Fabrication of Multiple Parallel Microchannels in a Single Microgroove via the Heating Assisted MIMIC Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Masters and PDMS Molds
2.3. HAMIMIC Method
2.4. Characterization Measurements
3. Results and Discussion
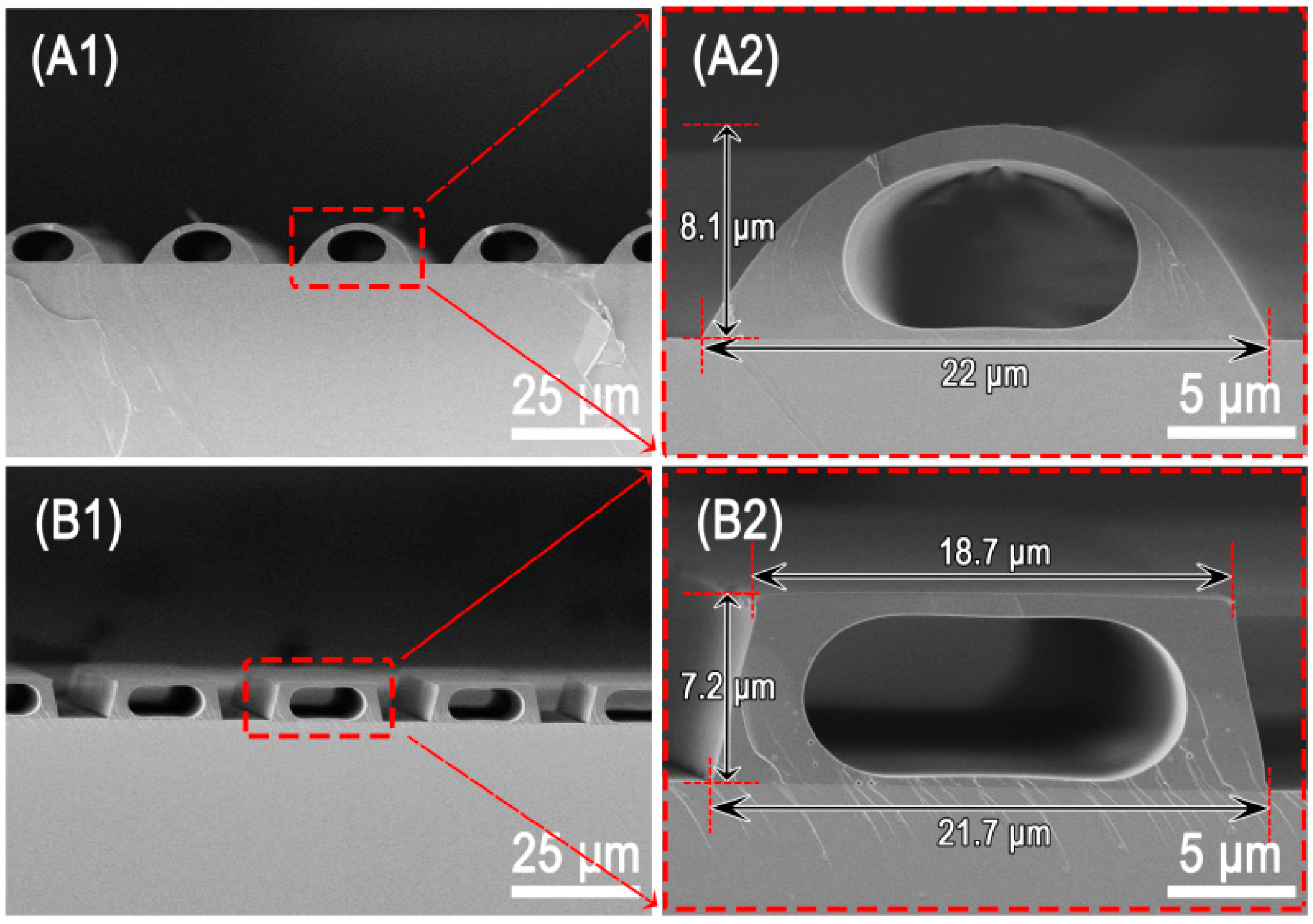
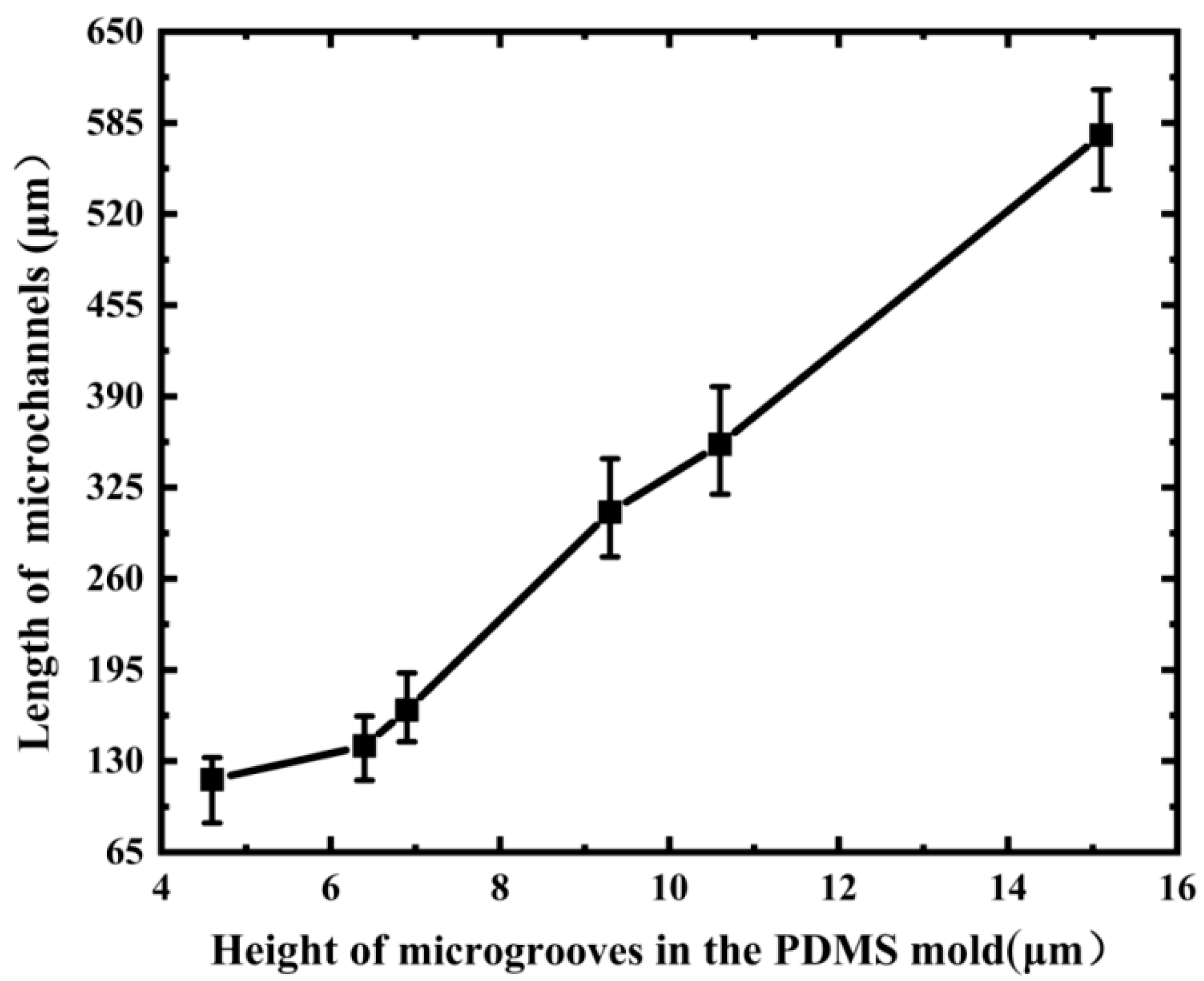
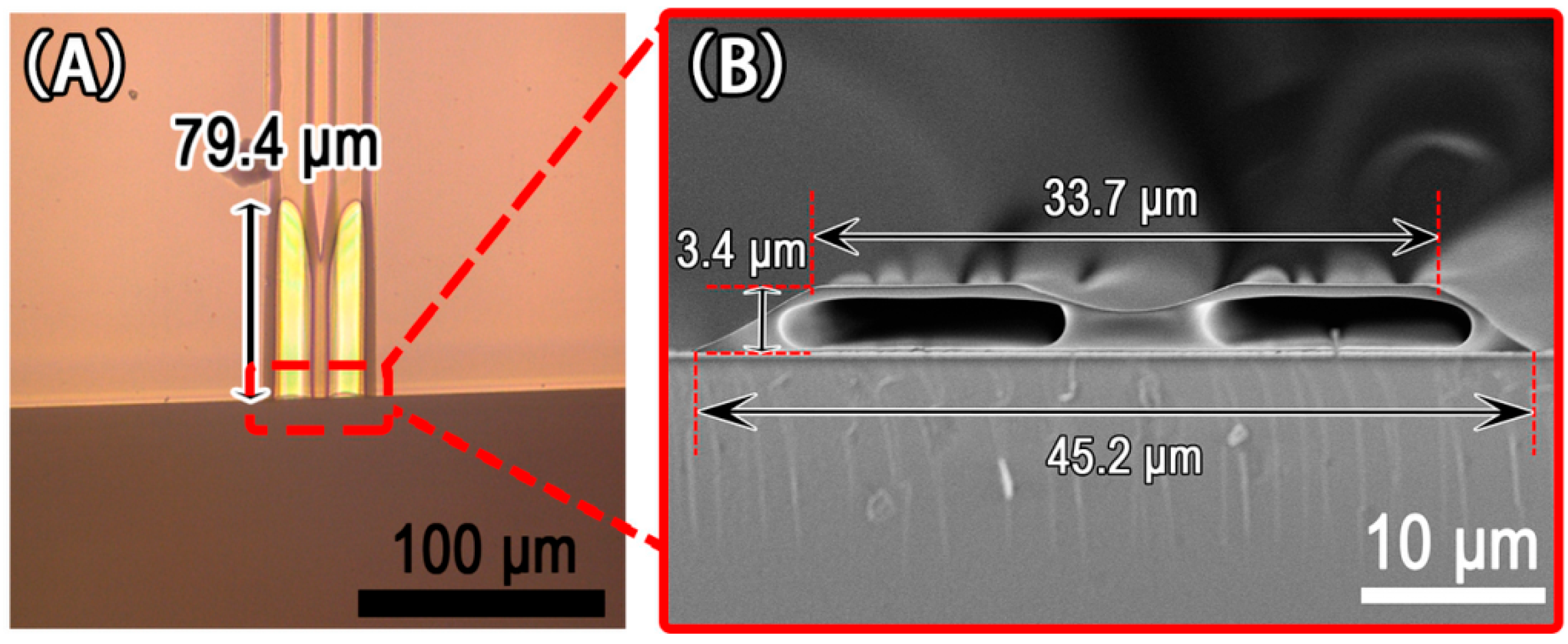
3.1. Single Microchannel
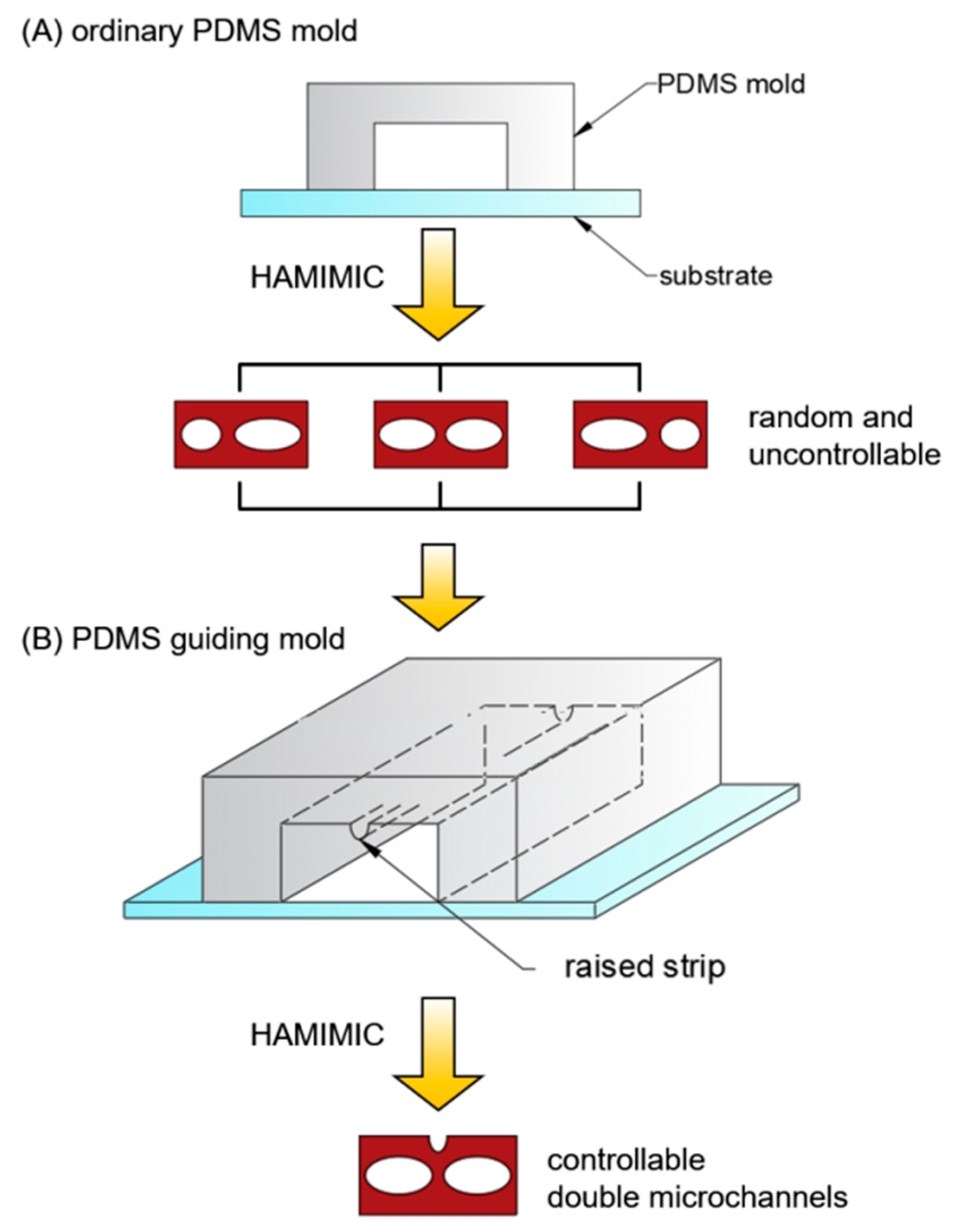
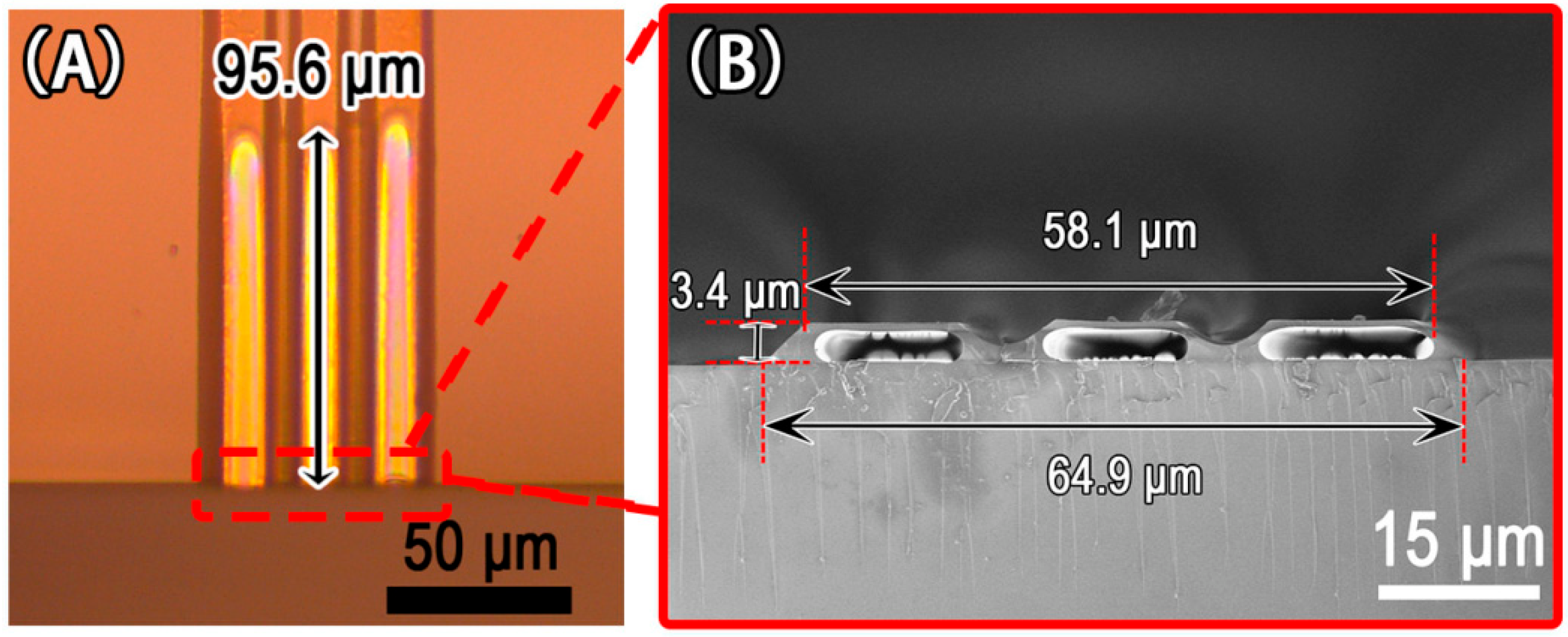
3.2. Double Microchannels
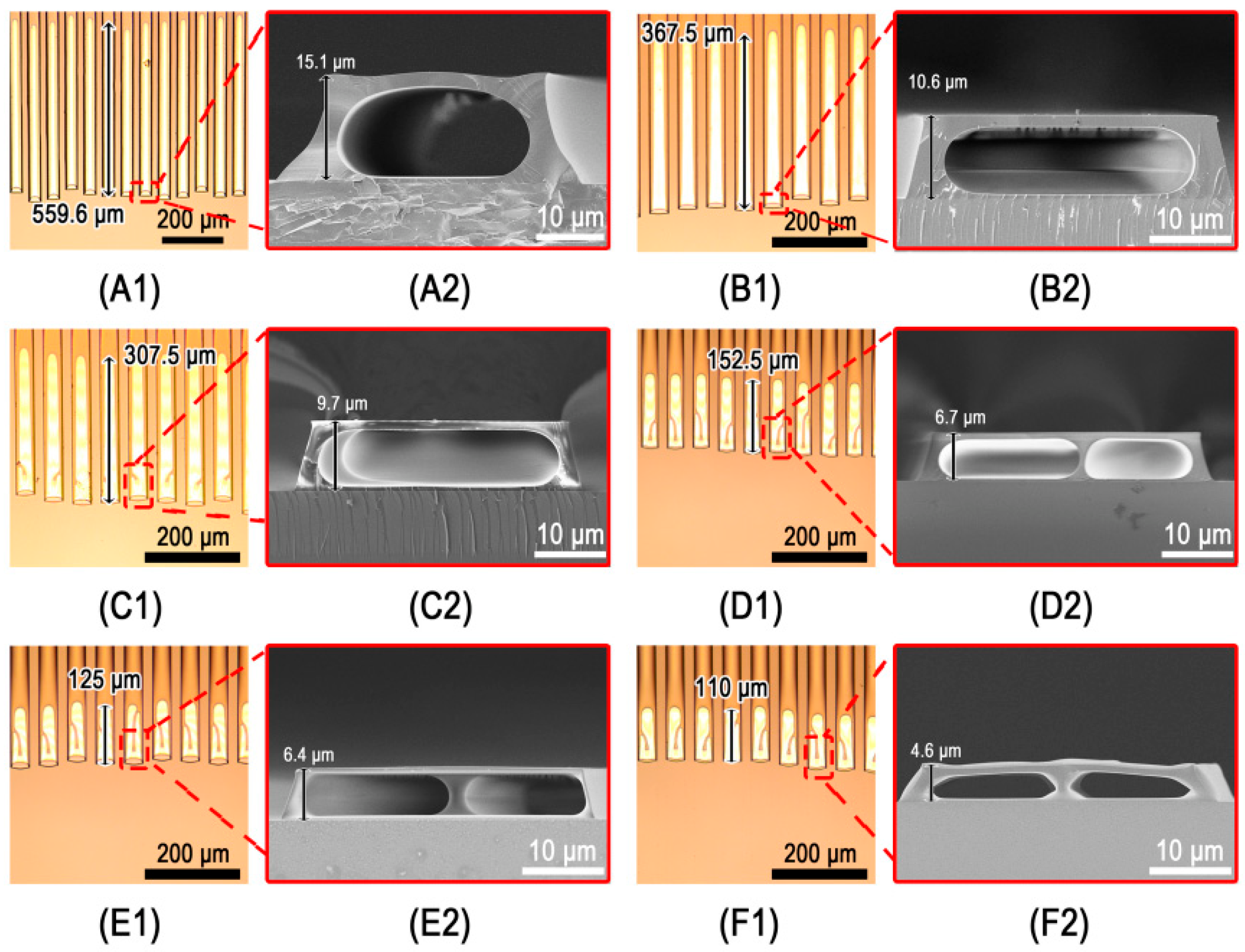
3.3. Mulitiple Parallel Microchannels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, K.S.; Baptista, M.; Moon, S.; Woodfield, T.B.; Rnjak-Kovacina, J. Microchannels in development, survival, and vascularisation of tissue analogues for regenerative medicine. Trends Biotechnol. 2019, 37, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Zheng, W.; Guan, L.; Ai, Y.; Liang, Q. Engineering of hydrogel materials with perfusable microchannels for building vascularized tissues. Small 2020, 16, 1902838. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.S.; Lin, R.L.; Montesano, G.; Durmus, N.G.; Lee, G.; Yoo, S.-S.; Kayaalp, E.; Hæggström, E.; Khademhosseini, A.; Demirci, U. Engineered 3D tissue models for cell-laden microfluidic channels. Anal. Bioanal. Chem. 2009, 395, 185–193. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab A Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [Green Version]
- Choi, N.W.; Cabodi, M.; Held, B.; Gleghorn, J.P.; Bonassar, L.J.; Stroock, A.D. Microfluidic scaffolds for tissue engineering. Nat. Mater. 2007, 6, 908–915. [Google Scholar] [CrossRef]
- Golden, A.P.; Tien, J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab A Chip 2007, 7, 720–725. [Google Scholar] [CrossRef]
- Jeong, J.H.; Chan, V.; Cha, C.; Zorlutuna, P.; Dyck, C.; Hsia, K.J.; Bashir, R.; Kong, H. “Living” microvascular stamp for patterning of functional neovessels; orchestrated control of matrix property and geometry. Adv. Mater. 2012, 24, 58–63. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wray, L.S.; Golinski, J.M.; Kaplan, D.L. Arrayed hollow channels in silk-based scaffolds provide functional outcomes for engineering critically sized tissue constructs. Adv. Funct. Mater. 2014, 24, 2188–2196. [Google Scholar] [CrossRef]
- Varoni, E.M.; Altomare, L.; Cochis, A.; GhalayaniEsfahani, A.; Cigada, A.; Rimondini, L.; De Nardo, L. Hierarchic micro-patterned porous scaffolds via electrochemical replica-deposition enhance neo-vascularization. Biomed. Mater. 2016, 11, 025018. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.; Deng, C.; Li, G.; Chang, J.; Zhang, Z.; Jiang, X.; Wu, C. 3D printing of Lotus root-like biomimetic materials for cell delivery and tissue regeneration. Adv. Sci. 2017, 4, 1700401. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: Beyond creating static networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Chen, X.; Huang, Q.; Du, C.; Ruan, S.; Wei, H. Enhancing the pressure sensitivity of a Fabry–Perot interferometer using a simplified hollow-core photonic crystal fiber with a microchannel. Appl. Phys. B 2015, 120, 461–467. [Google Scholar] [CrossRef]
- Zhou, K.; Yan, Z.; Zhang, L.; Bennion, I. Refractometer based on fiber Bragg grating Fabry-Pérot cavity embedded with a narrow microchannel. Opt. Express 2011, 19, 11769–11779. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhou, Y.; Wang, D.; He, F.; Rotello, V.M.; Carter, K.R.; Watkins, J.J.; Nugen, S.R. UV-nanoimprint lithography as a tool to develop flexible microfluidic devices for electrochemical detection. Lab A Chip 2015, 15, 3086–3094. [Google Scholar] [CrossRef]
- Knowlton, S.; Yu, C.H.; Ersoy, F.; Emadi, S.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic chips with patterned, cell-laden hydrogel constructs. Biofabrication 2016, 8, 025019. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Shi, Y.; Liu, X.; Han, Z.; Zhao, Z.; Chen, Y.; Xie, W.; Li, X. Enhanced fluorescence detection of proteins using ZnO nanowires integrated inside microfluidic chips. Biosens. Bioelectron. 2018, 99, 368–374. [Google Scholar] [CrossRef]
- Mulloni, V.; Capuano, A.; Adami, A.; Quaranta, A.; Lorenzelli, L. A dry film technology for the manufacturing of 3-D multi-layered microstructures and buried channels for lab-on-chip. Microsyst. Technol. 2019, 25, 3219–3233. [Google Scholar] [CrossRef]
- Hamilton, E.S.; Ganjalizadeh, V.; Wright, J.G.; Schmidt, H.; Hawkins, A.R. 3D Hydrodynamic focusing in microscale optofluidic channels formed with a single sacrificial layer. Micromachines 2020, 11, 349. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Kang, S.-W.; Kwon, T.-S.; Banerjee, D. In situ characterization of enhanced thermal performance by periodic nanostructures on the surface of a microchannel. Int. J. Heat Mass Transf. 2018, 124, 414–422. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Gao, Q. A novel method for rapid fabrication of PMMA microfluidic chip by laser cutting and sealing integration. Surf. Rev. Lett. 2019, 26, 1950042. [Google Scholar] [CrossRef]
- Piekiel, N.W.; Morris, C.J.; Currano, L.J.; Lunking, D.M.; Isaacson, B.; Churaman, W.A. Enhancement of on-chip combustion via nanoporous silicon microchannels. Combust. Flame 2014, 161, 1417–1424. [Google Scholar] [CrossRef]
- Fekete, Z.; Pongrácz, A.; Fürjes, P.; Battistig, G. Improved process flow for buried channel fabrication in silicon. Microsyst. Technol. 2012, 18, 353–358. [Google Scholar] [CrossRef]
- Chen, H.; Yu, W.; Cargill, S.; Patel, M.; Bailey, C.; Tonry, C.; Desmulliez, M. Self-encapsulated hollow microstructures formed by electric field-assisted capillarity. Microfluid. Nanofluidics 2012, 13, 75–82. [Google Scholar] [CrossRef]
- Parekh, D.P.; Ladd, C.; Panich, L.; Moussa, K.; Dickey, M.D. 3D printing of liquid metals as fugitive inks for fabrication of 3D microfluidic channels. Lab A Chip 2016, 16, 1812–1820. [Google Scholar] [CrossRef]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft lithography for microfluidics: A review. Biochip J. 2008, 2, 1–11. [Google Scholar]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Kim, E.; Xia, Y.; Whitesides, G.M. Polymer microstructures formed by moulding in capillaries. Nature 1995, 376, 581–584. [Google Scholar] [CrossRef]
- Mortensen, N.A.; Okkels, F.; Bruus, H. Reexamination of Hagen-Poiseuille flow: Shape dependence of the hydraulic resistance in microchannels. Phys. Rev. E 2005, 71, 057301. [Google Scholar] [CrossRef] [Green Version]
- Lui, M.; Gardiner, E.E.; Arthur, J.F.; Pinar, I.; Lee, W.M.; Ryan, K.; Carberry, J.; Andrews, R.K. Novel stenotic microchannels to study thrombus formation in shear gradients: Influence of shear forces and human platelet-related factors. Int. J. Mol. Sci. 2019, 20, 2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Piao, Y.; Seo, T.S. Fabrication of a circular PDMS microchannel for constructing a three-dimensional endothelial cell layer. Bioprocess Biosyst. Eng. 2013, 36, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Pitingolo, G.; Vecchione, R.; Falanga, A.P.; Guarnieri, D.; Netti, P.A. Fabrication of a modular hybrid chip to mimic endothelial-lined microvessels in flow conditions. J. Micromechanics Microeng. 2017, 27, 035014. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Park, W.-T. Fabrication method of multi-depth circular microchannels for investigating arterial thrombosis-on-a-chip. Sens. Actuators B Chem. 2020, 321, 128590. [Google Scholar] [CrossRef]
- Li, W.; Xing, W.; Zhao, F.; Zhang, L.; Huang, Y.; Li, J.; Zhu, L.; Xu, Z.; Zhang, D. Fabrication of hollow polymer microchannels using the MIMIC technique with subsequent heat treatment. Int. J. Precis. Eng. Manuf. 2021, 22, 1453–1460. [Google Scholar] [CrossRef]
- Kim, E.; Xia, Y.; Whitesides, G.M. Micromolding in capillaries: Applications in materials science. J. Am. Chem. Soc. 1996, 118, 5722–5731. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Xing, W.; Li, W.; Liu, S.; Dong, Y.; Zhang, L.; Zhao, F.; Wang, J.; Xu, Z. Fabrication of Multiple Parallel Microchannels in a Single Microgroove via the Heating Assisted MIMIC Technique. Micromachines 2022, 13, 364. https://doi.org/10.3390/mi13030364
Zhang D, Xing W, Li W, Liu S, Dong Y, Zhang L, Zhao F, Wang J, Xu Z. Fabrication of Multiple Parallel Microchannels in a Single Microgroove via the Heating Assisted MIMIC Technique. Micromachines. 2022; 13(3):364. https://doi.org/10.3390/mi13030364
Chicago/Turabian StyleZhang, Dengying, Wenqiang Xing, Weiren Li, Shengming Liu, Yanli Dong, Lichun Zhang, Fengzhou Zhao, Jun Wang, and Zheng Xu. 2022. "Fabrication of Multiple Parallel Microchannels in a Single Microgroove via the Heating Assisted MIMIC Technique" Micromachines 13, no. 3: 364. https://doi.org/10.3390/mi13030364
APA StyleZhang, D., Xing, W., Li, W., Liu, S., Dong, Y., Zhang, L., Zhao, F., Wang, J., & Xu, Z. (2022). Fabrication of Multiple Parallel Microchannels in a Single Microgroove via the Heating Assisted MIMIC Technique. Micromachines, 13(3), 364. https://doi.org/10.3390/mi13030364





