Engineering Atomic-to-Nano Scale Structural Homogeneity towards High Corrosion Resistance of Amorphous Magnesium-Based Alloys
Abstract
:1. Introduction
2. Experimental Method
2.1. Sample Preparation
2.2. Corrosion Resistance Test
2.3. Characterization Techniques
3. Results and Discussion
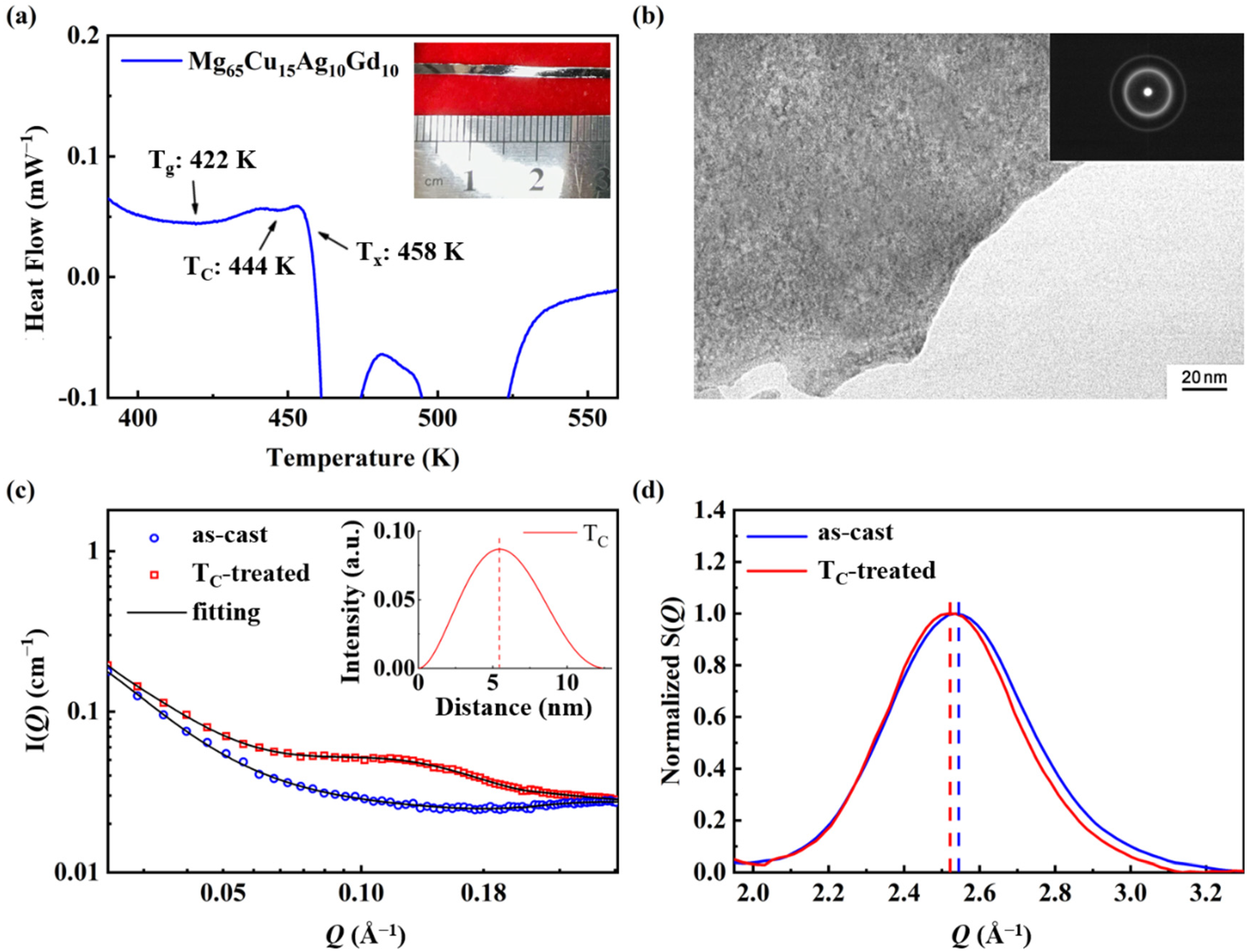
3.1. Microstructure and Thermal Analysis
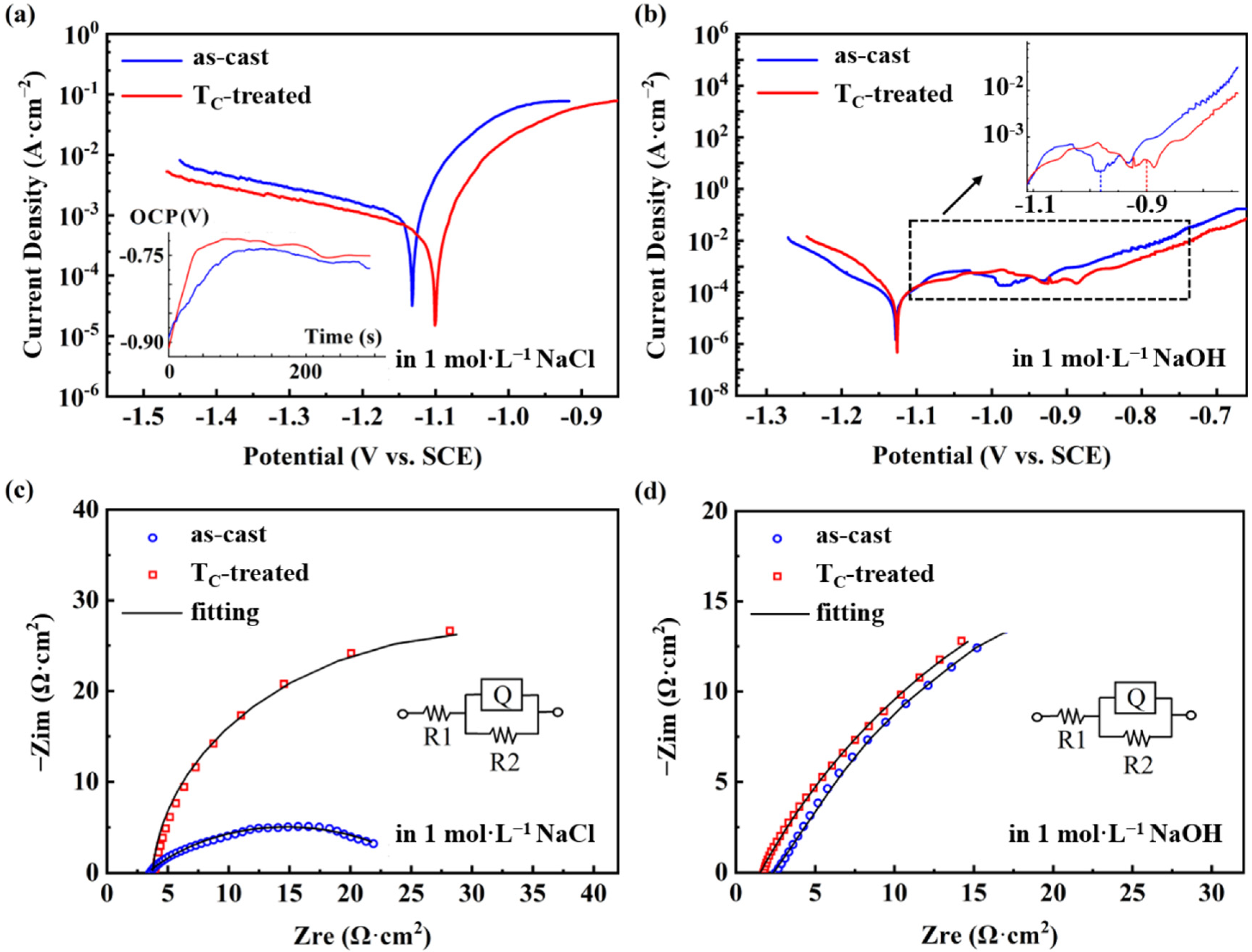
3.2. Analysis of Potentiodynamic Corrosion Behavior
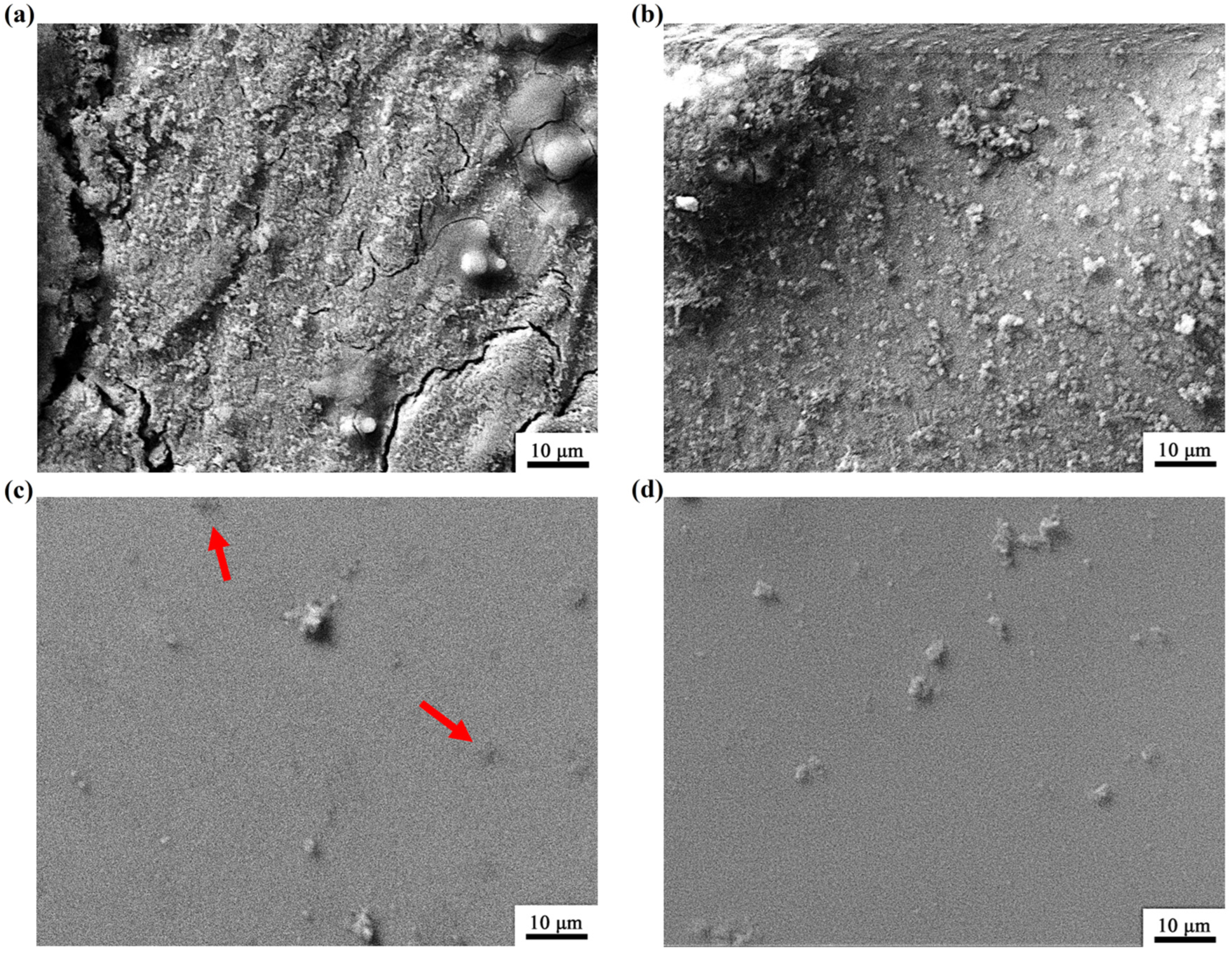
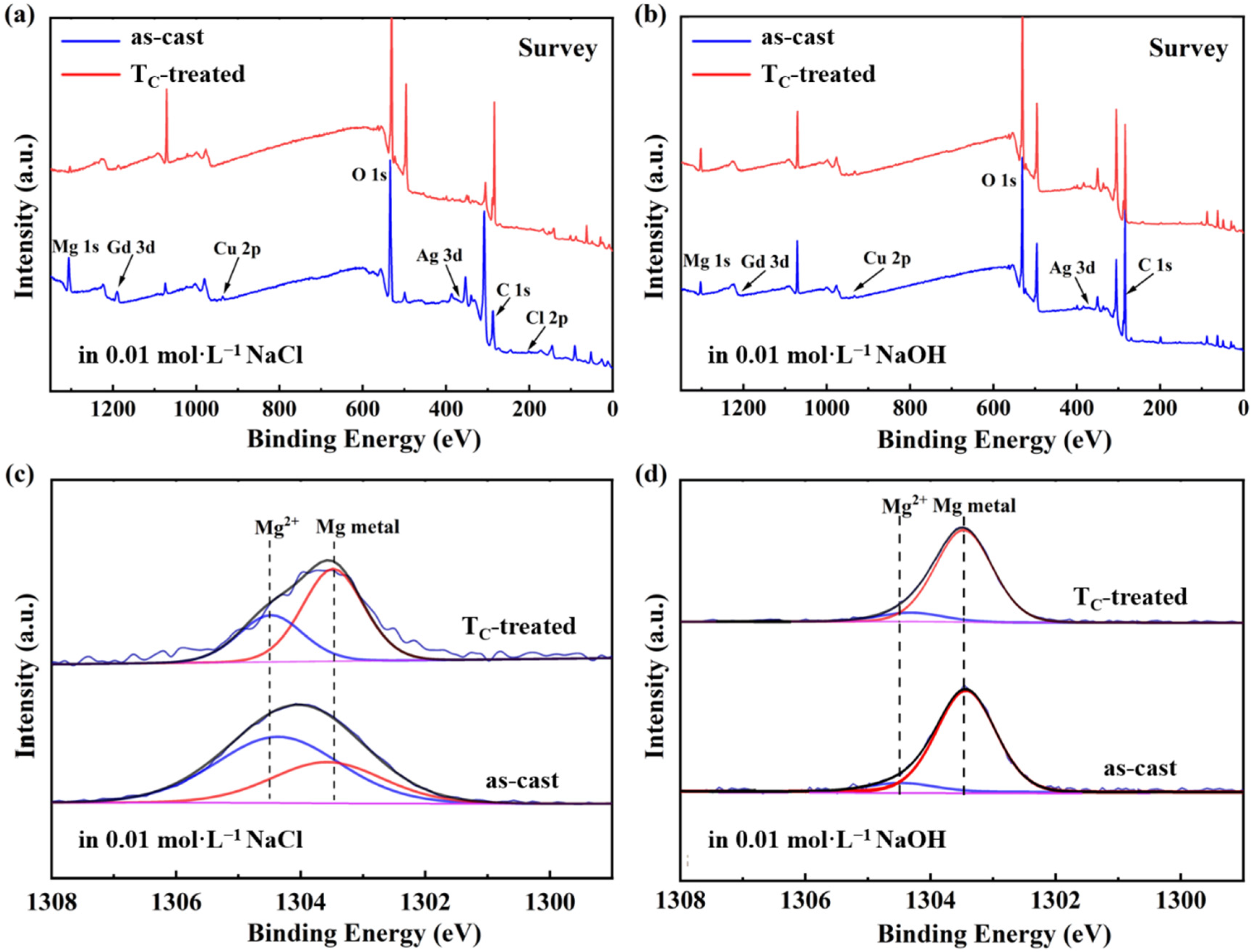
3.3. Analysis of Immersion Corrosion Behavior
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhattacharjee, T.; Suh, B.C.; Sasaki, T.T.; Ohkubo, T.; Kim, N.J.; Hono, K. High strength and formable Mg–6.2Zn–0.5Zr–0.2Ca alloy sheet processed by twin roll casting. Mater. Sci. Eng. A 2014, 609, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Nie, J.F.; Xu, S.W.; HJDavies, C.; Birbilis, N. Super-formable pure magnesium at room temperature. Nat. Commun. 2017, 8, 972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, B.; Zhao, X.A.; Zhang, X.; Miao, Y.; Yang, N.; Yang, B.; Zhang, L.; Kuang, W.; Li, J.; et al. Turning a native or corroded Mg alloy surface into an anti-corrosion coating in excited CO2. Nat. Commun. 2018, 9, 4058. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.C.; Liu, M.; Song, G.; Atrens, A. Influence of the β-phase morphology on the corrosion of the Mg alloy AZ91. Corros. Sci. 2008, 50, 1939–1953. [Google Scholar] [CrossRef]
- Inoue, A.; Ohtera, K.; Kita, K.; Masumoto, T. New Amorphous Mg-Ce-Ni Alloys with High Strength and Good Ductility. Jpn. J. Appl. Phys. 1988, 27, L2248. [Google Scholar] [CrossRef]
- Gonzalez, S.; Figueroa, I.A.; Todd, I. Influence of minor alloying additions on the glass-forming ability of Mg-Ni-La bulk metallic glasses. J. Alloys Compd. 2009, 484, 612–618. [Google Scholar] [CrossRef]
- Wang, C.; Shuai, Y.; Yang, Y.; Zeng, D.; Liang, X.; Peng, S.; Shuai, C. Amorphous magnesium alloy with high corrosion resistance fabricated by laser powder bed fusion. J. Alloys Compd. 2022, 897, 163247. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Wei, Y.; Guo, S.; Fusheng, P. Enhanced mechanical properties and corrosion resistance of a Mg-Zn-Ca bulk metallic glass composite by Fe particle addition. Mater. Lett. 2013, 91, 311–314. [Google Scholar] [CrossRef]
- Romzi, M.A.F.; Alias, J.; Ramli, M.I.M. Effect of zinc (Zn) on the microstructure and corrosion behaviour of magnesium (Mg). Mater. Today Proc. 2022, 48, 1873–1879. [Google Scholar] [CrossRef]
- Matias, T.B.; Roche, V.; Nogueira, R.P.; Asato, G.H.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J.; Jorge, A.M., Jr. Mg-Zn-Ca amorphous alloys for application as temporary implant: Effect of Zn content on the mechanical and corrosion properties. Mater. Des. 2016, 110, 188–195. [Google Scholar] [CrossRef]
- Chen, S.; Tu, J.; Hu, Q.; Xiong, X.; Wu, J.; Zou, J.; Zeng, X. Corrosion resistance and in vitro bioactivity of Si-containing coating prepared on a biodegradable Mg-Zn-Ca bulk metallic glass by micro-arc oxidation. J. Non-Cryst. Solids 2017, 456, 125–131. [Google Scholar] [CrossRef]
- Zai, W.; Sun, S.; Man, H.C.; Lian, J.; Zhang, Y. Preparation and anticorrosion properties of electrodeposited calcium phosphate (CaP) coatings on Mg-Zn-Ca metallic glass. Mater. Chem. Phys. 2022, 290, 126532. [Google Scholar] [CrossRef]
- Guan, W.; Cui-Xia, G.; Shu-Jie, P. Thermal stability, mechanical properties and corrosion behavior of a Mg–Cu–Ag–Gd metallic glass with Nb addition. Rare Met. 2017, 36, 183–187. [Google Scholar]
- Guoqiang, L.; Lijing, Z.; Huanxi, L. Corrosion behavior of the bulk amorphous Mg65Cu25Gd10 alloy. Rare Met. Mater. Eng. 2009, 38, 110–114. [Google Scholar]
- Sun, Y.; Li, Z.; Liu, J.; Liang, Z. Corrosion characteristics of Mg-Cu-Gd amorphous alloys. J. Nanjing Univ. Aeronaut. Astronaut. 2010, 42, 636–640. [Google Scholar]
- Zai, W.; Man, H.C.; Su, Y.; Li, G.; Lian, J. Impact of microalloying element Ga on the glass-forming ability (GFA), mechanical properties and corrosion behavior of Mg-Zn-Ca bulk metallic glass. Mater. Chem. Phys. 2020, 255, 123555. [Google Scholar] [CrossRef]
- Li, H.; Pang, S.; Liu, Y.; Sun, L.; Liaw, P.K.; Zhang, T. Biodegradable Mg-Zn-Ca-Sr bulk metallic glasses with enhanced corrosion performance for biomedical applications. Mater. Des. 2015, 67, 9–19. [Google Scholar] [CrossRef]
- Park, E.S.; Jeong, E.Y.; Lee, J.K.; Bae, J.C.; Kwon, A.R.; Gebert, A.; Schultz, L.; Chang, H.J.; Kim, D.H. In situ formation of two glassy phases in the Nd-Zr-Al-Co alloy system. Scr. Mater. 2007, 56, 197–200. [Google Scholar] [CrossRef]
- Sarlar, K.; Kucuk, I. Phase separation and glass forming ability of (Fe0.72Mo0.04B0.24)(100-x)Gd-x (x = 4, 8) bulk metallic glasses. J. Non-Cryst. Solids 2016, 447, 198–201. [Google Scholar] [CrossRef]
- Lan, S.; Ren, Y.; Wei, X.Y.; Wang, B.; Gilbert, E.P.; Shibayama, T.; Watanabe, S.; Ohnuma, M.; Wang, X.L. Hidden amorphous phase and reentrant supercooled liquid in Pd-Ni-P metallic glasses. Nat. Commun. 2017, 8, 14679. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; He, H.; Zhou, J.; Lu, C.; Dong, W.; Liu, S.; Lan, S.; Wu, Z.; Wang, A.; Wang, L.; et al. In-situ scattering study of a liquid-liquid phase transition in Fe-B-Nb-Y supercooled liquids and its correlation with glass-forming ability. J. Alloys Compd. 2019, 787, 831–839. [Google Scholar] [CrossRef]
- Liu, S.; Ge, J.; Ying, H.; Lu, C.; Ma, D.; Wang, X.L.; Zuo, X.; Ren, Y.; Feng, T.; Shen, J.; et al. In Situ Scattering Studies of Crystallization Kinetics in a Phase-Separated Zr-Cu-Fe-Al Bulk Metallic Glass. Acta Metall. Sin. Engl. Lett. 2022, 35, 103–114. [Google Scholar] [CrossRef]
- Lan, S.; Wei, X.; Zhou, J.; Lu, Z.; Wu, X.; Feygenson, M.; Neuefeind, J.; Wang, X.L. In-situ study of crystallization kinetics in ternary bulk metallic glass alloys with different glass forming abilities. Appl. Phys. Lett. 2014, 105, 201906. [Google Scholar] [CrossRef]
- Lan, S.; Dong, W.X.; Wang, X.L. The progress of research on anomalous exothermic phenomenon and hidden amorphous phase transition in metallic glasses. Chin. J. Nat. 2017, 39, 327–339. [Google Scholar]
- Sheng, H.W.; Liu, H.Z.; Cheng, Y.Q.; Wen, J.; Lee, P.L.; Luo, W.K.; Shastri, S.D.; Ma, E. Polyamorphism in a metallic glass. Nat. Mater. 2007, 6, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, L.; Ge, J.; Wu, Z.; Ke, Y.; Li, Q.; Sun, B.; Feng, T.; Wu, Y.; Wang, J.T.; et al. Deformation-enhanced hierarchical multiscale structure heterogeneity in a Pd-Si bulk metallic glass. Acta Mater. 2020, 200, 42–55. [Google Scholar] [CrossRef]
- Di, Y.X.; Wang, Y.M. Local Structure-Property Correlation of Fe-Based Amorphous Alloys: Based on Minor Alloying Research. Acta Metall. Sin. 2020, 56, 1558–1568. [Google Scholar]
- Li, K.H.; Ge, J.C.; Liu, S.N.; Fu, S.; Yin, Z.X.; Zhang, W.T.; Chen, G.X.; Wei, S.C.; Ji, H.; Feng, T.; et al. In situ scattering study of multiscale structural evolution during liquid–liquid phase transition in Mg-based metallic glasses. Rare Met. 2021, 40, 3107–3116. [Google Scholar] [CrossRef]
- Hu, X.; Ge, J.; Liu, S.; Fu, S.; Wu, Z.; Feng, T.; Liu, D.; Wang, X.; Lan, S. Combustion mechanism of Fe-Nb-B-Y amorphous alloys with anomalous exothermic phenomena. Acta Metall. Sin. 2021, 57, 542–552. [Google Scholar]
- Shin, S.S.; Kim, H.K.; Lee, J.C.; Park, I.M. Effect of Sub-T_g Annealing on the Corrosion Resistance of the Cu–Zr Amorphous Alloys. Acta Metall. Sin. Engl. Lett. 2018, 31, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Liu, X.; Zhou, Y.; Wei, Y.; Wei, X.; Xu, G.; Shen, J. Effects of Annealing Below Glass Transition Temperature on the Wettability and Corrosion Performance of Fe-based Amorphous Coatings. Acta Metall. Sin. Engl. Lett. 2022, 35, 243–253. [Google Scholar] [CrossRef]
- Ren, Y.; Babaie, E.; Bhaduri, S.B. Nanostructured amorphous magnesium phosphate/poly (lactic acid) composite coating for enhanced corrosion resistance and bioactivity of biodegradable AZ31 magnesium alloy. Prog. Org. Coat. 2018, 118, 1–8. [Google Scholar] [CrossRef]
- Eivani, A.R.; Mehdizade, M.; Chabok, S.; Zhou, J. Applying multi-pass friction stir processing to refine the microstructure and enhance the strength, ductility and corrosion resistance of WE43 magnesium alloy. J. Mater. Res. Technol. 2021, 12, 1946–1957. [Google Scholar] [CrossRef]
- Qiu, X.; Thompson, J.W.; Billinge, S.J. PDFgetX2: A GUI-driven program to obtain the pair distribution function from X-ray powder diffraction data. J. Appl. Crystallogr. 2004, 37, 678. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Ge, J.; Ke, Y.; Ying, H.; Zhu, L.; He, H.; Liu, S.; Lu, C.; Lan, S.; Almer, J.; et al. In-situ observation of an unusual phase transformation pathway with Guinier-Preston zone-like precipitates in Zr-based bulk metallic glasses. J. Alloys Compd. 2020, 819, 153049. [Google Scholar] [CrossRef]
- Lan, S.; Guo, C.; Zhou, W.; Ren, Y.; Almer, J.; Pei, C.; Hahn, H.; Liu, C.T.; Feng, T.; Wang, X.L.; et al. Engineering medium-range order and polyamorphism in a nanostructured amorphous alloy. Commun. Phys. 2019, 2, 117. [Google Scholar] [CrossRef]
- Chen, S.Q.; Hui, K.Z.; Dong, L.Z.; Li, Z.; Zhang, Q.H.; Gu, L.; Zhao, W.; Lan, S.; Ke, Y.; Shao, Y.; et al. Excellent long-term reactivity of inhomogeneous nanoscale Fe-based metallic glass in wastewater purification. Sci. China Mater. 2020, 63, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Miller, M.K.; Wang, X.L.; Liu, C.T.; Stoica, A.D.; Ma, D.; Almer, J.; Shi, D. Metallic Glasses: Nanoscale Solute Partitioning in Bulk Metallic Glasses. Adv. Mater. 2009, 21, 305–308. [Google Scholar] [CrossRef]
- Kelton, K.F. A new model for nucleation in bulk metallic glasses. Philos. Mag. Lett. 1998, 77, 337–344. [Google Scholar] [CrossRef]
- Duparc, O.H. The Preston of the Guinier-Preston Zones. Guinier. Metall. Mater. Trans. 2010, 41, 1873. [Google Scholar] [CrossRef]
- Oden, M.; Rogström, L.; Knutsson, A.; Terner, M.R.; Hedström, P.; Almer, J.; Ilavsky, J. In situ small-angle x-ray scattering study of nanostructure evolution during decomposition of arc evaporated TiAlN coatings. Appl. Phys. Lett. 2009, 94, 053114. [Google Scholar] [CrossRef]
- Ilavsky, J.; Jemian, P.R. Irena: Tool suite for modeling and analysis of small-angle scattering. J. Appl. Crystallogr. 2009, 42, 347–353. [Google Scholar] [CrossRef]
- Gleiter, H. Nanoglasses: A New Kind of Noncrystalline Material and the Way to an Age of New Technologies? Small 2016, 12, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Debye, P.; Anderson, H.R., Jr.; Brumberger, H. Scattering by an Inhomogeneous Solid. II. The Correlation Function and Its Application. J. Appl. Phys. 1957, 28, 679–683. [Google Scholar] [CrossRef]
- Fu, S.; Liu, S.; Ge, J.; Wang, J.; Ying, H.; Wu, S.; Yan, M.; Zhu, L.; Ke, Y.; Luan, J.; et al. In situ study on medium-range order evolution during the polyamorphous phase transition in a Pd-Ni-P nanostructured glass. J. Mater. Sci. Technol. 2022, 125, 145–156. [Google Scholar] [CrossRef]
- Lan, S.; Blodgett, M.; Kelton, K.F.; Ma, J.L.; Fan, J.; Wang, X.L. Structural crossover in a supercooled metallic liquid and the link to a liquid-to-liquid phase transition. Appl. Phys. Lett. 2016, 108, 211907. [Google Scholar] [CrossRef]
- Wessels, V.; Gangopadhyay, A.K.; Sahu, K.K.; Hyers, R.W.; Canepari, S.M.; Rogers, J.R.; Kramer, M.J.; Goldman, A.I.; Robinson, D.; Lee, J.W.; et al. Rapid chemical and topological ordering in supercooled liquid Cu46Zr54. Phys. Rev. B 2011, 83, 094116. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Wu, Z.; Ge, J.; Liu, S.; Lan, S.; Gilbert, E.P.; Ren, Y.; Ma, D.; Wang, X.L. In situ neutron scattering studies of a liquid–liquid phase transition in the supercooled liquid of a Zr–Cu–Al–Ag glass-forming alloy. Appl. Phys. Lett. 2021, 118, 191901. [Google Scholar] [CrossRef]
- Lan, S.; Zhu, L.; Wu, Z.; Gu, L.; Zhang, Q.; Kong, H.; Liu, J.; Song, R.; Liu, S.; Sha, G.; et al. A medium-range structure motif linking amorphous and crystalline states. Nat. Mater. 2021, 20, 1347. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Q.; Zhang, C.; Liu, Y.; Liang, J.; Wang, D.; Zhou, F. Synergistic effect of hydrophobic film and porous MAO membrane containing alkynol inhibitor for enhanced corrosion resistance of magnesium alloy. Surf. Coat. Technol. 2019, 357, 515–525. [Google Scholar] [CrossRef]
- Lopes, D.R.; Silva, C.L.; Soares, R.B.; Pereira, P.H.R.; Oliveira, A.C.; Figueiredo, R.B.; Langdon, T.G.; Lins, V.F. Cytotoxicity and Corrosion Behavior of Magnesium and Magnesium Alloys in Hank’s Solution after Processing by High-Pressure Torsion. Adv. Eng. Mater. 2019, 21, 1900391. [Google Scholar] [CrossRef]
- Kaur, H.; Tian, R.; Roy, A.; McCrystall, M.; Horvath, D.V.; Lozano Onrubia, G.; Smith, R.; Ruether, M.; Griffin, A.; Backes, C.; et al. Correction to Production of Quasi-2D Platelets of Nonlayered Iron Pyrite (FeS2) by Liquid-Phase Exfoliation for High Performance Battery Electrodes. ACS Nano 2021, 14, 13418–13432. [Google Scholar] [CrossRef]
- Vladescu, A.; Pruna, V.; Kulesza, S.; Braic, V.; Titorencu, I.; Bramowicz, M.; Gozdziejewska, A.; Parau, A.; Cotrut, C.M.; Pana, I.; et al. Influence of Ti, Zr or Nb carbide adhesion layers on the adhesion, corrosion resistance and cell proliferation of titania doped hydroxyapatite to the Ti6Al4V alloy substrate, utilizable for orthopaedic implants. Ceram. Int. 2018, 45, 1710–1723. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Shen, Z.C.; Zhnag, C.F.; Huang, D.P. Electrochemical Data Manual; Hunan Science & Technology Press: Changsha, China, 1985. [Google Scholar]
- Zhang, Y.; Yan, C.; Wang, F.; Li, W. Electrochemical behavior of anodized Mg alloy AZ91D in chloride containing aqueous solution. Corros. Sci. 2005, 47, 2816–2831. [Google Scholar] [CrossRef]
- McFeely, F.R.; Kowalczyk, S.P.; Ley, L.; Shirley, D.A. Multiplet splittings of the 4s and 5s core levels in the rare earth metals. Phys. Lett. A 1974, 49, 301–302. [Google Scholar] [CrossRef]
- Soma, M.; Seyama, H. Surface compositions of powdered rock samples studied by X-ray photoelectron spectroscopy. Chem. Geol. 1986, 55, 97–103. [Google Scholar] [CrossRef]
- Song, G. Corrosion of Magnesium Alloys; Woodhead Publishing Ltd.: Sawston, UK, 2011. [Google Scholar]
- Zhang, Y.; Fang, Y.; Feng, S.; Ma, D.; Zhou, Y.; Wang, L.M. Trapping mechanism of metastable β-Ga disclosed by its lattice stability optimization and nucleation behavior exploration. Calphad 2022, 79, 102475. [Google Scholar] [CrossRef]
- Li, Q. Formation of bulk ferromagnetic nanostructured Fe 40 Ni 40 P 14 B 6 alloys by metastable liquid spinodal decomposition. Sci. China Ser. E Technol. Sci. 2009, 52, 1919–1922. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Qi, L.; Wang, L.; Pan, S.; Ma, M.; Zhang, X.; Li, G.; Liu, R. Atomic structure of shear bands in Cu64Zr36 metallic glasses studied by molecular dynamics simulations. Acta Mater. 2015, 95, 236–243. [Google Scholar] [CrossRef]
- Xiang, S.; Li, Q.; Zuo, M.; Cao, D.; Li, H.; Sun, Y. Influence of the preparation cooling rate on crystallization kinetics of Fe 74 Mo 6 P 13 C 7 amorphous alloys. J. Non-Cryst. Solids 2017, 475, 116–120. [Google Scholar] [CrossRef]
| Q (μs0/Ω) | α | R1 (Ω·cm2) | R2 (Ω·cm2) | |
|---|---|---|---|---|
| as-cast in 1 M NaCl | 421.1 | 0.56 | 2.77 | 24.00 |
| TC-treated in 1 M NaCl | 1000.0 | 1.00 | 3.70 | 56.06 |
| as-cast in 1 M NaOH | 545.1 | 0.65 | 2.51 | 81.72 |
| TC-treated in 1 M NaOH | 77.99 | 0.77 | 2.45 | 84.24 |
| Mg | Cu | Ag | Gd | O | Cl | Total | |
|---|---|---|---|---|---|---|---|
| as-cast in 0.01 M NaCl | 13.49 | 1.32 | 0.25 | 0.31 | 83.02 | 1.61 | 100 |
| TC-treated in 0.01 M NaCl | 3.49 | 0.66 | 0.33 | 0.18 | 93.77 | 1.57 | 100 |
| as-cast in 0.01 M NaOH | 8.04 | 1.54 | 0.56 | 0.31 | 89.55 | - | 100 |
| TC-treated in 0.01 M NaOH | 11.07 | 1.14 | 0.43 | 0.16 | 87.20 | - | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Zhang, W.; Li, K.; Fu, S.; Lou, Y.; Liu, S.; Ge, J.; Ying, H.; Liu, W.-D.; Zuo, X.; et al. Engineering Atomic-to-Nano Scale Structural Homogeneity towards High Corrosion Resistance of Amorphous Magnesium-Based Alloys. Micromachines 2022, 13, 1992. https://doi.org/10.3390/mi13111992
Qin Y, Zhang W, Li K, Fu S, Lou Y, Liu S, Ge J, Ying H, Liu W-D, Zuo X, et al. Engineering Atomic-to-Nano Scale Structural Homogeneity towards High Corrosion Resistance of Amorphous Magnesium-Based Alloys. Micromachines. 2022; 13(11):1992. https://doi.org/10.3390/mi13111992
Chicago/Turabian StyleQin, Yuan, Wentao Zhang, Kanghua Li, Shu Fu, Yu Lou, Sinan Liu, Jiacheng Ge, Huiqiang Ying, Wei-Di Liu, Xiaobing Zuo, and et al. 2022. "Engineering Atomic-to-Nano Scale Structural Homogeneity towards High Corrosion Resistance of Amorphous Magnesium-Based Alloys" Micromachines 13, no. 11: 1992. https://doi.org/10.3390/mi13111992
APA StyleQin, Y., Zhang, W., Li, K., Fu, S., Lou, Y., Liu, S., Ge, J., Ying, H., Liu, W.-D., Zuo, X., Shen, J., Wei, S.-C., Hahn, H., Ren, Y., Wu, Z., Wang, X.-L., Zhu, H., & Lan, S. (2022). Engineering Atomic-to-Nano Scale Structural Homogeneity towards High Corrosion Resistance of Amorphous Magnesium-Based Alloys. Micromachines, 13(11), 1992. https://doi.org/10.3390/mi13111992








