Fabrication of Metal-Insulator-Metal Nanostructures Composed of Au-MgF2-Au and Its Potential in Responding to Two Different Factors in Sample Solutions Using Individual Plasmon Modes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Design of MIM Nanostrctures
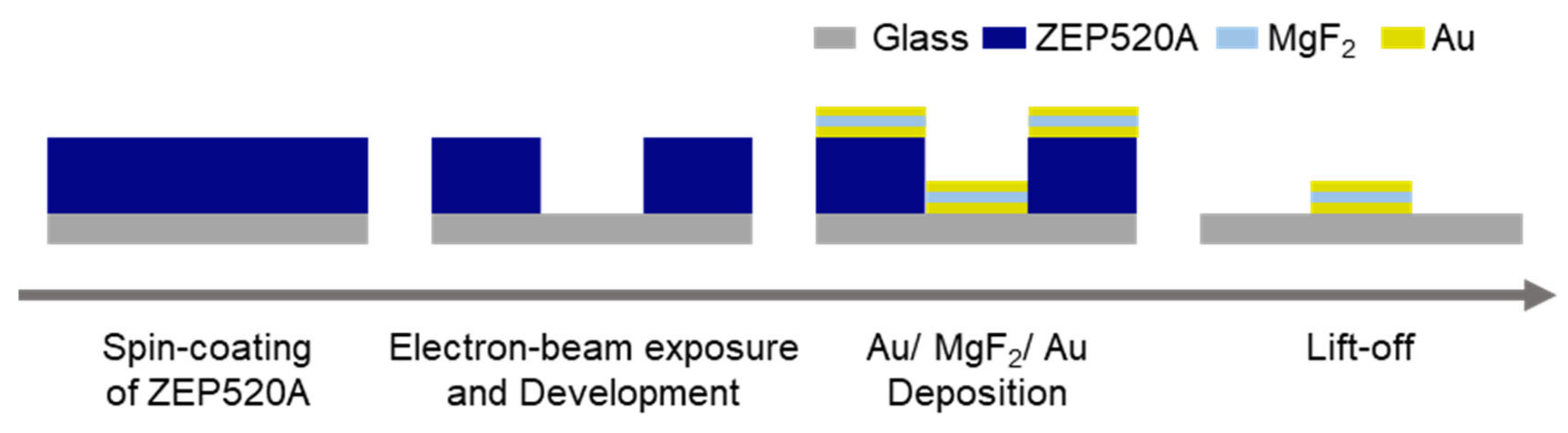
2.2.2. Fabrication of MIM Nanostructures
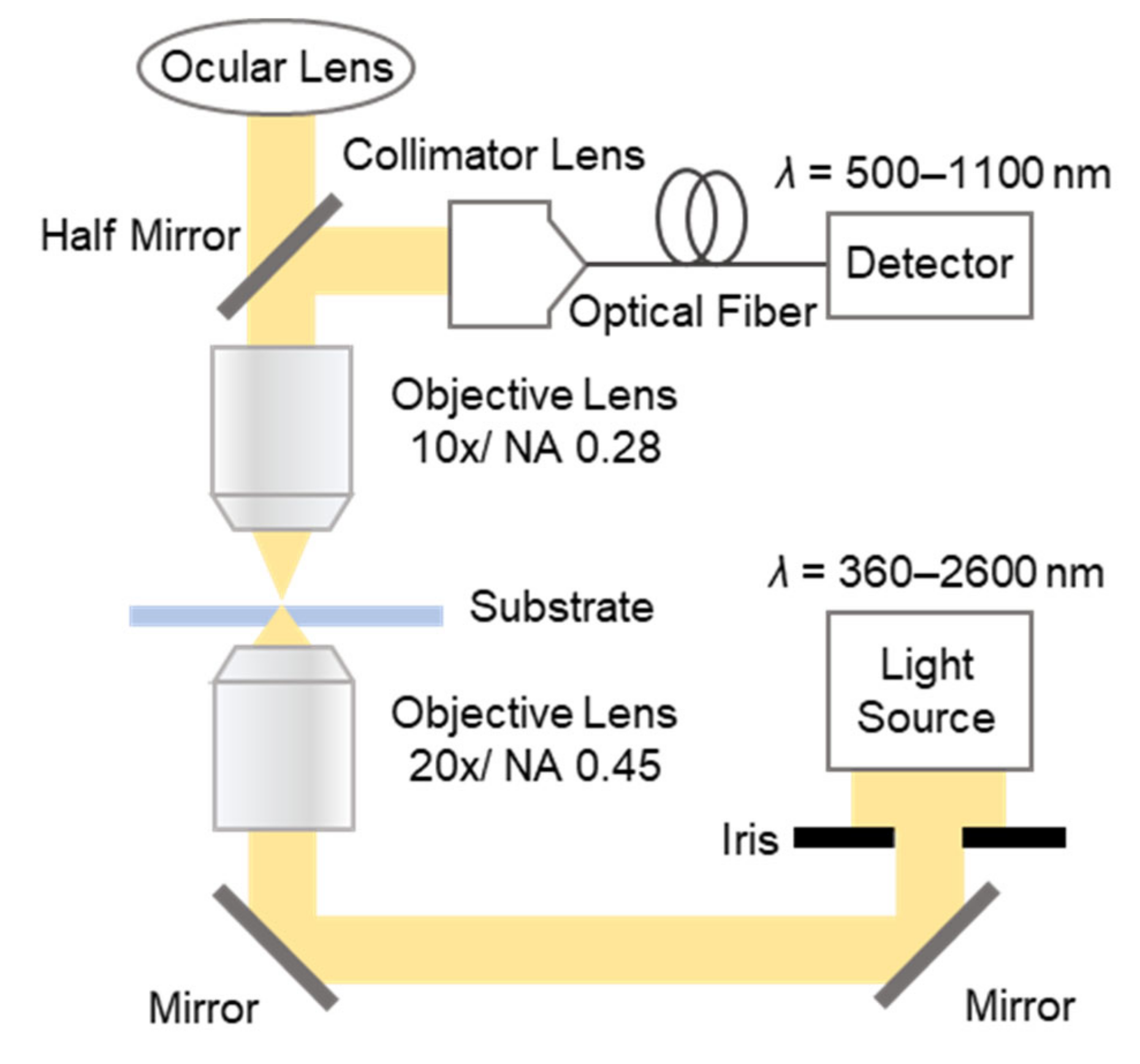
2.2.3. Evaluation of Optical Characteristics and Refractive Index Response of MIM Nanostructures
3. Results
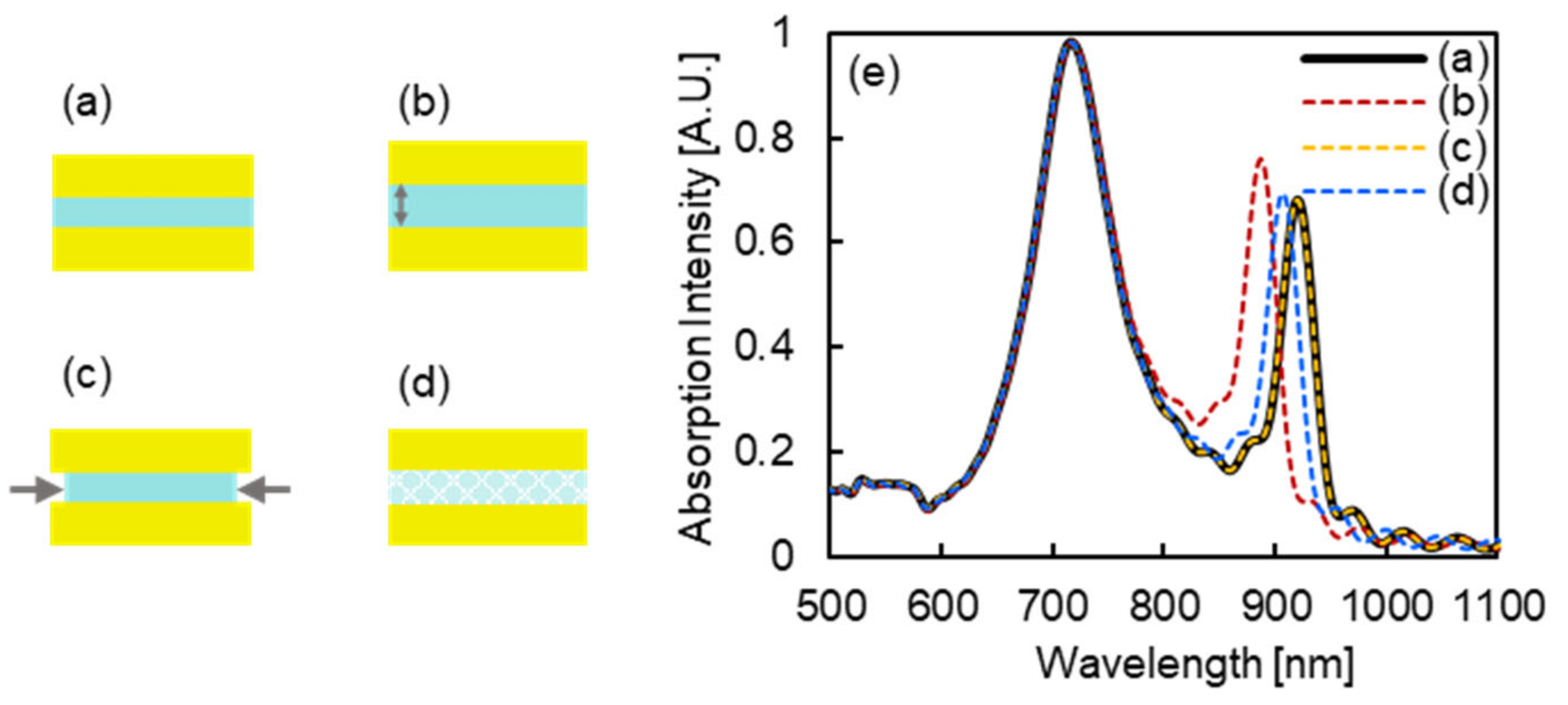
3.1. Simulated Optical Characteristics of Designed MIM Nanostructures
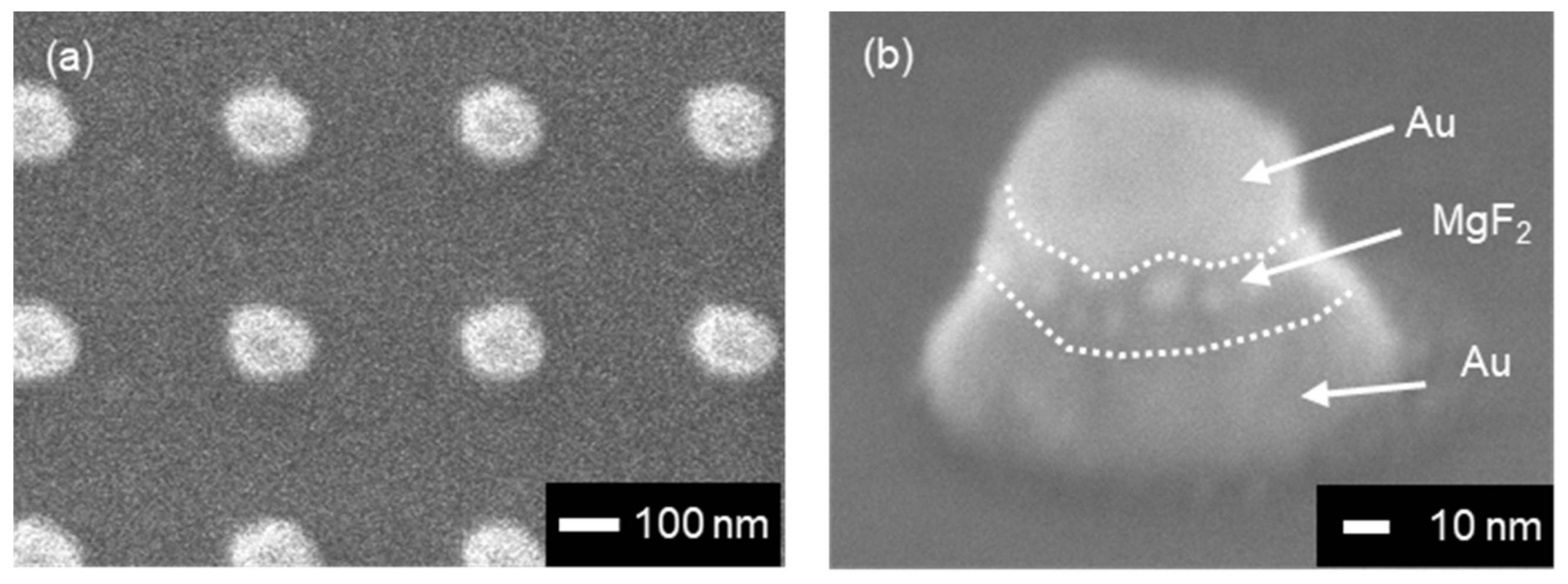
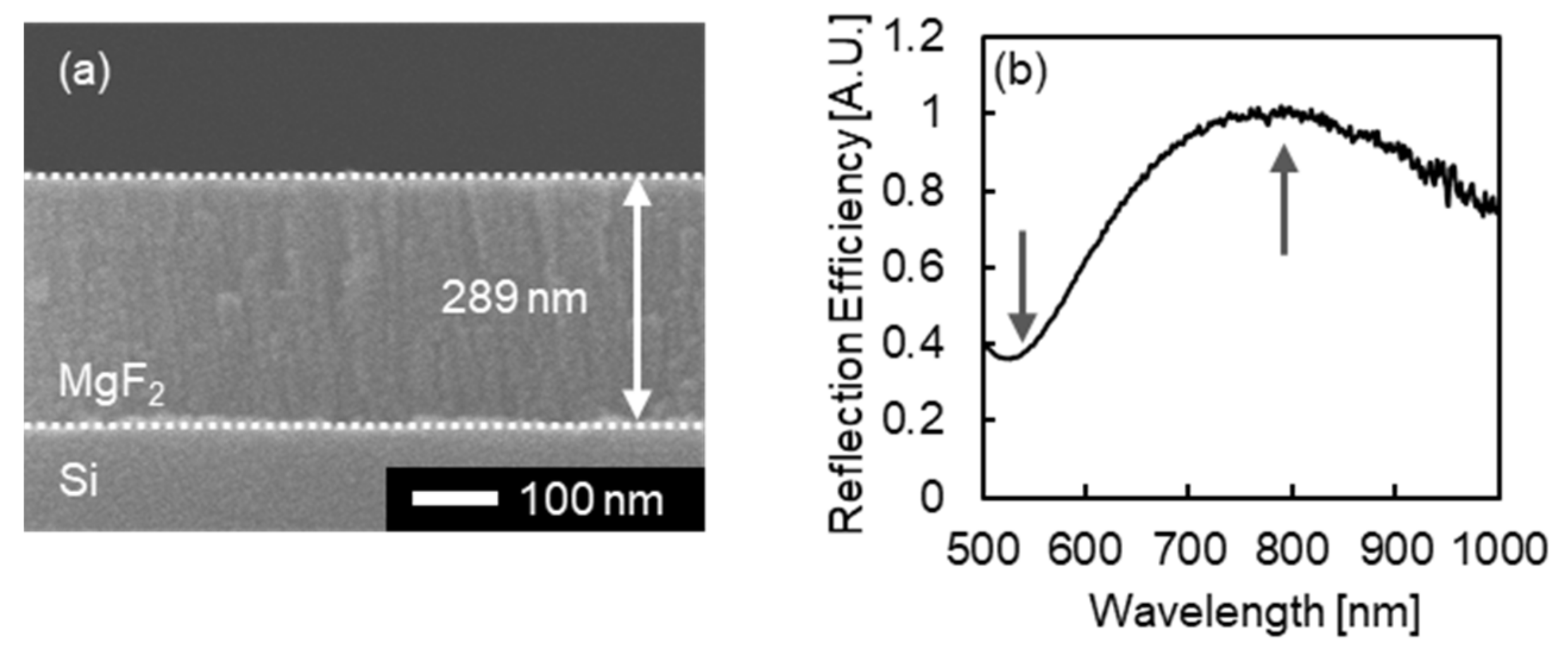
3.2. Structural and Optical Characterization of Fabricated MIM Nanostrctures
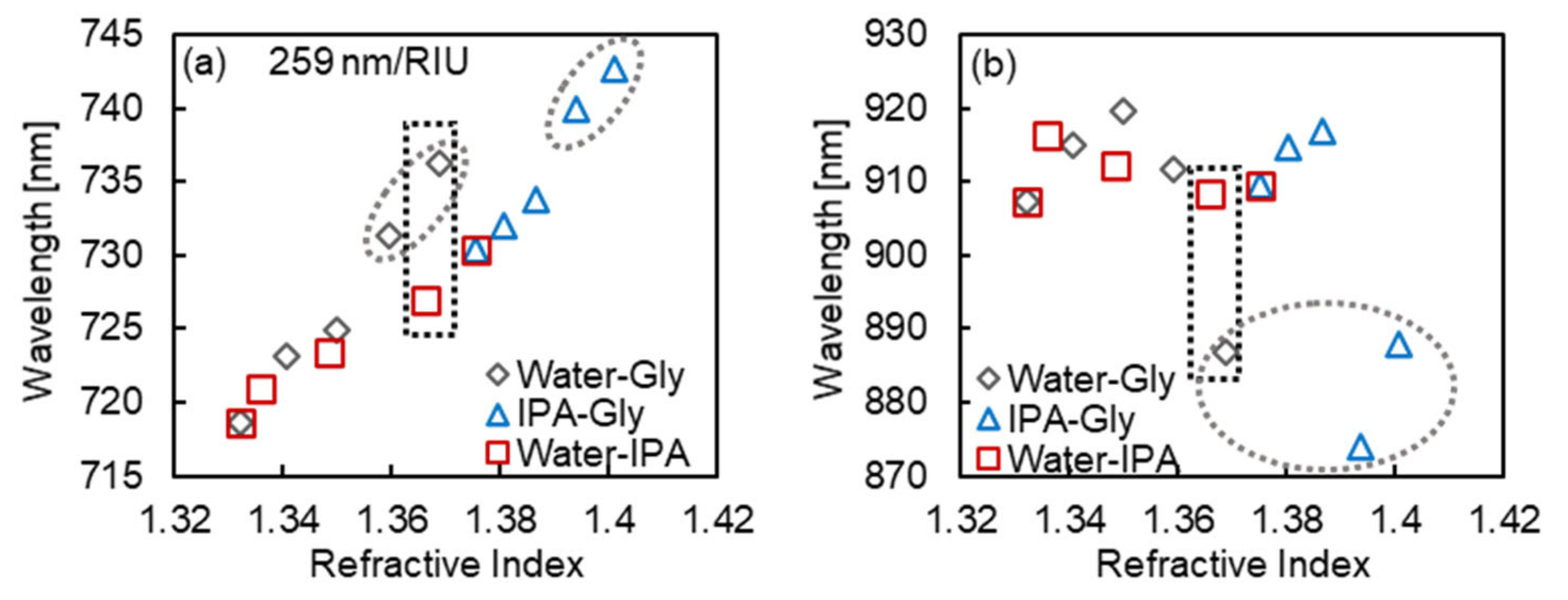
3.3. Surrounding Refractive Index Response of MIM Nanostructures to Sample Solutions with Different Components
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oka, H.; Ohdaira, Y. Simple Model of Saturable Localised Surface Plasmon. Sci. Rep. 2018, 8, 2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebsch, A. Surface-Plasmon Dispersion and Size Dependence of Mie Resonance: Silver versus Simple Metals. Phys. Rev. B 1993, 48, 11317–11328. [Google Scholar] [CrossRef] [PubMed]
- Blaber, M.G.; Henry, A.I.; Bingham, J.M.; Schatz, G.C.; van Duyne, R.P. LSPR Imaging of Silver Triangular Nanoprisms: Correlating Scattering with Structure Using Electrodynamics for Plasmon Lifetime Analysis. J. Phys. Chem. C 2012, 116, 393–403. [Google Scholar] [CrossRef]
- Grześkiewicz, B.; Ptaszyński, K.; Kotkowiak, M. Near and Far-Field Properties of Nanoprisms with Rounded Edges. Plasmonics 2014, 9, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Qin, F.; Yi, Z.; Yao, W.; Liu, Z.; Wu, X.; Wu, P. Ultra-Wideband and Wide-Angle Perfect Solar Energy Absorber Based on Ti Nanorings Surface Plasmon Resonance. Phys. Chem. Chem. Phys. 2021, 23, 17041–17048. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, Y.; Luo, Y.; Zhang, J.; Yi, Z.; Wu, X.; Cheng, S.; Yang, W.; Yu, Y.; Wu, P. A Four-Band and Polarization-Independent BDS-Based Tunable Absorber with High Refractive Index Sensitivity. Phys. Chem. Chem. Phys. 2021, 23, 26864–26873. [Google Scholar] [CrossRef]
- Prodan, E.; Nordlander, P. Structural Tunability of the Plasmon Resonances in Metallic Nanoshells. Nano Lett. 2003, 3, 543–547. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef]
- Abumazwed, A.; Kubo, W.; Tanaka, T.; Kirk, A.G. Improved Method for Estimating Adlayer Thickness and Bulk RI Change for Gold Nanocrescent Sensors. Sci. Rep. 2018, 8, 6683. [Google Scholar] [CrossRef]
- Haes, A.J.; Zou, S.; Schatz, G.C.; van Duyne, R.P. Nanoscale Optical Biosensor: Short Range Distance Dependence of the Localized Surface Plasmon Resonance of Noble Metal Nanoparticles. J. Phys. Chem. B 2004, 108, 6961–6968. [Google Scholar] [CrossRef]
- Verellen, N.; van Dorpe, P.; Huang, C.; Lodewijks, K.; Vandenbosch, G.A.E.; Lagae, L.; Moshchalkov, V.V. Plasmon Line Shaping Using Nanocrosses for High Sensitivity Localized Surface Plasmon Resonance Sensing. Nano Lett. 2011, 11, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Sutherland, J.N.; Maynard, J.A.; Oh, S.H. Nanohole-Based Surface Plasmon Resonance Instruments with Improved Spectral Resolution Quantify a Broad Range of Antibody-Ligand Binding Kinetics. Anal. Chem. 2012, 84, 1941–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohapatra, S.; Moirangthem, R.S. Fabrication of Flexible and Economical Plasmonic Biosensor Using Gold Nanograting Imprinted on Hot-Melt Adhesive Film for Label-Free Sensing of Immunoglobulin Proteins. Sens. Actuators B Chem. 2019, 301, 127070. [Google Scholar] [CrossRef]
- Menezes, J.W.; Ferreira, J.; Santos, M.J.L.; Cescato, L.; Brolo, A.G. Large-Area Fabrication of Periodic Arrays of Nanoholes in Metal Films and Their Application in Biosensing and Plasmonic-Enhanced Photovoltaics. Adv. Funct. Mater. 2010, 20, 3918–3924. [Google Scholar] [CrossRef]
- Svavarsson, H.G.; Yoon, J.W.; Song, S.H.; Magnusson, R. Fabrication of Large Plasmonic Arrays of Gold Nanocups Using Inverse Periodic Templates. Plasmonics 2011, 6, 741–744. [Google Scholar] [CrossRef]
- Mori, T.; Mori, T.; Tanaka, Y.; Suzaki, Y.; Yamaguchi, K. Fabrication of Single-Crystalline Plasmonic Nanostructures on Transparent and Flexible Amorphous Substrates. Sci. Rep. 2017, 7, 42859. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yu, H.; Shan, X.; Wang, W.; Liu, X.; Wang, S.; Tao, N. Label-Free Tracking of Single Organelle Transportation in Cells with Nanometer Precision Using a Plasmonic Imaging Technique. Small 2015, 11, 2878–2884. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Li, H.; Yang, M.; Pang, S.W. High Sensitivity Plasmonic Biosensor Based on Nanoimprinted Quasi 3D Nanosquares for Cell Detection. Nanotechnology 2016, 27, 295101. [Google Scholar] [CrossRef]
- Cetin, A.E.; Topkaya, S.N.; Yalcin-Ozuysal, O.; Khademhosseini, A. Refractive Index Sensing for Measuring Single Cell Growth. ACS Nano 2021, 15, 10710–10721. [Google Scholar] [CrossRef]
- Kim, D.K.; Kerman, K.; Saito, M.; Sathuluri, R.R.; Endo, T.; Yamamura, S.; Kwon, Y.S.; Tamiya, E. Label-Free DNA Biosensor Based on Localized Surface Plasmon Resonance Coupled with Interferometry. Anal. Chem. 2007, 79, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ye, J.; Wang, S.; Stakenborg, T.; Lagae, L. Gold Nanoring as a Sensitive Plasmonic Biosensor for On-Chip DNA Detection. Appl. Phys. Lett. 2012, 100, 173114. [Google Scholar]
- Lednický, T.; Bonyár, A. Large Scale Fabrication of Ordered Gold Nanoparticle-Epoxy Surface Nanocomposites and Their Application as Label-Free Plasmonic DNA Biosensors. ACS Appl. Mater. Interfaces 2020, 12, 4804–4814. [Google Scholar] [CrossRef]
- Hiep, H.M.; Nakayama, T.; Saito, M.; Yamamura, S.; Takamura, Y.; Tamiya, E. A Microfluidic Chip Based on Localized Surface Plasmon Resonance for Real-Time Monitoring of Antigen-Antibody Reactions. Jpn. J. Appl. Phys. 2008, 47, 1337–1341. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.H.; Erdene, N.; Park, J.H.; Jeong, D.H.; Lee, H.Y.; Lee, S.K. Real-Time Label-Free Immunoassay of Interferon-Gamma and Prostate-Specific Antigen Using a Fiber-Optic Localized Surface Plasmon Resonance Sensor. Biosens. Bioelectron. 2013, 39, 346–351. [Google Scholar] [CrossRef]
- Ha, S.J.; Park, J.H.; Lee, B.; Kim, M.G. Label-Free Direct Detection of Saxitoxin Based on a Localized Surface Plasmon Resonance Aptasensor. Toxins 2019, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Ng, S.P.; Liang, X.; Ding, N.; Chen, X.; Wu, C.M.L. Label-Free LSPR Detection of Trace Lead(II) Ions in Drinking Water by Synthetic Poly(MPD-Co-ASA) Nanoparticles on Gold Nanoislands. Anal. Chem. 2017, 89, 1985–1993. [Google Scholar] [CrossRef]
- Atapour, M.; Amoabediny, G.; Ahmadzadeh-Raji, M. Integrated Optical and Electrochemical Detection of Cu2+ Ions in Water Using a Sandwich Amino Acid-Gold Nanoparticle-Based Nano-Biosensor Consisting of a Transparent-Conductive Platform. RSC Adv. 2019, 9, 8882–8893. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, F.; Nie, L. Integrating Multiple “omics” Analysis for Microbial Biology: Application and Methodologies. Microbiology 2010, 156, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Wilmes, A.; Limonciel, A.; Aschauer, L.; Moenks, K.; Bielow, C.; Leonard, M.O.; Hamon, J.; Carpi, D.; Ruzek, S.; Handler, A.; et al. Application of Integrated Transcriptomic, Proteomic and Metabolomic Profiling for the Delineation of Mechanisms of Drug Induced Cell Stress. J. Proteom. 2013, 79, 180–194. [Google Scholar] [CrossRef]
- Cappuccio, A.; Zollinger, R.; Schenk, M.; Walczak, A.; Servant, N.; Barillot, E.; Hupé, P.; Modlin, R.L.; Soumelis, V. Combinatorial Code Governing Cellular Responses to Complex Stimuli. Nat. Commun. 2015, 6, 6847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Cao, G.; Wu, Y.; Zhou, X.; Liao, W. Theoretical Description of Dynamic Transmission Characteristics in MDM Waveguide Aperture-Side-Coupled with Ring Cavity. Plasmonics 2015, 10, 1537–1543. [Google Scholar] [CrossRef]
- Deng, Y.; Cao, G.; Yang, H.; Zhou, X.; Wu, Y. Dynamic Control of Double Plasmon-Induced Transparencies in Aperture-Coupled Waveguide-Cavity System. Plasmonics 2018, 13, 345–352. [Google Scholar] [CrossRef]
- Laible, F.; Horneber, A.; Fleischer, M. Mechanically Tunable Nanogap Antennas: Single-Structure Effects and Multi-Structure Applications. Adv. Opt. Mater. 2021, 9, 210036. [Google Scholar] [CrossRef]
- Pham, T.T.; Pham, D.D.; Nguyen, T.A.H.; Vu, M.T.; Thi Nghiem, L.H.; van Nguyen, T.; Tanaka, D.; Nguyen, D.C. Synthesis and Optical Characterization of Asymmetric Multilayer Metal-Insulator Nanocrescent in Aqueous Solutions. Appl. Phys. Express 2020, 13, 122004. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Plasmonic Coupling in Noble Metal Nanostructures. Chem. Phys. Lett. 2010, 487, 153–164. [Google Scholar] [CrossRef]
- Schaffernak, G.; Krug, M.K.; Belitsch, M.; Gašparić, M.; Ditlbacher, H.; Hohenester, U.; Krenn, J.R.; Hohenau, A. Plasmonic Dispersion Relations and Intensity Enhancement of Metal-Insulator-Metal Nanodisks. ACS Photonics 2018, 5, 4823–4827. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Guo, Y.; Seo, J.; Shuai, Y.; Lee, J.; Lee, B.J. Absorption Characteristics of a Metal-Insulator-Metal Nanodisk for Solar Thermal Applications. Opt. Express 2020, 28, 15731. [Google Scholar] [CrossRef]
- Horrer, A.; Krieg, K.; Freudenberger, K.; Rau, S.; Leidner, L.; Gauglitz, G.; Kern, D.P.; Fleischer, M. Plasmonic Vertical Dimer Arrays as Elements for Biosensing. Anal. Bioanal. Chem. 2015, 407, 8225–8231. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Hall, P.G. Adsorption of Water Vapour by Magnesium Fluoride. J. Chem. Soc. Farad. Trans. 1976, 72, 610–618. [Google Scholar] [CrossRef]
- Cen, J.; Liang, F.; Chen, D.L.; Zhang, L.; Yang, N.; Zhu, W. Adsorption of Water Molecule on Calcium Fluoride and Magnesium Fluoride Surfaces: A Combined Theoretical and Experimental Study. J. Phys. Chem. C 2020, 124, 7853–7859. [Google Scholar] [CrossRef]
- Hohenau, A.; Ditlbacher, H.; Lamprecht, B.; Krenn, J.R.; Leitner, A.; Aussenegg, F.R. Electron Beam Lithography, a Helpful Tool for Nanooptics. Microelectron. Eng. 2006, 83, 1464–1467. [Google Scholar] [CrossRef]
- Aćimović, S.S.; Kreuzer, M.P.; González, M.U.; Quidant, R. Plasmon Near-Field Coupling in Metal Dimers as a Step toward Single-Molecule Sensing. ACS Nano 2009, 3, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, G.F. E-Beam Lithography of Nano-Interdigital Transducers on Insulating and Semiconducting Substrates. Microsyst. Technol. 2010, 16, 2023–2027. [Google Scholar] [CrossRef]
- Iriarte, G.F.; Rodriguez-Madrid, J.G.; Calle, F. Fabrication of Sub-100 nm IDT SAW Devices on Insulating, Semiconducting and Conductive Substrates. J. Mater. Processing Technol. 2012, 212, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Lucas, J.; Smektala, F.; Luc Adam, J. Fluorine in Optics. J. Fluor. Chem. 2002, 114, 113–118. [Google Scholar] [CrossRef]
- Kedawat, G.; Srivastava, S.; Jain, V.K.; Kumar, P.; Kataria, V.; Agrawal, Y.; Gupta, B.K.; Vijay, Y.K. Fabrication of Artificially Stacked Ultrathin ZnS/MgF2 Multilayer Dielectric Optical Filters. ACS Appl. Mater. Interfaces 2013, 5, 4872–4877. [Google Scholar] [CrossRef]




| Samples | Glycerol Concentration [wt.%] for Water-Gly and IPA-Gly | ||||
|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | |
| IPA Concentration [wt.%] for Water-IPA | |||||
| 0 | 3.5 | 18 | 50 | 100 | |
| Water-Gly | 1.3324 | 1.3407 | 1.3499 | 1.3594 | 1.3690 |
| IPA-Gly | 1.3753 | 1.3805 | 1.3865 | 1.3938 | 1.4009 |
| Water-IPA | 1.3324 | 1.3359 | 1.3484 | 1.3661 | 1.3753 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, H.; Kawasaki, D.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Fabrication of Metal-Insulator-Metal Nanostructures Composed of Au-MgF2-Au and Its Potential in Responding to Two Different Factors in Sample Solutions Using Individual Plasmon Modes. Micromachines 2022, 13, 257. https://doi.org/10.3390/mi13020257
Yamada H, Kawasaki D, Sueyoshi K, Hisamoto H, Endo T. Fabrication of Metal-Insulator-Metal Nanostructures Composed of Au-MgF2-Au and Its Potential in Responding to Two Different Factors in Sample Solutions Using Individual Plasmon Modes. Micromachines. 2022; 13(2):257. https://doi.org/10.3390/mi13020257
Chicago/Turabian StyleYamada, Hirotaka, Daiki Kawasaki, Kenji Sueyoshi, Hideaki Hisamoto, and Tatsuro Endo. 2022. "Fabrication of Metal-Insulator-Metal Nanostructures Composed of Au-MgF2-Au and Its Potential in Responding to Two Different Factors in Sample Solutions Using Individual Plasmon Modes" Micromachines 13, no. 2: 257. https://doi.org/10.3390/mi13020257






