Microelectromechanical Systems Based on Magnetic Polymer Films
Abstract
:1. Introduction
2. Common Polymers Used in Developing Magnetic Composite Films
| Polymer | Specific Properties | Ref. * |
|---|---|---|
| Polydimethylsiloxane (PDMS) | Ease of fabrication by rapid prototyping and good sealing, transparency in the UV-visible regions, low polarity, low electrical conductivity, and elasticity/flexibility; density of 970 kg/m3; Young’s modulus = 0.36–0.87 GPa, tensile or fracture strength is 3.5–7.65 MPa while elongation to break is 76%; Good chemical stability, being compatible with the following solvents: water, nitromethane, dimethyl sulfoxide, ethylene glycol, perfluorotributylamine, perfluorodecalin, acetonitrile, and propylene carbonate. Higher swelling ratios are reported for diisopropylamine, triethylamine, pentane, and xylenes (1.41–2.13 defined as follow: S = D/D0, where D is the length of PDMS in the solvent and D0 is the length of the dry PDMS). A major drawback of the PDMS-based materials is related to the high deformability. For instance, in the case of the microfluidic systems with thin wall, the diameter can be enlarged several times before failure. | [27,28,29,30] |
| Polymethyl methacrylate (PMMA) | Good transparency in the visible regions, but filters the UV light bellow 300 nm; durable density of 1180 kg/m3; the glass transition occurs between 100 and 130 °C; water absorption is 0.3%; Young Modulus is 2855 MPa, tensile or fracture strength is 70 MPa while the elongation to break is of 4.5%; PMMA is biocompatible and also biodegradable. Generally, PMMA is stable in most inorganic chemicals, aliphatic hydrocarbons, cycloaliphatic compounds, fats and oils at room temperature, and also to diluted acids and concentrated solutions of most alkalis at temperatures up to 60 °C but is attacked by chlorinated hydrocarbons, ketones, esters, ethers, alcohols and aromatic compounds. | [31] |
| Parylene (PAR) | Transparent material, the glass transition temperature <90 °C; depending on the composition, the melting point can vary between 290 and 420 °C; tensile strength is 45–69 MPa for Parylene N/C, the Young’s modulus is 2.4–3.0 GPa (N-C-F) while the elongation to break is 20–200% for Parylene C, can reach 250% for Parylene N while for Parylene F 10–50% at most. Good water absorption (<0.1%); good barrier properties in general; inert to most solvents up to 150 °C; parylene C became soluble in chloro-naphtalene at 175 °C while parylene N at 265 °C (solvent boiling point); diluted inorganic reagents (including acids, alkali, etc.) have no effect bellow 75 °C but, under severe conditions (concentrated acids, 75 °C for 30mins) swelling is observed (ranging from 0.7% for HCl to 8.2% for chromic acid); these polymers are biocompatible. | [32,33] |
| Polyimides (PI) | Strong dependence of the properties and performances of the polyimides can be correlated with the composition and synthesis/processing; aromatic polyimides are usually dielectric, tensile strength 72 MPa, their Young modulus can be 3.8–12.2 GPa while the elongation to break is only 8% depending on composition and processing, transparent material in the visible range (80–92% transmittance in the 420–900 nm); excellent thermal stability but also good chemical properties; The solubility is strongly dependent on the nature of the polyimides; there are some polyimides soluble in polar solvents (such as dimethyl acetamide, dimethyl formamide, N-methyl pyrolidone, m-cresol, as well as in conventional polar solvents such as tetrahydrofuran and chloroform) but other polyimides can be very stable. Loading polyimides with 0.5–1.4% carbon-based materials can greatly approve electrical properties; good biocompatibility and can act as an electrically triggering drug delivery support (if loaded with C). | [34,35,36,37] |
| Polyethylene terephthalate (PET) | Strong dependence of the properties and performances with the synthesis/ processing; Young Modulus is 2.8–3.17 GPa, tensile or fracture strength is 60–85 MPa while the elongation to break is of 20% but is also strongly dependent on the composition; transparent material in the visible range; good thermal stability, the melting point is 255–265 while the Tg is 67–140 °C; PET is insoluble in water, ethyl ether and most organic solvents but soluble in trifluoro acetic acid, DMSO, nitrobenzene, phenol and o-chlorophenol. The chemical stability in concentrated acids or alkali is poor, thus, limiting the use of this polymer. | [38] |
| Polyethersulphone (PES) | Polyethersulfone is a transparent material resistant to acids, alkalis, oils, greases, and aliphatic hydrocarbons and alcohols. It is attacked by ketones, ester, some halogenated and aromatic hydrocarbons, pyridine and aniline; it is very stable, being obtained up to 400 °C, being not oxidized up to 150–190 °C; the Tg is 190–290 °C; Young Modulus is 2.6 GPa, tensile or fracture strength is 83–85 MPa while the elongation to break is of 25–80% being strongly dependent on the composition and processing. | [39] |
| Polystyrene (PS) | Transparent, brittle, flammable, thermoplastic, stiff and hard material, obtained by polimerization of styrene. Polystyrene can be copolymerized or blended with other polymers, lending hardness and rigidity to many plastic materials. Flows when heated over 100 °C. Refractive index is 1.6. Soluble in benzene, toluene, ethylacetate, acetone, chloroform, trichloroethylene, cyclohexanone, MEK, THF, etc. Insoluble in water. | [40,41] |
| Polyaniline (PANI) | It is one of the best known, studied and applicable conducting polymer. Its properties strongly depend on the type of dopant and its concentration. PANI has many amine functional groups which interact with negative charge anion owing to its inherent cationic nature, such as easy chemical/electrochemical synthesis in a large scale, nontoxicity and good environmental stability. | [42,43] |
3. MEMS Type Devices Based on Magnetic Polymer Films
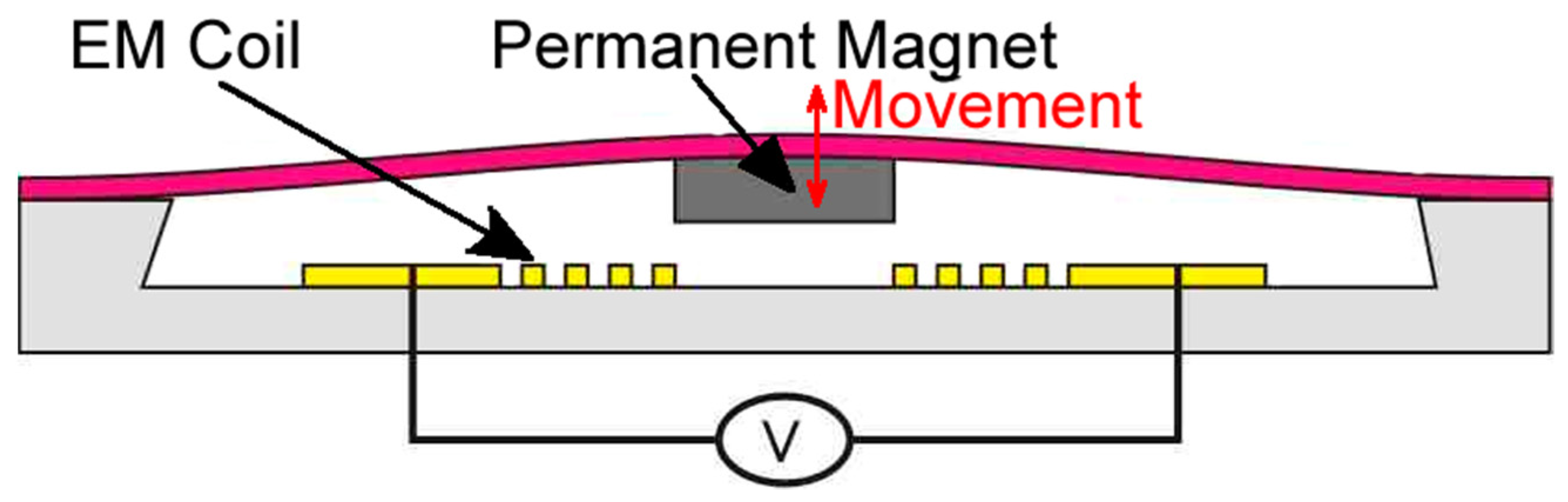
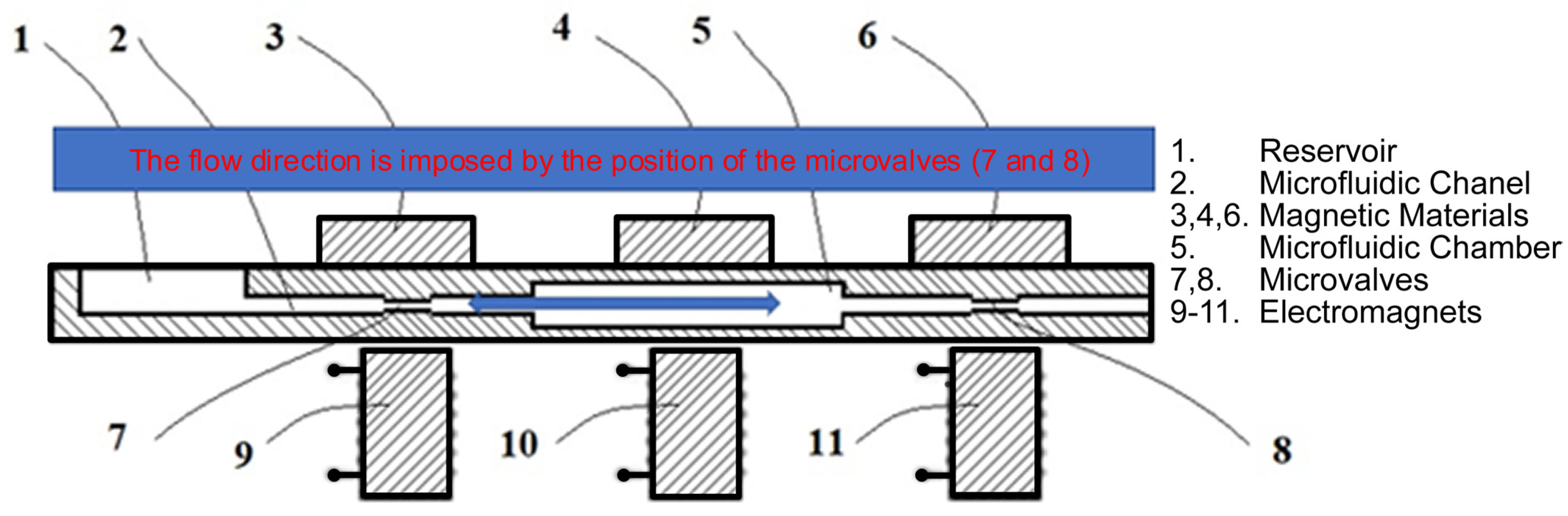
3.1. Microvalves
3.2. Micromixers
3.3. Microsensors
3.4. Magnetic Labeling and Separation
3.5. Micropumps
3.6. Drug Delivery Micro-Systems
| Crt. No. | Film | Composition | Applications | Reference |
|---|---|---|---|---|
| 1 | PDMS-(Nd-Ce)FeB | Magnetic composite polymers based on PDMS and hard rare earth magnetic powder, (Nd0.7Ce0.3)10.5Fe83.9B5.6 at a ratio of 20:80 (wt) | Delivery, mixer | [48] |
| 2 | Silicon rubber-NbFeB | Silicon rubber loaded with NbFeB | Transport and mixing | [83] |
| 3 | PDMS-NdFeB-polyelectrolytes | PDMS membranes and 10%, 30%, 50% or 70%) NdFeB powder were obtained, proving the possibility of using these films as film-shaped microrobots. | Delivery and Transport | [52] |
| 4 | shape memory polymer/polyimide laminate actuator | Shape memory polymer/polyimide laminate actuator, wirelessly activated by external RF electromagnetic field at specific frequency of heater. Cu-clad PI. | Drug Delivery | [55] |
| 5 | Fe3O4/PDMS | PDMS-based composite film loaded with 5% Fe3O4 is used in a bidirectional approach using two identical coils with 15 turns and operated alternatively. | Micropump, Drug Delivery | [3] |
| 6 | Silicone rubber-Fe | Silicon rubber membranes loaded with 30% (w/w) atomized iron powder. | Transdermal Delivery | [53] |
| 7 | PDMS-NdFeB | NdFeB magnets were entrapped into PDMS film | Intramedullary delivery | [54] |
| 8 | Stimuli responsive polymer—Ni | Different stimuli (pH and temperature) responsive hydrogels are patterned at the center axis of the electroplated Ni rotor. By changing the conditions, these hydrogel rings shrink expansion and, thus, assure, within a microfluidic chamber, a mixing process. | Sample Preparation | [8] |
| 9 | PDMS-Fe(CO)5 | PDMS-based film is loaded with iron carbonyl and used for the manipulation (capture) of the magnetically labeled cells. | Cell and Particle Manipulator Chemical Release of Antibiotics | [77,81] |
| 10 | Polystyrene-Fe3O4 | Magnetic Fe3O4 nanoparticles were encapsulated by polystyrene. | Microelectromechanical systems (MEMS) | [78] |
| 11 | PANI-Fe2O3 | Magnetic nanoparticles are coated with PANI | Signal transducer for application in a disposable membrane strip biosensor. | [88] |
4. Design and Fabrication of the Microfluidic Systems
5. Conclusions
6. Challenges and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piedade, A.P.; Romeu, F.; Branco, R.; Morais, P.V. Thin films for medical and environmental applications. In Methods for Film Synthesis and Coating Procedures; Nánai, L., Samantara, A., Fábián, L., Ratha, S., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Liu, Y.-W.; Zhan, Q.-F.; Li, R.-W. Fabrication, properties, and applications of flexible magnetic films. Chin. Phys. B 2013, 22, 127502. [Google Scholar] [CrossRef]
- Tahmasebipour, M.; Paknahad, A.A. Unidirectional and bidirectional valveless electromagnetic micropump with PDMS-Fe3O4 nanocomposite magnetic membrane. J. Micromech. Microeng. 2019, 29, 075014. [Google Scholar] [CrossRef]
- Hatch, A.; Kamholz, A.; Holman, G.; Yager, P.; Bohringer, K. A ferrofluidic magnetic micropump. J. Microelectromech. Syst. 2001, 10, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Khoo, M.; Liu, C. Micro magnetic silicone elastomer membrane actuator. Sens. Actuators A Phys. 2001, 89, 259–266. [Google Scholar] [CrossRef]
- Gad-el-Hak, M. Micropumps, microturbines, and flow physics in microdevices. In Smart Structures and Materials 2003: Smart Electronics, Mems, Biomems, and Nanotechnology; International Society for Optics and Photonics: Bellingham, WA, USA, 2003; Volume 5055, pp. 242–257. [Google Scholar]
- Liu, C.X.; Guo, M.; Chen, X.F.; Cheng, J. Low voltage driven miniaturized pump with high back pressure. In Microfluidics, Biomems, and Medical Microsystems; International Society for Optics and Photonics: Bellingham, WA, USA, 2003; Volume 4982, pp. 344–355. [Google Scholar]
- Agarwal, A.; Sridharamurthy, S.; Beebe, D.; Jiang, H. Programmable autonomous micromixers and micropumps. J. Microelectromech. Syst. 2005, 14, 1409–1421. [Google Scholar] [CrossRef]
- Chang, H.T.; Lee, C.Y.; Wen, C.Y. Design and modeling of a MEMS-based valveless pump driven by an electromagnetic force. arXiv 2006, arXiv:0711.3320. [Google Scholar]
- Hauptmann, P.R. Selected examples of intelligent (micro) sensor systems: State-of-the-art and tendencies. Meas. Sci. Technol. 2006, 17, 459–466. [Google Scholar] [CrossRef]
- Sun, Y.; Kwok, Y.C.; Nguyen, N.T. A Novel Circular Ferro-Fluid Driven Flow-Through Microchip for Rapid DNA Amplification. In Proceedings of the TRANSDUCERS 2007–2007 International Solid-State Sensors, Actuators and Microsystems Conference, Lyon, France, 10–14 June 2007; Volumes 1 and 2, pp. 383–386. [Google Scholar] [CrossRef]
- Sun, Y.; Kwok, Y.C.; Nguyen, N.T. A circular ferrofluid driven microchip for rapid polymerase chain reaction. Lab Chip 2007, 7, 1012–1017. [Google Scholar] [CrossRef]
- Feldmann, A.; Demming, S.; Lesche, C.; Buttgenbach, S. Novel electromagnetic micropump. In BioMEMS and Nanotechnology III; International Society for Optics and Photonics: Bellingham, WA, USA, 2008; Volume 6799, p. 67990T. [Google Scholar]
- Khaderi, S.N.; Ioan, D.; den Toonder, J.M.J.; Onck, P.R. Nature-inspired microfluidic manipulation using magnetic actuators. In Proceedings of the Symposium on Microelectromechanical Systems-Materials and Devices Held at the 2007 MRS Fall Meeting, Tangier, Morocco, 26–28 November 2007; Materials Research Society: Warrendale, PA, USA, 2008; pp. 297–303. [Google Scholar]
- Al-Fandi, M.; Jaradat, M.; Fandi, K.; Beech, J.; Tegenfeldt, J.; Yih, T. Nano-engineered living bacterial motors for active microfluidic mixing. IET Nanobiotechnol. 2010, 4, 61–71. [Google Scholar] [CrossRef]
- Jackson, W.C.; Rabinovich, E.; Tran, H.D.; O’Brien, M.J.; Lopez, G.P. Rapid prototyping of active microfluidic components based on magnetically modified elastomeric materials. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2001, 19, 596. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Tsai, Y.-W.; Tsai, C.-H.; Lee, C.-Y.; Fu, L.-M. Design and Analysis of Impedance Pumps Utilizing Electromagnetic Actuation. Sensors 2010, 10, 4040–4052. [Google Scholar] [CrossRef] [PubMed]
- Yunas, J.; Mulyanti, B.; Hamidah, I.; Said, M.M.; Pawinanto, R.E.; Ali, W.A.F.W.; Subandi, A.; Hamzah, A.A.; Latif, R.; Majlis, B.Y. Polymer-Based MEMS Electromagnetic Actuator for Biomedical Application: A Review. Polymers 2020, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Bußmann, A.; Leistner, H.; Zhou, D.; Wackerle, M.; Congar, Y.; Richter, M.; Hubbuch, J. Piezoelectric Silicon Micropump for Drug Delivery Applications. Appl. Sci. 2021, 11, 8008. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Goharpey, F.; Velankar, S. Interfacially compatibilized PI/PDMS blends with reduced octadecylamine-functionalized graphene oxide: Morphological and rheological properties. Soft Matter 2021, 17, 9670–9681. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Das, S.; Mohanty, S.; Nayak, S.K. A facile preparation of epoxy-polydimethylsiloxane (EP-PDMS) polymer coatings for marine applications. J. Mater. Res. 2019, 34, 2881–2894. [Google Scholar] [CrossRef]
- Borók, A.; Laboda, K.; Bonyár, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J.; Sun, Y.; Bock, C.; Chen, Q. Thickness-dependent mechanical properties of polydimethylsiloxane membranes. J. Micromech. Microeng. 2009, 19, 035028. [Google Scholar] [CrossRef]
- Versaci, M.; Jannelli, A.; Morabito, F.; Angiulli, G. A Semi-Linear Elliptic Model for a Circular Membrane MEMS Device Considering the Effect of the Fringing Field. Sensors 2021, 21, 5237. [Google Scholar] [CrossRef]
- Angiulli, G.; Jannelli, A.; Morabito, F.C.; Versaci, M. Reconstructing the membrane detection of a 1D electrostatic-driven MEMS device by the shooting method: Convergence analysis and ghost solutions identification. Comput. Appl. Math. 2018, 37, 4484–4498. [Google Scholar] [CrossRef]
- Steeves, C.A.; Young, Y.L.; Liu, Z.; Bapat, A.; Bhalerao, K.; Soboyejo, A.B.O.; Soboyejo, W.O. Membrane thickness design of implantable bio-MEMS sensors for the in-situ monitoring of blood flow. J. Mater. Sci. Mater. Electron. 2007, 18, 25–37. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, A.C.; Whitesides, G.M. Solvent Compatibility of Poly(dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.-J.; Kim, J.; Kim, J.-A.; Jang, H.; Seo, S.; Lee, W. PDMS-Parylene Hybrid, Flexible Microfluidicsfor Real-Time Modulation of 3D HelicalInertial Microfluidics. Micromachines 2018, 9, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskin, D.; Mokabbar, T.; Pei, Y.; Van Rijn, P. The Relationship between Bulk Silicone and Benzophenone-Initiated Hydrogel Coating Properties. Polymers 2018, 10, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghe, M.; Blionas, S.; Ragoussis, J.; Galvin, P. Evaluation of Silicon and Polymer substrates for fabrication of integrated microfluidic microsystems for DNA extraction and amplification. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2482–2485. [Google Scholar]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Noh, H.-S.; Moon, K.-S.; Cannon, A.; Hesketh, P.J.; Wong, C.P. Wafer bonding using microwave heating of parylene intermediate layers. J. Micromech. Microeng. 2004, 14, 625–631. [Google Scholar] [CrossRef]
- Parylene Conformal Coating Specifications & Properties. Available online: https://engineering.tufts.edu/microfab/documents/Parylene.pdf (accessed on 30 November 2021).
- Georgiev, A.; Dimov, D.; Spassova, E.; Assa, J.; Dineff, P.; Danev, G. Chemical and Physical Properties of Polyimides: Biomedical and Engineering Applications. In High Performance Polymers—Polyimides Based—From Chemistry to Applications; Abadie, M.J.M., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Lyulin, S.V.; Larin, S.V.; Gurtovenko, A.A.; Nazarychev, V.M.; Falkovich, S.G.; Yudin, V.E.; Svetlichnyi, V.M.; Gofman, I.V.; Lyulin, A.V. Thermal properties of bulk polyimides: Insights from computer modeling versus experiment. Soft Matter 2014, 10, 1224–1232. [Google Scholar] [CrossRef]
- Ree, M.; Kim, K.; Woo, S.H.; Chang, H. Structure, chain orientation, and properties in thin films of aromatic polyimides with various chain rigidities. J. Appl. Phys. 1997, 81, 698–708. [Google Scholar] [CrossRef]
- Ghosh, A.; Sen, S.K.; Banerjee, S.; Voit, B. Solubility improvements in aromatic polyimides by macromolecular engineering. RSC Adv. 2012, 2, 5900–5926. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Ida, S. PES (Poly(ether sulfone)), Polysulfone. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Philippova, O.; Barabanova, A.; Molchanov, V.; Khokhlov, A. Magnetic polymer beads: Recent trends and developments in synthetic design and applications. Eur. Polym. J. 2011, 47, 542–559. [Google Scholar] [CrossRef] [Green Version]
- Taddei, C.; Sansone, L.; Ausanio, G.; Iannotti, V.; Pepe, G.P.; Giordano, M.; Serra, C.A. Fabrication of polystyrene-encapsulated magnetic iron oxide nanoparticles via batch and microfluidic-assisted production. Colloid Polym. Sci. 2019, 297, 861–870. [Google Scholar] [CrossRef]
- Babel, V.; Hiran, B.L. A review on polyaniline composites: Synthesis, characterization, and applications. Polym. Compos. 2021, 42, 3142–3157. [Google Scholar] [CrossRef]
- Bhadra, J.; Alkareem, A.; Al-Thani, N. A review of advances in the preparation and application of polyaniline based thermoset blends and composites. J. Polym. Res. 2020, 27, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Arias, F.; Oliver, S.; Xu, B.; Holmlin, R.; Whitesides, G. Fabrication of metallic heat exchangers using sacrificial polymer mandrils. J. Microelectromech. Syst. 2001, 10, 107–112. [Google Scholar] [CrossRef]
- Koydemir, H.C.; Kulah, H.; Ozgen, C. Solvent Compatibility of Parylene C Film Layer. J. Microelectromech. Syst. 2013, 23, 298–307. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Bao, M. Introduction to MEMS Devices. In Analysis and Design Principles of MEMS Devices; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–32. [Google Scholar]
- Currie, C.E.; Gray, B.L. Bidirectional Magnetic Polymer Membrane Actuators Integrated into Thermoplastic Microfluidics. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 8–22 January 2020; pp. 1056–1059. [Google Scholar] [CrossRef]
- Amokrane, W.; Belharet, K.; Ferreira, A. Design and modeling of a two-magnet actuator for robotic micromanipulation. Sens. Actuators A Phys. 2020, 316, 112391. [Google Scholar] [CrossRef]
- Vignali, E.; Manigrasso, Z.; Gasparotti, E.; Biffi, B.; Landini, L.; Positano, V.; Capelli, C.; Celi, S. Design, simulation, and fabrication of a three-dimensional printed pump mimicking the left ventricle motion. Int. J. Artif. Organs 2019, 42, 539–547. [Google Scholar] [CrossRef]
- Lee, M.; Park, T.; Kim, C.; Park, S.-M. Characterization of a magneto-active membrane actuator comprising hard magnetic particles with varying crosslinking degrees. Mater. Des. 2020, 195, 108921. [Google Scholar] [CrossRef]
- Iacovacci, V.; Lucarini, G.; Innocenti, C.; Comisso, N.; Dario, P.; Ricotti, L.; Menciassi, A. Polydimethylsiloxane films doped with NdFeB powder: Magnetic characterization and potential applications in biomedical engineering and microrobotics. Biomed. Microdevices 2015, 17, 1–7. [Google Scholar] [CrossRef]
- Jayaneththi, V.; Aw, K.; Sharma, M.; Wen, J.; Svirskis, D.; McDaid, A. Controlled transdermal drug delivery using a wireless magnetic microneedle patch: Preclinical device development. Sens. Actuators B Chem. 2019, 297, 126708. [Google Scholar] [CrossRef]
- Chen, Z.; Noh, S.; Prisby, R.D.; Lee, J.-B. An Implanted Magnetic Microfluidic Pump for In Vivo Bone Remodeling Applications. Micromachines 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zainal, M.A.; Ahmad, A.; Ali, M.S.M. Frequency-controlled wireless shape memory polymer microactuator for drug delivery application. Biomed. Microdevices 2017, 19, 8. [Google Scholar] [CrossRef]
- Oh, K.W.; Ahn, C.H. A review of microvalves. J. Micromech. Microeng. 2006, 16, R13–R39. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Hou, C.-W.; Li, X.-J.; Jin, Z.-J. Actuation Mechanism of Microvalves: A Review. Micromachines 2020, 11, 172. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xing, D.; Li, Y. Micropumps, microvalves, and micromixers within PCR microfluidic chips: Advances and trends. Biotechnol. Adv. 2007, 25, 483–514. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suzuki, H. Switchable Microvalves Employing a Conducting Polymer and Their Automatic Operation in Conjunction with Micropumps with a Superabsorbent Polymer. ACS Appl. Mater. Interfaces 2020, 12, 37741–37749. [Google Scholar] [CrossRef]
- D’Eramo, L.; Chollet, B.; Leman, M.; Martwong, E.; Li, M.; Geisler, H.; Dupire, J.; Kerdraon, M.; Vergne, C.; Monti, F.; et al. Microfluidic actuators based on temperature-responsive hydrogels. Microsyst. Nanoeng. 2018, 4, 17069. [Google Scholar] [CrossRef]
- Amrani, I.; Cheriet, A.; Feliachi, M. Design and experimental investigation of a bi-directional valveless electromagnetic micro-pump. Sens. Actuators A Phys. 2018, 272, 310–317. [Google Scholar] [CrossRef]
- Alam, M.W.; Samad, M.F. Design and Optimization of Electromagnetically Actuated Bidirectional Microvalve. In Proceedings of the 3rd International Conference on Electrical, Computer and Telecommunication Engineering, ICECTE, Rajshahi, Bangladesh, 26–28 December 2019; pp. 248–251. [Google Scholar]
- Campbell, C.J.; Grzybowski, B.A. Microfluidic mixers: From microfabricated to self-assembling devices. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2004, 362, 1069–1086. [Google Scholar] [CrossRef]
- Wang, G.R.; Yang, F.; Zhao, W. There can be turbulence in microfluidics at low Reynolds number. Lab Chip 2014, 14, 1452–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Shen, J. Simulation and experimental analysis of a SAR micromixer with F-shape mixing units. Anal. Methods 2017, 9, 1885–1890. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Wu, Z.; Zheng, Y. Numerical study on stagger Koch fractal baffles micromixer. Int. J. Heat Mass Transf. 2019, 133, 1065–1073. [Google Scholar] [CrossRef]
- Yin, B.; Yue, W.; Sohan, A.S.M.M.F.; Zhou, T.; Qian, C.; Wan, X. Micromixer with Fine-Tuned Mathematical Spiral Structures. ACS Omega 2021, 6, 30779–30789. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Chen, X. Simulation and experimental research of an effective SAR multilayer interlaced micromixer based on Koch fractal geometry. Microfluid. Nanofluidics 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Lee, C.-J.; Hsu, Y.-H. Vacuum pouch microfluidic system and its application for thin-film micromixers. Lab Chip 2019, 19, 2834–2843. [Google Scholar] [CrossRef]
- Arayanarakool, R.; See, H.H.; Marshall, S.D.; Virik, N.S.; Wang, H.; Lee, P.S.; Chen, P.C.Y. Rapid Prototyping of Polymer-Based Rolled-Up Microfluidic Devices. Micromachines 2018, 9, 516. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J. A Review on Micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef]
- Rahbar, M.; Shannon, L.; Gray, B.L. Microfluidic active mixers employing ultra-high aspect-ratio rare-earth magnetic nano-composite polymer artificial cilia. J. Micromech. Microeng. 2013, 24, 025003. [Google Scholar] [CrossRef]
- Pawinanto, R.E.; Yunas, J.; Hashim, A.M. Micropillar based active microfluidic mixer for the detection of glucose concentration. Microelectron. Eng. 2020, 234, 111452. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Alcacer, A.; Bausells, J.; Jaffrezic-Renault, N.; Zine, N.; Errachid, A. Electrochemical Impedance Spectroscopy Microsensor Based on Molecularly Imprinted Chitosan Film Grafted on a 4-Aminophenylacetic Acid (CMA) Modified Gold Electrode, for the Sensitive Detection of Glyphosate. Front. Chem. 2021, 9, 621057. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.-H.-N.; Martincic, E.; Dufour-Gergam, E.; Joubert, P.-Y. Mechanical Characterization of PDMS Films for the Optimization of Polymer Based Flexible Capacitive Pressure Microsensors. J. Sens. 2017, 2017, 8235729. [Google Scholar] [CrossRef]
- Simonov, V.; Vlasov, D. Piezoresonance Chemical Sensors on Elastic Polymer Films. Meas. Tech. 2019, 62, 294–298. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Wang, L.; Yi, X.; Hui, Y.S.; Qin, J.; Wen, W. Microfluidic Device for Controllable Chemical Release via Field-Actuated Membrane Incorporating Nanoparticles. J. Nanomater. 2013, 2013, 5. [Google Scholar] [CrossRef]
- Omidi, M.H.; Alibeygi, M.; Piri, F.; Masoudifarid, M. Polystyrene/magnetite nanocomposite synthesis and characterization: Investigation of magnetic and electrical properties for using as microelectromechanical systems (MEMS). Mater. Sci. 2017, 35, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-J.; Duan, F.-F.; Wu, P.-C.; Lee, W. Liquid crystal–photopolymer composite films for label-free single-substrate protein quantitation and immunoassay. Biomed. Opt. Express 2020, 11, 4915–4927. [Google Scholar] [CrossRef]
- Goudu, S.R.; Kim, H.; Hu, X.; Lim, B.; Kim, K.; Torati, S.R.; Ceylan, H.; Sheehan, D.; Sitti, M.; Kim, C. Mattertronics for programmable manipulation and multiplex storage of pseudo-diamagnetic holes and label-free cells. Nat. Commun. 2021, 12, 3024. [Google Scholar] [CrossRef]
- Faivre, M.; Gelszinnis, R.; Degouttes, J.; Terrier, N.; Rivière, C.; Ferrigno, R.; Deman, A.-L. Magnetophoretic manipulation in microsystem using carbonyl iron-polydimethylsiloxane microstructures. Biomicrofluidics 2014, 8, 054103. [Google Scholar] [CrossRef] [Green Version]
- Suna, A.; Gulle, M.; Erten, A. Comparison of Magnetic Particle Incorporated PDMS Membrane Actuators. In Proceedings of the 2019 11th International Conference on Electrical and Electronics Engineering (ELECO), Bursa, Turkey, 28–30 November 2019; pp. 475–478. [Google Scholar]
- Wang, X.; Zhang, Q.; Liu, P.; Zhu, X.; Wu, C.; Wang, J.; Liu, C.; Wang, J.; Gao, Y.; Song, A.; et al. An ultrafast response and precisely controllable soft electromagnet actuator based on Ecoflex rubber film filled with neodymium-iron-boron. J. Micromech. Microeng. 2020, 31, 025010. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.B.; Kim, B.H.; Lee, C.; Cho, Y.M.; Kim, S.N.; Park, C.G.; Cho, Y.C.; Choy, Y.B. Implantable batteryless device for on-demand and pulsatile insulin administration. Nat. Commun. 2017, 8, 15032. [Google Scholar] [CrossRef] [Green Version]
- Sönmez, M.; Ficai, D.; Ficai, A.; Alexandrescu, L.; Georgescu, M.; Trusca, R.; Gurau, D.; Titu, M.A.; Andronescu, E. Applications of mesoporous silica in biosensing and controlled release of insulin. Int. J. Pharm. 2018, 549, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Barbari, G.R.; Dorkoosh, F.; Amini, M.; Javan, N.B.; Sharifzadeh, M.; Atyabi, F.; Balalaie, A.; Tehrani, N.R.; Tehrani, M.R. Synthesis and characterization of a novel peptide-grafted Cs and evaluation of its nanoparticles for the oral delivery of insulin, in vitro, and in vivo study. Int. J. Nanomed. 2018, 13, 5127–5138, Retracted in Int. J. Nanomed. 2020, 15, 1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Park, J. Magnetic micropump embedded in contact lens for on-demand drug delivery. Micro Nano Syst. Lett. 2020, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yuk, J.S.; Rose, J.; Alocilja, E.C. Characterization of polyaniline-coated magnetic nanoparticles for application in a disposable membrane strip biosensor. Eur. Phys. J. Appl. Phys. 2010, 50, 11401. [Google Scholar] [CrossRef]
- Said, M.M.; Yunas, J.; Bais, B.; Hamzah, A.A.; Majlis, B.Y. The Design, Fabrication, and Testing of an Electromagnetic Micropump with a Matrix-Patterned Magnetic Polymer Composite Actuator Membrane. Micromachines 2017, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Gheorghe, M.; Ficai, A.; Andronescu, E.; Ficai, D.; Dolete, D.; Mihailescu, B. Electromagnetic micropump and process for its realization (Micropompa electromagnetica si procedeu de realizare a acesteia). In NanoMems SRL; OSIM, Ed.; POLITEHNICA University of Bucharest: Bucharest, Romania, 2021. [Google Scholar]
- Lee, Y.-S.; Bhattacharjee, N.; Folch, A. 3D-printed Quake-style microvalves and micropumps. Lab Chip 2018, 18, 1207–1214. [Google Scholar] [CrossRef]
- Zhou, Z.; He, G.; Zhang, K.; Zhao, Y.; Sun, D. 3D-printed membrane microvalves and microdecoder. Microsyst. Technol. 2019, 25, 4019–4025. [Google Scholar] [CrossRef]
- Preuss, J.; Nguyen, G.N.; Berk, V.; Bahnemann, J. Miniaturized free-flow electrophoresis: Production, optimization, and application using 3D printing technology. Electrophoresis 2021, 42, 305–314. [Google Scholar] [CrossRef]
- Balakrishnan, H.K.; Doeven, E.H.; Merenda, A.; Dumée, L.F.; Guijt, R.M. 3D printing for the integration of porous materials into miniaturised fluidic devices: A review. Anal. Chim. Acta 2021, 1185, 338796. [Google Scholar] [CrossRef]
- Thomas, D.; Tehrani, Z.; Redfearn, B. 3-D printed composite microfluidic pump for wearable biomedical applications. Addit. Manuf. 2016, 9, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Fleck, E.; Sunshine, A.; DeNatale, E.; Keck, C.; McCann, A.; Potkay, J. Advancing 3D-Printed Microfluidics: Characterization of a Gas-Permeable, High-Resolution PDMS Resin for Stereolithography. Micromachines 2021, 12, 1266. [Google Scholar] [CrossRef] [PubMed]
- Fiering, J.; Mescher, M.J.; Swan, E.E.L.; Holmboe, M.E.; Murphy, B.A.; Chen, Z.; Peppi, M.; Sewell, W.F.; McKenna, M.J.; Kujawa, S.G.; et al. Local drug delivery with a self-contained, programmable, microfluidic system. Biomed. Microdevices 2008, 11, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.W.; Chen, X.M.; Zhang, J.; Zhang, X.H.; Xie, J.; Jin, L.; Liu, Z.H.; Zhuang, J.; Ren, W.; Ye, Z.G. A wearable, nozzle-diffuser microfluidic pump based on high-performance ferroelectric nanocomposites. Sens. Actuators B Chem. 2021, 347, 130611. [Google Scholar] [CrossRef]
- Ahmad, N.F.N.; Ghazali, N.N.N.; Wong, Y.H. Wearable patch delivery system for artificial pancreas health diagnostic-therapeutic application: A review. Biosens. Bioelectron. 2021, 189, 113384. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Huang, T.; Chung, J.H.; Chen, C. 3D-Printed, Modular, and Parallelized Microfluidic System with Customizable Scaffold Integration to Investigate the Roles of Basement Membrane Topography on Endothelial Cells. ACS Biomater. Sci. Eng. 2021, 7, 1600–1607. [Google Scholar] [CrossRef]
- Goldstein, Y.; Spitz, S.; Turjeman, K.; Selinger, F.; Barenholz, Y.; Ertl, P.; Benny, O.; Bavli, D. Breaking the Third Wall: Implementing 3D-Printing Techniques to Expand the Complexity and Abilities of Multi-Organ-on-a-Chip Devices. Micromachines 2021, 12, 627. [Google Scholar] [CrossRef]
- Hilber, W.; Clara, S.; Jakoby, B. Microfluidic Pumping Utilizing a PDMS Membrane with an Integrated Nonuniform Open-Porous Foam. IEEE Sens. J. 2015, 15, 5109–5114. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ficai, D.; Gheorghe, M.; Dolete, G.; Mihailescu, B.; Svasta, P.; Ficai, A.; Constantinescu, G.; Andronescu, E. Microelectromechanical Systems Based on Magnetic Polymer Films. Micromachines 2022, 13, 351. https://doi.org/10.3390/mi13030351
Ficai D, Gheorghe M, Dolete G, Mihailescu B, Svasta P, Ficai A, Constantinescu G, Andronescu E. Microelectromechanical Systems Based on Magnetic Polymer Films. Micromachines. 2022; 13(3):351. https://doi.org/10.3390/mi13030351
Chicago/Turabian StyleFicai, Denisa, Marin Gheorghe, Georgiana Dolete, Bogdan Mihailescu, Paul Svasta, Anton Ficai, Gabriel Constantinescu, and Ecaterina Andronescu. 2022. "Microelectromechanical Systems Based on Magnetic Polymer Films" Micromachines 13, no. 3: 351. https://doi.org/10.3390/mi13030351
APA StyleFicai, D., Gheorghe, M., Dolete, G., Mihailescu, B., Svasta, P., Ficai, A., Constantinescu, G., & Andronescu, E. (2022). Microelectromechanical Systems Based on Magnetic Polymer Films. Micromachines, 13(3), 351. https://doi.org/10.3390/mi13030351









