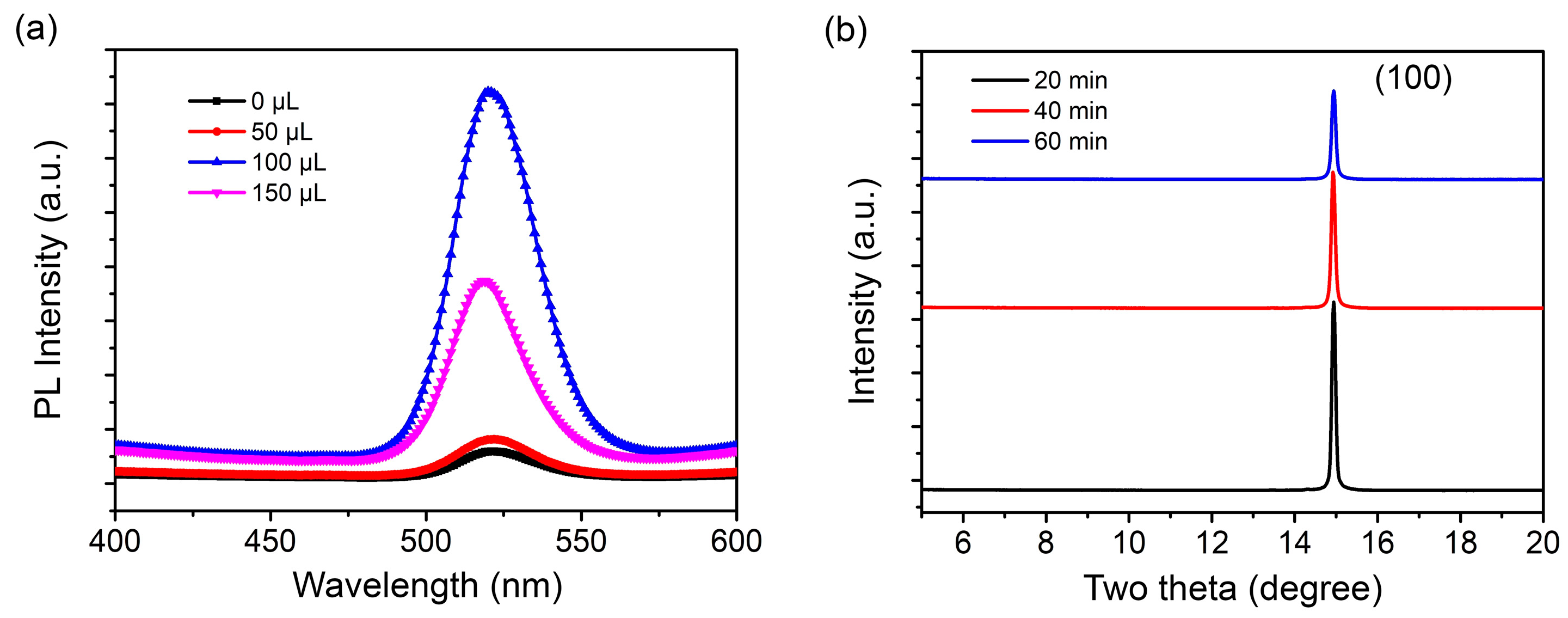
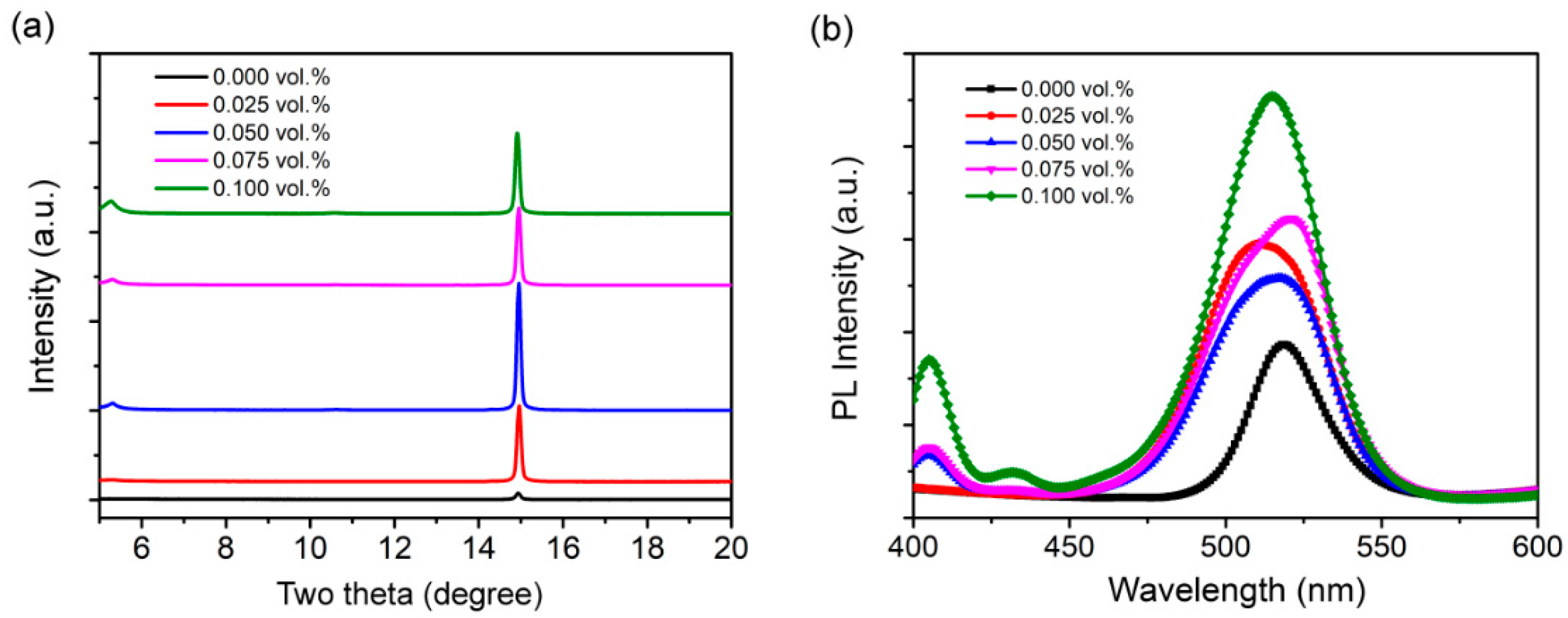
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quan, L.N.; de Arquer, F.P.G.; Sabatini, R.P.; Sargent, E.H. Perovskites for light emission. Adv. Mater. 2018, 30, 1801996. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shi, W.X.; Zhou, Y.M.; Cao, X.A. Impact of dopant aggregation on the EL of blue fluorescent host-dopant emitters. IEEE Electron Device Lett. 2019, 40, 750–753. [Google Scholar] [CrossRef]
- Shi, W.X.; Liu, N.; Zhou, Y.M.; Cao, X.A. Effects of postannealing on the characteristics and reliability of polyfluorene organic light-emitting diodes. IEEE Trans. Electron Devices 2019, 66, 1057. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Mei, S.J.; Sun, D.W.; Liu, N.; Mei, F.; Xu, J.X.; Cao, X.A. Improved charge injection and transport of light-emitting diodes based on two-dimensional materials. Appl. Sci. 2019, 9, 4140. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Mei, S.J.; Sun, D.W.; Shi, W.X.; Feng, J.H.; Zhou, Y.M.; Mei, F.; Xu, J.X.; Jiang, Y.; Cao, X.A. Effects of charge transport materials on blue fluorescent organic light-emitting diodes with a host-dopant system. Micromachines 2019, 10, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.H.; Sun, D.W.; Mei, S.J.; Shi, W.X.; Mei, F.; Zhou, Y.M.; Xu, J.X.; Jiang, Y.; Wu, L.Z. Plasmonic-enhanced organic light-emitting diodes based on a graphene oxide/Au nanoparticles composite hole injection layer. Front. Mater. 2018, 5, 75. [Google Scholar] [CrossRef]
- Pena, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Lin, K.B.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.W.; Lu, J.X.; Xie, L.Q.; Zhao, W.J.; Zhang, D.; Yan, C.Z.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Zhou, L.; Ou, Q.D.; Chen, J.D.; Shen, S.; Tang, J.X.; Li, Y.Q.; Lee, S.T. Light manipulation for organic optoelectronics using bio-inspired moth’s eye nanostructures. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Deschler, F.; Price, M.; Pathak, S.; Klintberg, L.E.; Jarausch, D.D.; Higler, R.; Huttner, S.; Leijtens, T.; Stranks, S.D.; Snaith, H.J.; et al. High photoluminescence efficiency and optically pumped lasing in solution-processed mixed halide perovskite semiconductors. J. Phys. Chem. Lett. 2014, 5, 1421–1426. [Google Scholar] [CrossRef]
- Sun, D.W.; Zhang, K.; Mei, S.J.; Xu, J.X.; Jiang, Y.; Xiao, X.H.; Zhou, Y.M.; Mei, F. High performance perovskite LEDs via SPR and enhanced hole injection by incorporated MoS2. J. Phys. D Appl. Phys. 2021, 54, 214002. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Mei, S.J.; Feng, J.J.; Sun, D.W.; Mei, F.; Xu, J.X.; Cao, X.A. Effects of PEDOT:PSS:GO composite hole transport layer on the luminescence of perovskite light-emitting diodes. RSC Adv. 2020, 10, 26381–26387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.M.; Mei, S.J.; Sun, D.W.; Liu, N.; Shi, W.X.; Feng, J.H.; Mei, F.; Xu, J.X.; Jiang, Y.; Cao, X.A. Improved efficiency of perovskite light-emitting diodes using a three-step spin-coated CH3NH3PbBr3 emitter and a PEDOT:PSS/MoO3-ammonia composite hole transport layer. Micromachines 2019, 10, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, J.Q.; Schubert, M.F.; Kim, J.K.; Schubert, E.F.; Chen, M.F.; Lin, S.Y.; Liu, W.; Smart, J.A. Optical thin-film materials with low refractive index for broadband elimination of Fresnel reflection. Nat. Photonics 2007, 1, 176–179. [Google Scholar] [CrossRef]
- Chen, J.D.; Zhou, L.; Ou, Q.D.; Li, Y.Q.; Shen, S.; Lee, S.T.; Tang, J.X. Enhanced light harvesting in organic solar cells featuring a biomimetic active layer. Adv. Energy Mater. 2014, 4, 1301777. [Google Scholar] [CrossRef]
- Jiang, J.; Chu, Z.M.; Yin, Z.; Li, J.Z.; Yang, Y.G.; Chen, J.R.; Wu, J.L.; You, J.B.; Zhang, X.W. Red perovskite light-emitting diodes with efficiency exceeding 25% realized by co-spacer cations. Adv. Mater. 2022, 34, 2204460. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, W.D.; Peng, X.M.; Sun, G.W.; Liu, X.Y.; Liu, D.H.; Li, Z.C.; He, F.R.; Shen, C.Y.; Gu, Q.; et al. Perovskite light-emitting diodes with eqe exceeding 28% through a synergetic dual-additive strategy for defect passivation and nanostructure regulation. Adv. Mater. 2021, 33, 2103268. [Google Scholar] [CrossRef]
- Nie, W.Y.; Tsai, H.H.; Asadpour, R.; Blancon, J.C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.C.; Jeong, S.H.; Park, M.H.; Kim, Y.H.; Wolf, C.; Lee, C.L.; Heo, J.H.; Sadhanala, A.; Myoung, N.; Yoo, S.; et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225. [Google Scholar] [CrossRef]
- Li, J.Q.; Shan, X.; Bade, S.G.R.; Geske, T.; Jiang, Q.L.; Yang, X.; Yu, Z.B. Single-layer halide perovskite light-emitting diodes with sub-band gap turn-on voltage and high brightness. J. Phys. Chem. Lett. 2016, 7, 4059–4066. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, X.W.; Deng, J.X.; Chu, Z.M.; Jiang, Q.; Meng, J.H.; Wang, P.Y.; Zhang, L.Q.; Yin, Z.G.; You, J.B. Efficient green light-emitting diodes based on quasi-two-dimensional composition and phase engineered perovskite with surface passivation. Nat. Commun. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.X.; Lin, K.B.; Sargent, E.H. Distribution control enables efficient reduced-dimensional perovskite LEDs. Nature 2021, 599, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.L.; Wu, L.; Liu, X.P.; Yang, X.K.; Zhou, P.; Yu, X.X.; Qin, T.S.; Shi, J.J.; Wang, S.; Li, S.S.; et al. Synergic interface optimization with green solvent engineering in mixed perovskite solar cells. Adv. Energy Mater. 2017, 7, 1700576. [Google Scholar] [CrossRef]
- Kong, X.Y.; Jiang, Y.; Li, Z.X.; Zhou, Y.S.; Xu, Z.J.; Cong, C.; Gao, X.S.; Lu, X.B.; Zhou, G.F.; Liu, J.M.; et al. Highly reproducible fabrication of perovskite films with an ultrawide antisolvent dripping window for large-scale flexible solar cells. Sol. RRL 2021, 5, 2000646. [Google Scholar] [CrossRef]
- Tao, J.Q.; Zhou, J.T.; Yao, Z.J.; Jiao, Z.B.; Wei, B.; Tan, R.Y.; Li, Z. Multi-shell hollow porous carbon nanoparticles with excellent microwave absorption properties. Carbon 2021, 172, 542–555. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.H.; Zhou, B.; Jia, X.G.; Ma, Q.S.; Yuan, N.Y.; Zheng, X.J.; Ding, J.N.; Zhang, W.H. Green anti-solvent processed planar perovskite solar cells with efficiency beyond 19%. Sol. RRL 2018, 2, 1700213. [Google Scholar] [CrossRef]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.B.; Huang, J.S. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.W.; Apergi, S.; Li, N.; Chen, M.Y.; Yin, C.Y.; Yuan, Z.C.; Gao, F.; Xie, F.Y.; Brocks, G.; Tao, S.X.; et al. Phenylalkylammonium passivation enables perovskite light emitting diodes with record high-radiance operational lifetime: The chain length matters. Nat. Commun. 2021, 12, 644. [Google Scholar] [CrossRef]
- Wang, F.; Geng, W.; Zhou, Y.; Fang, H.H.; Tong, C.J.; Loi, M.A.; Liu, L.M.; Zhao, N. Phenylalkylamine passivation of organolead halide perovskites enabling high-efficiency and air-stable photovoltaic cells. Adv. Mater. 2016, 28, 9986–9992. [Google Scholar] [CrossRef]
- Li, X.; Bi, D.Q.; Yi, C.Y.; Decoppet, J.D.; Luo, J.S.; Zakeeruddin, S.M.; Hagfeldt, A.; Gratzel, M. A vacuum flash-assisted solution process for high-efficiency large-area perovskite solar cells. Science 2016, 353, 58–62. [Google Scholar] [CrossRef]
- Konstantakou, M.; Perganti, D.; Falaras, P.; Stergiopoulos, T. Anti-solvent crystallization strategies for highly efficient perovskite solar cells. Crystals 2017, 7, 291. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.D.; Huang, F.Z.; Huang, W.C.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.B.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. Int. Ed. 2014, 53, 9898–9903. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Huang, J. Ion migration in organometal trihalide perovskite and its impact on photovoltaic efficiency and stability. Acc. Chem. Res. 2016, 49, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Park, J.H.; Nam, Y.S.; Lee, B.R.; Zhao, B.D.; Di Nuzzo, D.; Jung, E.D.; Jeon, H.; Kim, J.Y.; Jeong, H.Y.; et al. Growth of nanosized single crystals for efficient perovskite light-emitting diodes. ACS Nano 2018, 12, 3417–3423. [Google Scholar] [CrossRef]
- Yu, J.C.; Park, J.H.; Lee, S.Y.; Song, M.H. Effect of perovskite film morphology on device performance of perovskite light-emitting diodes. Nanoscale 2019, 11, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Dai, J.; Yu, Z.H.; Shao, Y.C.; Zhou, Y.; Xiao, X.; Zeng, X.C.; Huang, J.S. Tailoring passivation molecular structures for extremely small open-circuit voltage loss in perovskite solar cells. J. Am. Chem. Soc. 2019, 141, 5781–5787. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.H.; Tan, Y.; Yang, M.F.; Jiang, Y.; Lei, B.X.; Wang, L.Z.; Wu, W.Q. Small amines bring big benefits to perovskite-based solar cells and light-emitting diodes. Chem 2021, 8, 351–383. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.H.; Lee, B.R.; Jung, E.D.; Yu, J.C.; Di Nuzzo, D.; Friend, R.H.; Song, M.H. Song Amine-based passivating materials for enhanced optical properties and performance of organic-inorganic perovskites in light-emitting diodes. J. Phys. Chem. Lett. 2017, 8, 1784–1792. [Google Scholar] [CrossRef]
- Wang, N.N.; Cheng, L.; Si, J.J.; Liang, X.Y.; Jin, Y.Z.; Wang, J.P.; Huang, W. Morphology control of perovskite light-emitting diodes by using amino acid self-assembled monolayers. Appl. Phys. Lett. 2016, 108, 141102. [Google Scholar] [CrossRef]


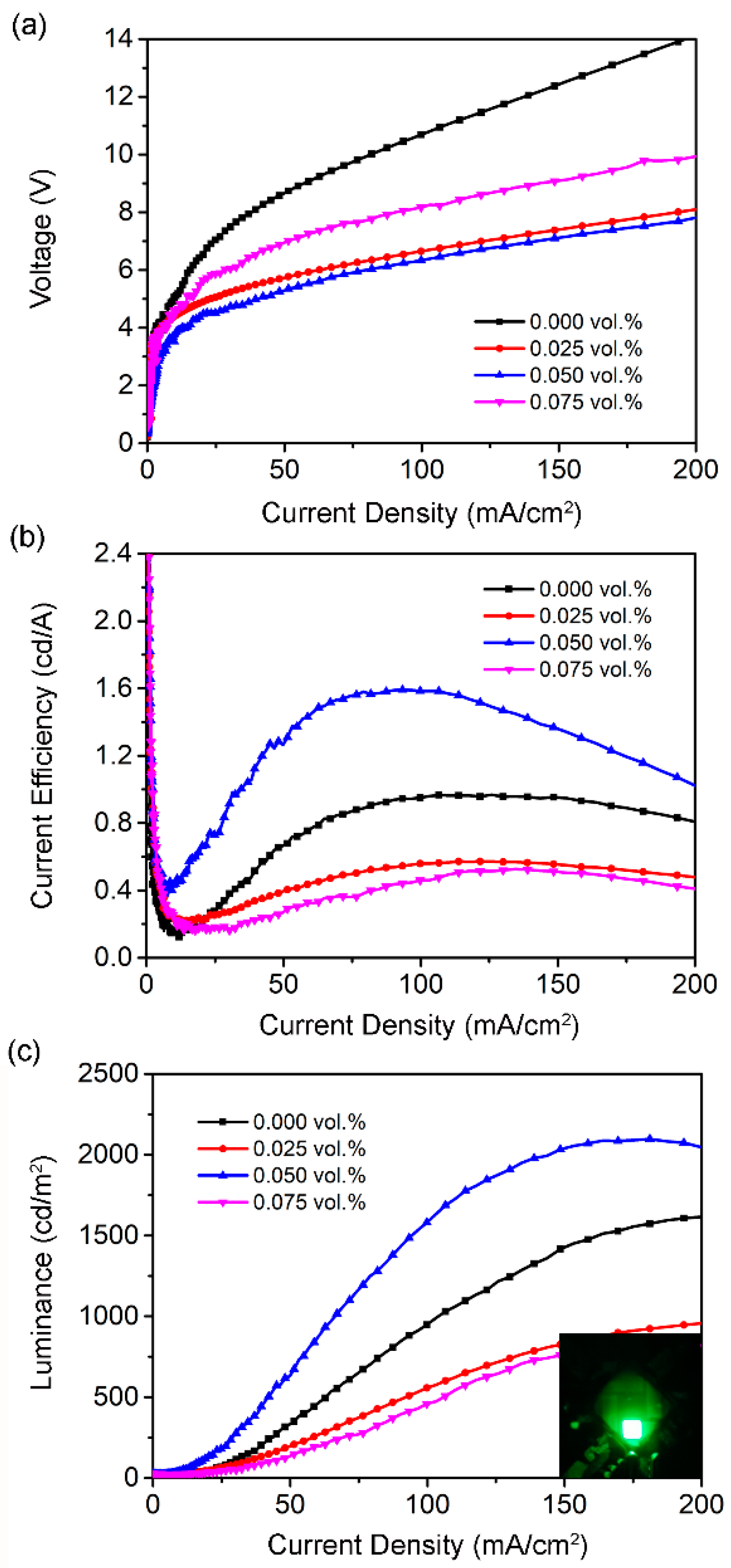
| PMA Doping Content | w/o | 0.025% | 0.050% | 0.075% |
|---|---|---|---|---|
| Lmax (cd/m2) | 1615 | 956 | 2098 | 827 |
| CEmax (cd/A) | 0.966 | 0.572 | 1.592 | 0.527 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Zhang, K.; Cai, X.; Tu, P.; Zhou, Y.; Mei, F. Enhanced Performance of Perovskite Light-Emitting Diodes via Phenylmethylamine Passivation. Micromachines 2022, 13, 1857. https://doi.org/10.3390/mi13111857
Yu S, Zhang K, Cai X, Tu P, Zhou Y, Mei F. Enhanced Performance of Perovskite Light-Emitting Diodes via Phenylmethylamine Passivation. Micromachines. 2022; 13(11):1857. https://doi.org/10.3390/mi13111857
Chicago/Turabian StyleYu, Shisong, Kai Zhang, Xiangcheng Cai, Peng Tu, Yuanming Zhou, and Fei Mei. 2022. "Enhanced Performance of Perovskite Light-Emitting Diodes via Phenylmethylamine Passivation" Micromachines 13, no. 11: 1857. https://doi.org/10.3390/mi13111857




