Development of a Highly Specific Fluoroimmunoassay for the Detection of Doxycycline Residues in Water Environmental and Animal Tissue Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of the Labeled Antibodies
2.3. QD-cFIA Procedure Used for DOX Detection
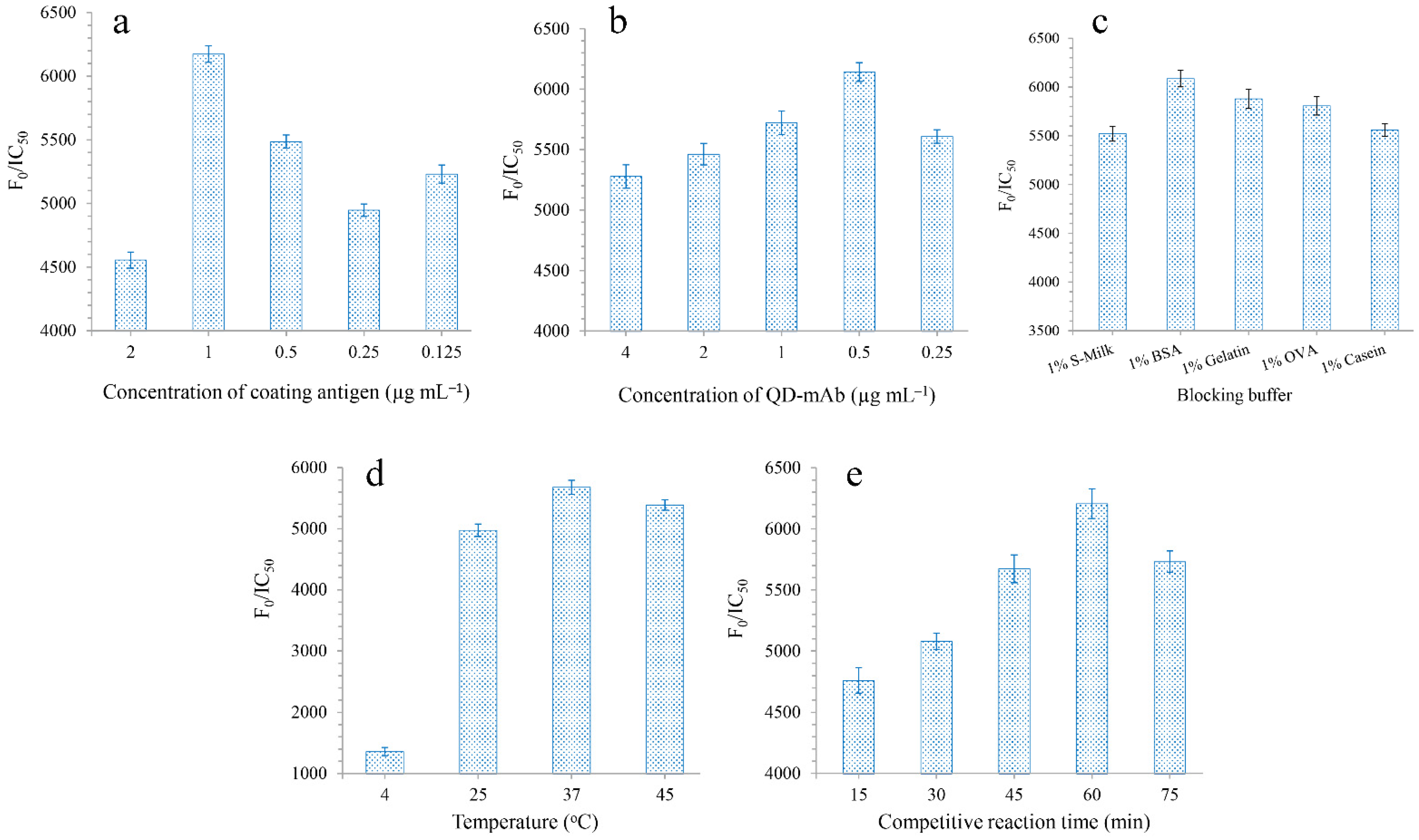
2.4. Optimization of Experimental Conditions
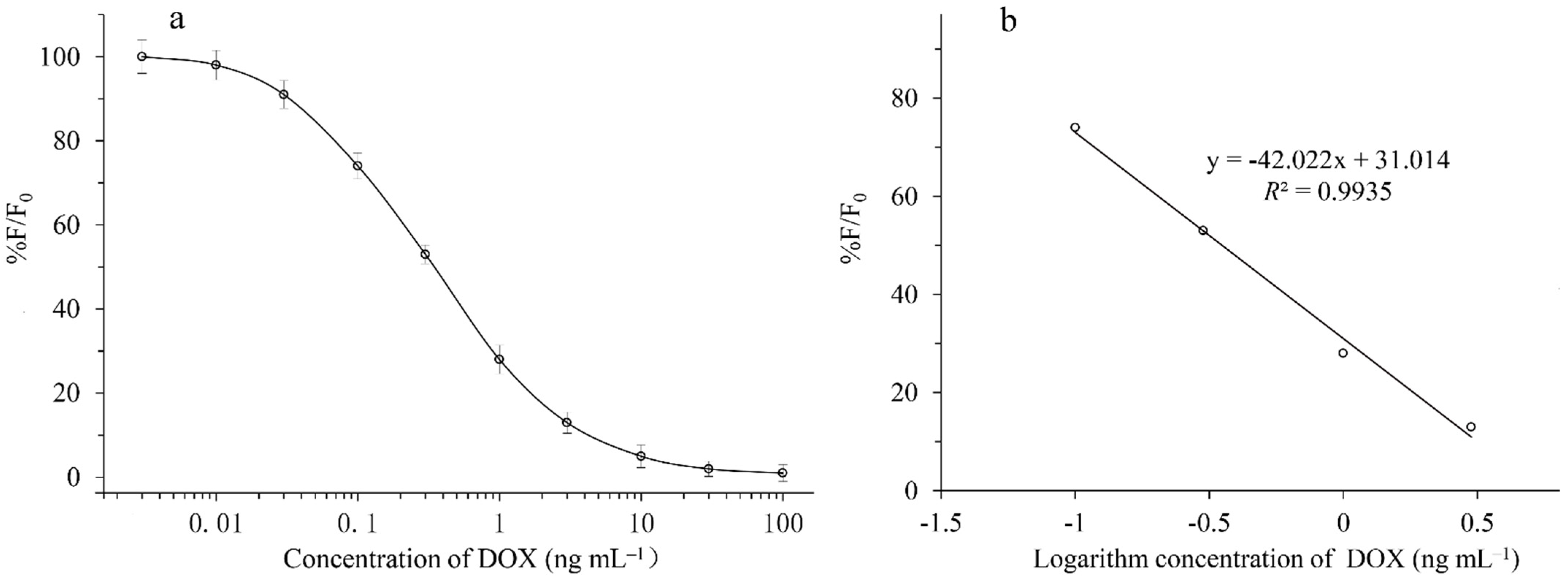
2.5. Standard Curve and Specificity of QD-cFIA
2.6. Accuracy and Precision Studies
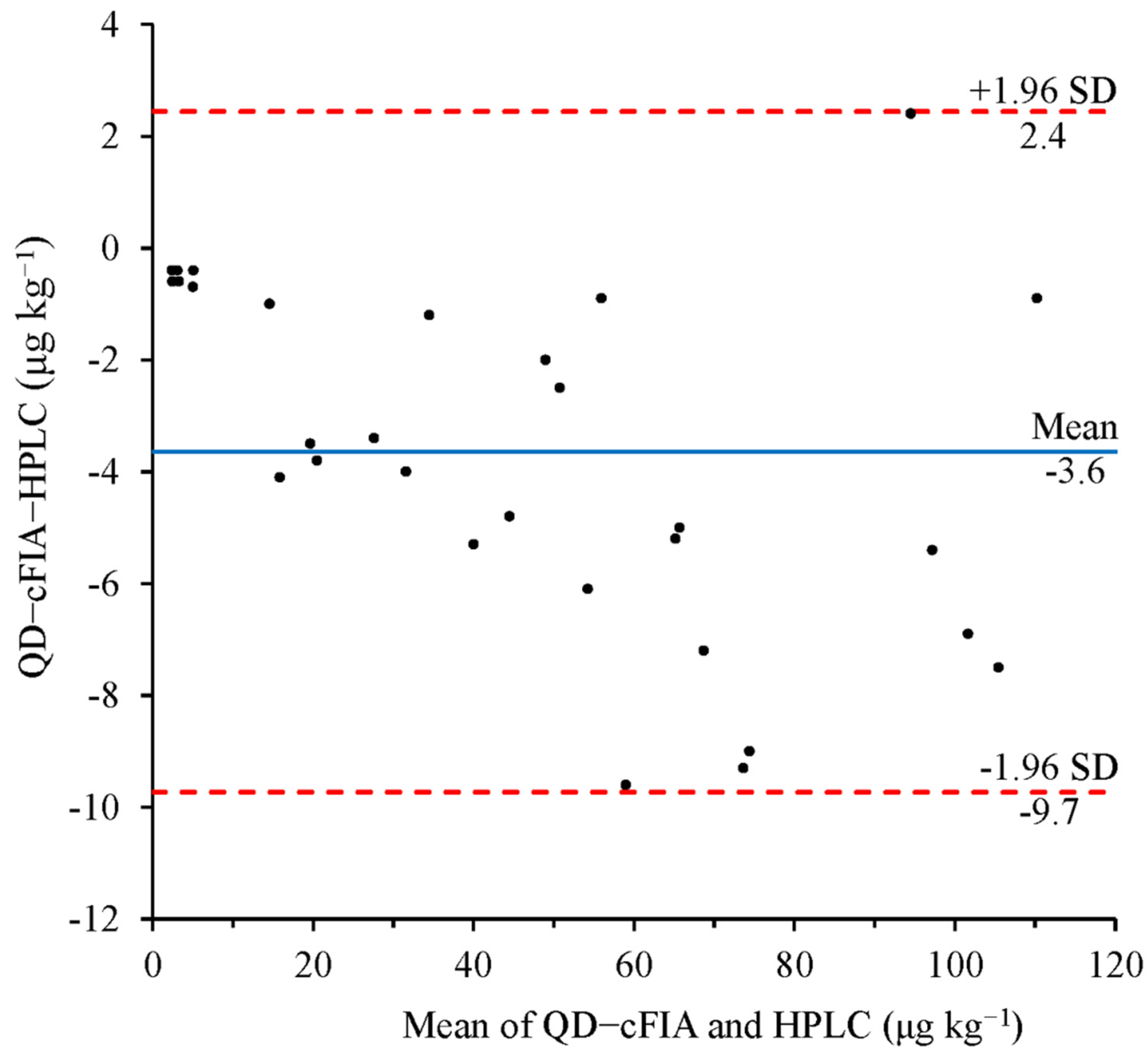
2.7. The Correlation of QD-cFIA with HPLC
3. Results and Discussion
3.1. Characterization of the QD-Labeled Antibodies
3.2. Optimization of QD-cFIA
3.3. Standard Curve and Specificity of QD-cFIA
3.4. Accuracy and Precision
3.5. Correction of Immunoassays and HPLC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, C.Q.; Zhang, N. Enzyme-amplified lanthanide luminescence based on complexation reaction--a new technique for the determination of doxycycline. J. Pharmaceut. Biomed. 2004, 35, 1301–1306. [Google Scholar] [CrossRef]
- Le, T.; Zhao, Z.; Wei, W.; Bi, D. Development of a highly sensitive and specific monoclonal antibody-based enzyme-linked immunosorbent assay for determination of doxycycline in chicken muscle, liver and egg. Food Chem. 2012, 134, 2442–2446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Zhang, L.F.; Zhang, K.Y.; Wang, X.Y.; Xue, F.Q. Determination of tetracyclines and their epimers in agricultural soil fertilized with swine manure by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Integr. Agric. 2012, 11, 1189–1198. [Google Scholar] [CrossRef]
- Lyu, N.; Yin, F.H.; Chen, Y.; Gao, Z.J.; Liu, Y.; Shi, L. Effects of elevated atmospheric CO2 and nitrogen application on cotton biomass, nitrogen utilization and soil urease activity. J. Appl. Ecol. 2015, 26, 3337–3344. [Google Scholar]
- Yan, Q.; Li, X.; Ma, B.; Zou, Y.; Wang, Y.; Liao, X.; Liang, J.; Mi, J.; Wu, Y. Different Concentrations of Doxycycline in Swine Manure Affect the Microbiome and Degradation of Doxycycline Residue in Soil. Front. Microbiol. 2018, 9, 3129. [Google Scholar] [CrossRef]
- Aga, D.S.; O’Connor, S.; Ensley, S.; Payero, J.O.; Snow, D.; Tarkalson, D. Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2005, 53, 7165–7171. [Google Scholar] [CrossRef]
- Girma, K.; Tilahun, Z.; Haimanot, D.; Tadele, T. Review on detection of antimicrobial residues in raw bulk milk in dairy farms. J. Basic. Appl. Sci. 2014, 6, 87–97. [Google Scholar]
- Cinquina, A.L.; Longo, F.; Anastasi, G.; Giannetti, L.; Cozzani, R. Validation of a high-performance liquid chromatography method for the determination of oxytetracycline, tetracycline, chlortetracycline and doxycycline in bovine milk and muscle. J. Chromatogr. A 2003, 987, 227–233. [Google Scholar] [CrossRef]
- Xu, N.; Dong, J.; Zhou, W.; Liu, Y.T.; Ai, X.H. Determination of Doxycycline, 4-epidoxycycline, and 6-epidoxycycline in Aquatic Animal Muscle Tissue by an Optimized Extraction Protocol and Ultra-performance Performance Liquid Chromatography with Ultraviolet Detection. Anal. Lett. 2019, 52, 452–464. [Google Scholar] [CrossRef]
- Gajda, A.; Posyniak, A.; Zmudzki, J.; Tomczyk, G. Determination of doxycycline in chicken fat by liquid chromatography with UV detection and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2013, 928, 113–120. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Ahlborn, J.; Hamscher, G. Simultaneous determination of 14 sulfonamides and tetracyclines in biogas plants by liquid-liquid-extraction and liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, V.G.; Oliveira, M.; de Almeida, C.A.A.; Hoff, R.; Mallmann, C.A. Liquid Chromatography-Tandem Mass Spectrometry Determination and Depletion Profile of Chlortetracycline, Doxycycline, and Oxytetracycline in Broiler Chicken Muscle After Oral Administration. Food Anal. Methods 2018, 11, 2181–2194. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.L.; Yang, S.; Hu, Q.W.; Cheng, H.B.; Liu, H.Y.; Qiu, Y.S. High-performance liquid chromatography using pressurized liquid extraction for the determination of seven tetracyclines in egg, fish and shrimp. J. Chromatogr. B 2013, 15, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tao, Y.; Chen, D.; Wang, Y.; Yuan, Z. Development of an HPLC–UV method for the simultaneous determination of tetracyclines in muscle and liver of porcine, chicken and bovine with accelerated solvent extraction. Food Chem. 2011, 124, 1131–1138. [Google Scholar] [CrossRef]
- Le, T.; Yu, H.; Guo, Y.; Ngom, B.; Shen, Y.; Bi, D. Development of an indirect competitive ELISA for the detection of doxycycline residue in animal edible tissues. Food Agric. Immunol. 2009, 20, 111–124. [Google Scholar] [CrossRef]
- Le, T.; Yu, H.; Wang, X.; Ngom, B.; Guo, Y.; Bi, D. Development and validation of an immunochromatographic test strip for rapid detection of doxycycline residues in swine muscle and liver. Food Agric. Immunol. 2011, 22, 235–246. [Google Scholar] [CrossRef][Green Version]
- Le, T.; Yi, S.; Wei, S.; Liu, J. A competitive dual-label time-resolved fluoroimmunoassay for the simultaneous detection of chlortetracycline and doxycycline in animal edible tissues. Food Agric. Immunol. 2015, 26, 804–812. [Google Scholar] [CrossRef]
- Zhu, Y.; Chao, J.; Zhu, F.; Zhu, N.; Zhang, Q.; Gyimah, E.; Yakubu, S.; Zou, Y.; Zhang, Z. Ratiometric fluorescence immunoassay based on FAM-DNA-functionalized CdSe/ZnS QDs for the sensitive detection of tetrabromobisphenol A in foodstuff and the environment. Anal. Bioanal. Chem. 2020, 412, 3605–3613. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, B.; Zhang, Y.; Wang, J.; Lu, Y.; Deng, J.; Wang, S. Application of CdTe/CdS/ZnS quantum dot in immunoassay for aflatoxin B1 and molecular modeling of antibody recognition. Anal. Chim. Acta 2019, 1047, 139–149. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.A.; Abad-Somovilla, A.; Quinones-Reyes, G.; Agullo, C.; Mercader, J.V.; Abad-Fuentes, A. Monoclonal antibody-based immunoassays for cyprodinil residue analysis in QuEChERS-based fruit extracts. Food Chem. 2015, 187, 530–536. [Google Scholar] [CrossRef]
- Le, T.; Zhu, L.; Yu, H. Dual-label quantum dot-based immunoassay for simultaneous determination of Carbadox and Olaquindox metabolites in animal tissues. Food Chem. 2016, 199, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, L.; Sun, Q.; Zhang, Z.; Le, T. Immunochromatographic Assay for Simultaneous and Quantitative Detection of 3-Methyl-Quinoxaline-2-Carboxylic Acid and Quinoxaline-2-Carboxylic Acid Residues in Animal Tissues Based on Highly Luminescent Quantum Dot Beads. Food Anal. Methods 2018, 11, 334–342. [Google Scholar] [CrossRef]
- Le, T.; Xie, Y.; Zhu, L.Q.; Zhang, L. Rapid and sensitive detection of 3-amino-2-oxazolidinone using a quantum dot-based immunochromatographic fluorescent biosensor. J. Agric. Food Chem. 2016, 64, 8678–8683. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, L.; Yang, X.; Le, T. Development of a quantum dot-based immunochromatography test strip for rapid screening of oxytetracycline and 4-epioxytetracycline in edible animal tissues. Food Addit. Contam. A 2017, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Meng, X.; Qiao, B.; Hu, P.; Meng, X.; Lu, S.; Ren, H.; Liu, Z.; Zhou, Y. Fluorescence-linked immunosorbent assay for detection of phenanthrene and its homolog. Anal. Biochem. 2018, 547, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Ding, Y.; Yang, J.; Ma, M.; Shi, H.; Wang, M. Direct competitive fluoroimmunoassays for detection of imidaclothiz in environmental and agricultural samples using quantum dots and europium as labels. Sci. Total Environ. 2017, 583, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Grande-Martínez, Á.; Moreno-González, D.; Arrebola-Liébanas, F.J.; Garrido-Frenich, A.; García-Campaña, A.M. Optimization of a modified QuEChERS method for the determination of tetracyclines in fish muscle by UHPLC-MS/MS. J. Pharmaceut. Biomed. 2018, 155, 27–32. [Google Scholar] [CrossRef]

| Method | Antibody Type | IC50 (ng mL−1) | LOD (ng mL−1) | Sample | References |
|---|---|---|---|---|---|
| Enzyme-linked immunosorbent assay | Monoclonal antibody | 1.32 | 0.14 | liver, muscle and egg | [2] |
| Enzyme-linked immunosorbent assay | Polyclonal antibody | 8.74 | 1.96 | Liver and muscle | [15] |
| Immunochromatographic test strip | Polyclonal antibody | 22.0 | 7.0 | Liver and muscle | [16] |
| Time-resolved fluroimmunoassay | Polyclonal antibody | 1.06 | 0.04 | liver, muscle and egg | [17] |
| QD-cFIA | Monoclonal antibody | 0.35 | 0.039 | Chicken (liver, muscle), fish muscle, tap water, river, water and contaminated water | This work |
| Compound | IC50 (ng m L−1) | CR (%) |
|---|---|---|
| DOX | 0.35 | 100 |
| 4-epi-doxycycline | 0.68 | 51.5 |
| Oxytetracycline | >10,000 | <0.01 |
| 4-epi- oxytetracycline | >10,000 | <0.01 |
| Tetracycline | >10,000 | <0.01 |
| 4-epi-tetracycline | >10,000 | <0.01 |
| Chlortetracycline | >10,000 | <0.01 |
| 4-epi-chlortetracycline chlortetracycline | >10,000 | <0.01 |
| Demeclocycline | >10,000 | <0.01 |
| Sample | Spiked (ng mL−1, ng g−1) | Mean Recovery ±SD (%) | RSD (%) |
|---|---|---|---|
| Chicken liver | 150 | 105.4 ± 10.4 | 9.9 |
| 300 | 98.7 ± 10.8 | 10.9 | |
| 600 | 88.5 ± 9.7 | 11.0 | |
| Chicken muscle | 50 | 98.8 ± 6.6 | 6.7 |
| 100 | 109.8 ± 9.4 | 8.6 | |
| 200 | 108.4 ± 7.5 | 6.9 | |
| Fish muscle | 100 | 81.3 ± 9.7 | 11.9 |
| 200 | 91.8 ± 7.2 | 7.8 | |
| 400 | 109.3 ± 11.2 | 10.3 | |
| Tap water | 10 | 102.3 ± 6.4 | 6.3 |
| 50 | 103.1 ± 7.6 | 7.4 | |
| 100 | 98.8 ± 4.6 | 4.7 | |
| River water | 10 | 103.3 ± 4.3 | 4.2 |
| 50 | 101.5 ± 9.5 | 9.4 | |
| 100 | 99.6 ± 11.2 | 11.2 | |
| Contaminated water | 10 | 106.3 ± 9.6 | 9.0 |
| 50 | 100.8 ± 7.9 | 7.8 | |
| 100 | 97.3 ± 9.5 | 9.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.; Xu, R.; Yang, L.; Xie, Y. Development of a Highly Specific Fluoroimmunoassay for the Detection of Doxycycline Residues in Water Environmental and Animal Tissue Samples. Micromachines 2022, 13, 1864. https://doi.org/10.3390/mi13111864
Le T, Xu R, Yang L, Xie Y. Development of a Highly Specific Fluoroimmunoassay for the Detection of Doxycycline Residues in Water Environmental and Animal Tissue Samples. Micromachines. 2022; 13(11):1864. https://doi.org/10.3390/mi13111864
Chicago/Turabian StyleLe, Tao, Rongli Xu, Lulan Yang, and Yong Xie. 2022. "Development of a Highly Specific Fluoroimmunoassay for the Detection of Doxycycline Residues in Water Environmental and Animal Tissue Samples" Micromachines 13, no. 11: 1864. https://doi.org/10.3390/mi13111864
APA StyleLe, T., Xu, R., Yang, L., & Xie, Y. (2022). Development of a Highly Specific Fluoroimmunoassay for the Detection of Doxycycline Residues in Water Environmental and Animal Tissue Samples. Micromachines, 13(11), 1864. https://doi.org/10.3390/mi13111864










