A Microfluidic Eye Facsimile System to Examine the Migration of Stem-like Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Fluorescent Labeling and Immunocytochemical (ICC) Staining
2.3. Preparation of Cells
2.4. External Stimuli
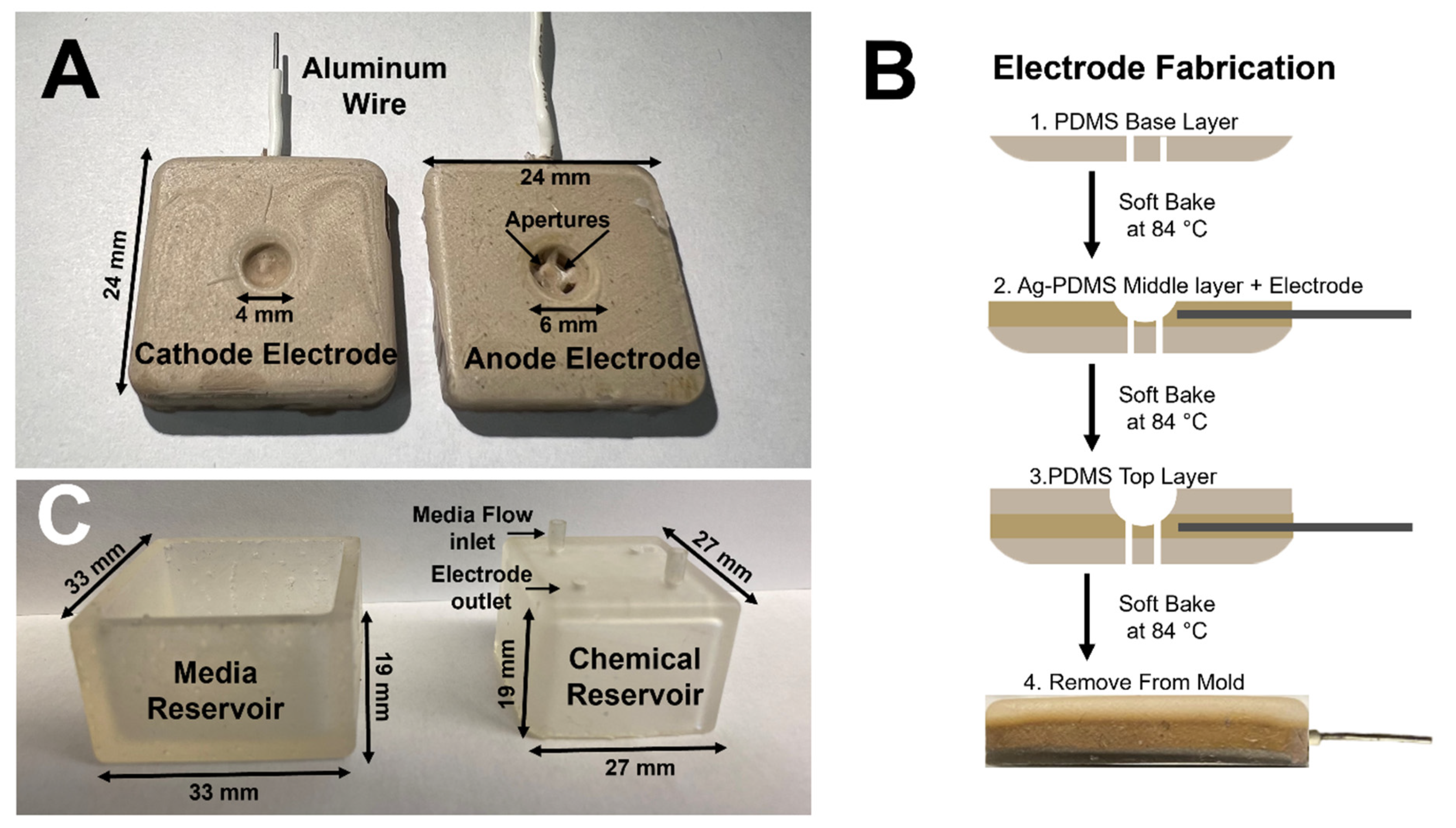
2.5. Eye Facsimiles
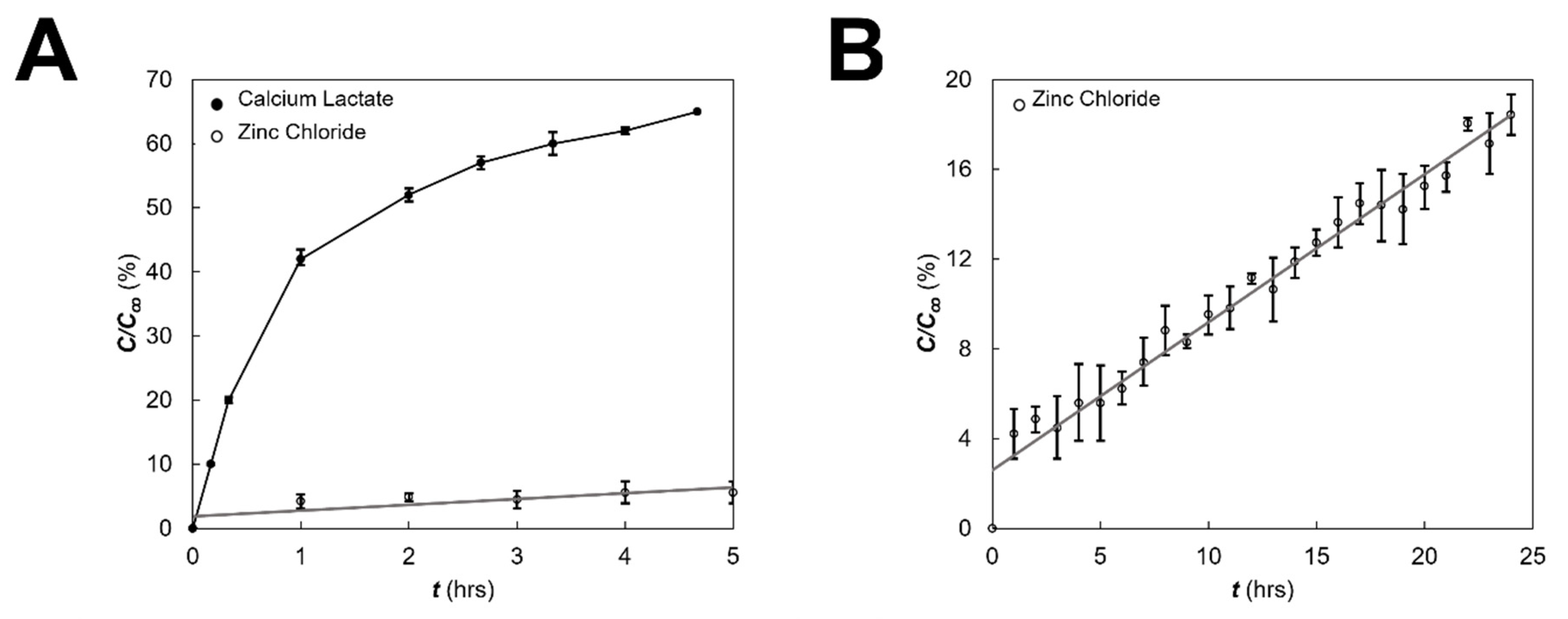
2.6. Molecular Transport across Eye Facsimiles
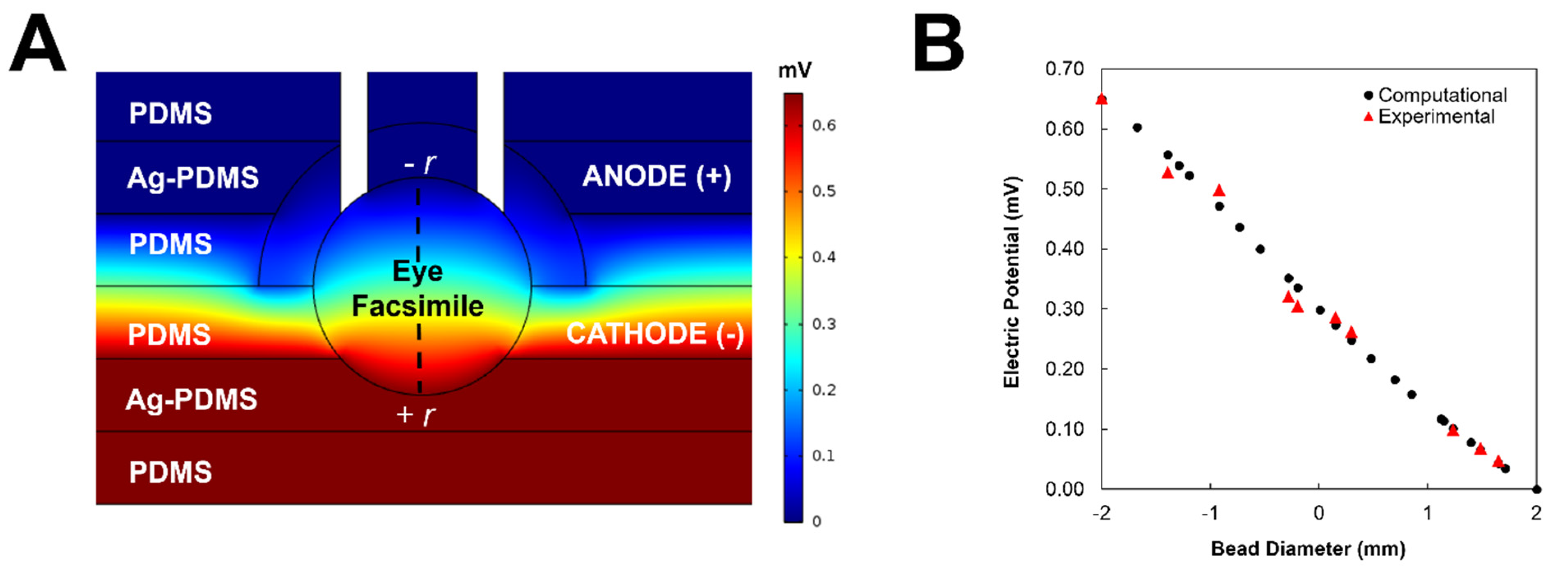
2.7. Finite Element Modeling
2.8. Fabrication of System Components
2.9. Cryosection and Imaging
2.10. Data Analyses and Statistics
3. Results
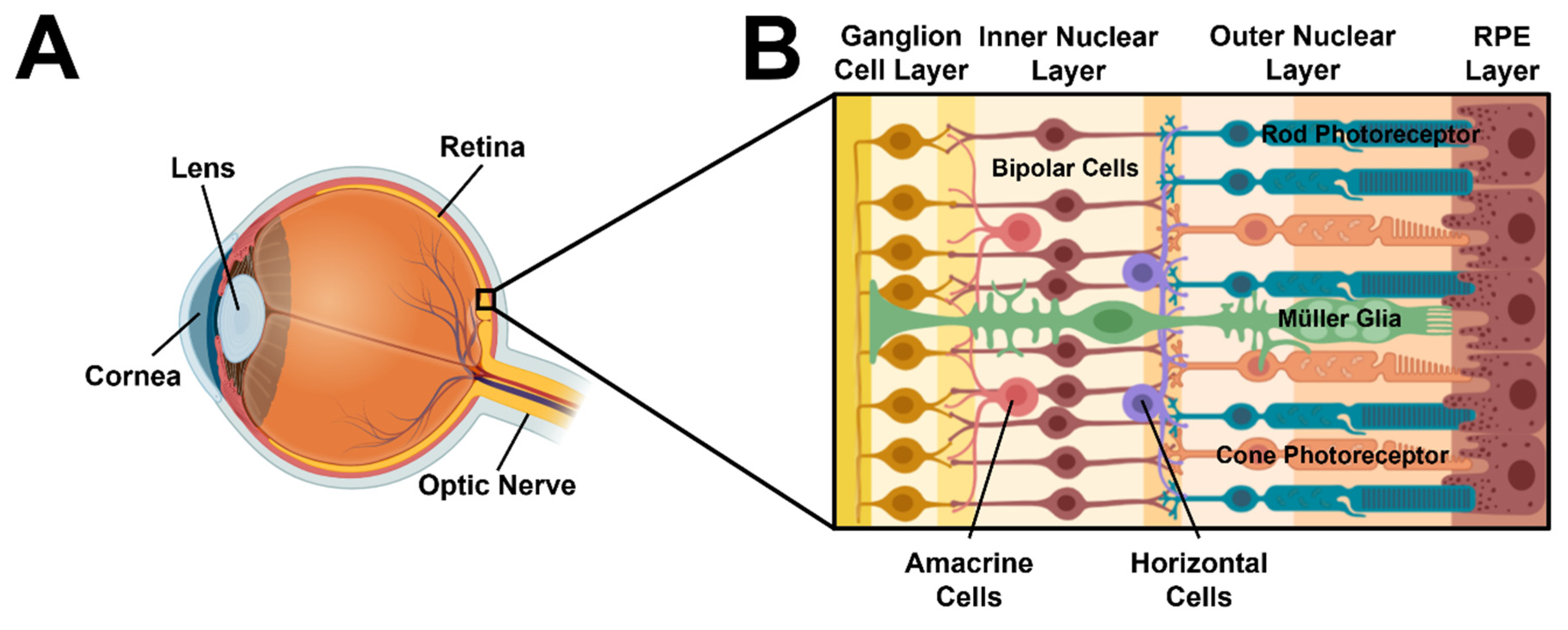
3.1. μ-Eye Design and Operation
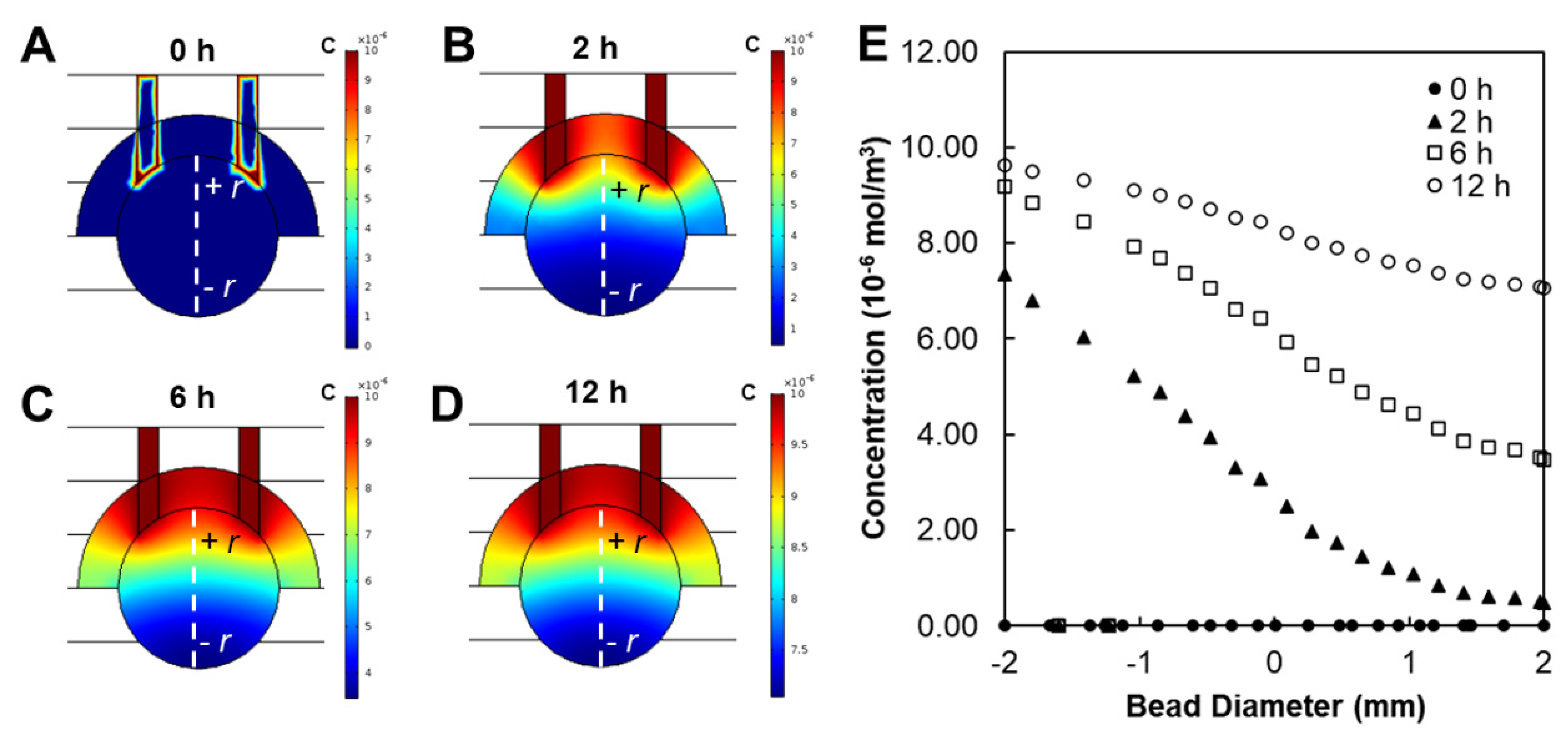
3.2. Modeling and Validation of External Fields
3.3. Migration of SCs within Eye Facsimiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Morizane, Y.; Morimoto, N.; Fujiwara, A.; Kawasaki, R.; Yamashita, H.; Ogura, Y.; Shiraga, F. Incidence and causes of visual impairment in Japan: The first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn. J. Ophthalmol. 2019, 63, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Friedman, D.S.; Bradley, C.; Massof, R. Estimates of Incidence and Prevalence of Visual Impairment, Low Vision, and Blindness in the United States. JAMA Ophthalmol. 2018, 136, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pezzullo, L.; Streatfeild, J.; Simkiss, P.; Shickle, D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv. Res. 2018, 18, 63. [Google Scholar] [CrossRef] [Green Version]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [Green Version]
- Grossniklaus, H.E.; Geisert, E.E.; Nickerson, J.M. Introduction to the Retina. Prog. Mol. Biol. Transl. Sci. 2015, 134, 383–396. [Google Scholar] [CrossRef]
- Care, R.A.; Anastassov, I.A.; Kastner, D.B.; Kuo, Y.M.; Della Santina, L.; Dunn, F.A. Mature Retina Compensates Functionally for Partial Loss of Rod Photoreceptors. Cell Rep. 2020, 31, 107730. [Google Scholar] [CrossRef]
- Shen, N.; Wang, B.; Soto, F.; Kerschensteiner, D. Homeostatic Plasticity Shapes the Retinal Response to Photoreceptor Degeneration. Curr. Biol. 2020, 30, 1916–1926.e1913. [Google Scholar] [CrossRef]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Tucker, J.; Marc, R.E. Retinal Remodeling and Metabolic Alterations in Human AMD. Front. Cell Neurosci. 2016, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, A.L.; Rojas-Roldan, L.; Coffin, J. Vision Loss in Older Adults. Am. Fam. Physician 2016, 94, 219–226. [Google Scholar]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [Green Version]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal stem cell transplantation: Balancing safety and potential. Prog. Retin. Eye Res. 2020, 75, 100779. [Google Scholar] [CrossRef]
- Gagliardi, G.; Ben M’Barek, K.; Goureau, O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: A pluripotent stem cell-based approach. Prog. Retin. Eye Res. 2019, 71, 1–25. [Google Scholar] [CrossRef]
- Jin, Z.B.; Gao, M.L.; Deng, W.L.; Wu, K.C.; Sugita, S.; Mandai, M.; Takahashi, M. Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 2019, 69, 38–56. [Google Scholar] [CrossRef]
- West, E.L.; Ribeiro, J.; Ali, R.R. Development of Stem Cell Therapies for Retinal Degeneration. Cold Spring Harb. Perspect. Biol. 2020, 12, a035683. [Google Scholar] [CrossRef]
- DePamphilis, L.M.S.; Shinbrot, T.; Vazquez, M. Opportunities for Agent Based Modeling of Retinal Stem Cell Transplantation. Neural Regen. Res. 2022, 17, 1978–1980. [Google Scholar] [CrossRef]
- Viringipurampeer, I.A.; Yanai, A.; Nizamudheen, V.S.; Gregory-Evans, C.Y.; Gregory-Evans, K. Photoreceptor precursor cell integration into rodent retina after treatment with novel glycopeptide PKX-001. J. Tissue Eng. Regen. Med. 2021, 15, 556–566. [Google Scholar] [CrossRef]
- Ludwig, A.L.; Gamm, D.M. Outer Retinal Cell Replacement: Putting the Pieces Together. Transl. Vis. Sci. Technol. 2021, 10, 15. [Google Scholar] [CrossRef]
- Rohiwal, S.S.; Ellederova, Z.; Ardan, T.; Klima, J. Advancement in Nanostructure-Based Tissue-Engineered Biomaterials for Retinal Degenerative Diseases. Biomedicines 2021, 9, 1005. [Google Scholar] [CrossRef]
- Warre-Cornish, K.; Barber, A.C.; Sowden, J.C.; Ali, R.R.; Pearson, R.A. Migration, integration and maturation of photoreceptor precursors following transplantation in the mouse retina. Stem Cells Dev. 2014, 23, 941–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesaresi, M.; Bonilla-Pons, S.A.; Sebastian-Perez, R.; Di Vicino, U.; Alcoverro-Bertran, M.; Michael, R.; Cosma, M.P. The Chemokine Receptors Ccr5 and Cxcr6 Enhance Migration of Mesenchymal Stem Cells into the Degenerating Retina. Mol. Ther. 2021, 29, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.L.; Tang, S.B. Differentiation of retinal ganglion cells from induced pluripotent stem cells: A review. Int. J. Ophthalmol. 2019, 12, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Akiba, R.; Matsuyama, T.; Tu, H.Y.; Hashiguchi, T.; Sho, J.; Yamamoto, S.; Takahashi, M.; Mandai, M. Quantitative and Qualitative Evaluation of Photoreceptor Synapses in Developing, Degenerating and Regenerating Retinas. Front. Cell Neurosci. 2019, 13, 16. [Google Scholar] [CrossRef]
- Santos-Ferreira, T.; Postel, K.; Stutzki, H.; Kurth, T.; Zeck, G.; Ader, M. Daylight vision repair by cell transplantation. Stem Cells 2015, 33, 79–90. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Liu, Y.; Fernandez de Castro, J.; Ezashi, T.; Telugu, B.P.; Roberts, R.M.; Kaplan, H.J.; Dean, D.C. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells 2011, 29, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Santos-Ferreira, T.; Llonch, S.; Borsch, O.; Postel, K.; Haas, J.; Ader, M. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat. Commun. 2016, 7, 13028. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Chang, K.C.; Nahmou, M.; Goldberg, J.L. Induced Pluripotent Stem Cells Promote Retinal Ganglion Cell Survival After Transplant. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1571–1576. [Google Scholar] [CrossRef]
- Lamba, D.A.; McUsic, A.; Hirata, R.K.; Wang, P.R.; Russell, D.; Reh, T.A. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE 2010, 5, e8763. [Google Scholar] [CrossRef]
- Pearson, R.A.; Barber, A.C.; Rizzi, M.; Hippert, C.; Xue, T.; West, E.L.; Duran, Y.; Smith, A.J.; Chuang, J.Z.; Azam, S.A.; et al. Restoration of vision after transplantation of photoreceptors. Nature 2012, 485, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tenerelli, K.; Wu, S.; Xia, X.; Yokota, S.; Sun, C.; Galvao, J.; Venugopalan, P.; Li, C.; Madaan, A.; et al. Cell transplantation of retinal ganglion cells derived from hESCs. Restor. Neurol. Neurosci. 2020, 38, 131–140. [Google Scholar] [CrossRef]
- Rong, L.; Gu, X.; Xie, J.; Zeng, Y.; Li, Q.; Chen, S.; Zou, T.; Xue, L.; Xu, H.; Yin, Z.Q. Bone Marrow CD133(+) Stem Cells Ameliorate Visual Dysfunction in Streptozotocin-induced Diabetic Mice with Early Diabetic Retinopathy. Cell Transplant. 2018, 27, 916–936. [Google Scholar] [CrossRef] [Green Version]
- Kruczek, K.; Gonzalez-Cordero, A.; Goh, D.; Naeem, A.; Jonikas, M.; Blackford, S.J.I.; Kloc, M.; Duran, Y.; Georgiadis, A.; Sampson, R.D.; et al. Differentiation and Transplantation of Embryonic Stem Cell-Derived Cone Photoreceptors into a Mouse Model of End-Stage Retinal Degeneration. Stem Cell Rep. 2017, 8, 1659–1674. [Google Scholar] [CrossRef] [Green Version]
- Reh, T.A. Photoreceptor Transplantation in Late Stage Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFg1–ORSFg7. [Google Scholar] [CrossRef] [Green Version]
- Tsai, E.L.S.; Ortin-Martinez, A.; Gurdita, A.; Comanita, L.; Yan, N.; Smiley, S.; Delplace, V.; Shoichet, M.S.; Nickerson, P.E.B.; Wallace, V.A. Modeling of Photoreceptor Donor-Host Interaction Following Transplantation Reveals a Role for Crx, Muller Glia, and Rho/ROCK Signaling in Neurite Outgrowth. Stem Cells 2019, 37, 529–541. [Google Scholar] [CrossRef]
- Li, Y.; Hao, H.; Swerdel, M.R.; Cho, H.Y.; Lee, K.B.; Hart, R.P.; Lyu, Y.L.; Cai, L. Top2b is involved in the formation of outer segment and synapse during late-stage photoreceptor differentiation by controlling key genes of photoreceptor transcriptional regulatory network. J. Neurosci. Res. 2017, 95, 1951–1964. [Google Scholar] [CrossRef] [Green Version]
- Javed, A.; Cayouette, M. Temporal Progression of Retinal Progenitor Cell Identity: Implications in Cell Replacement Therapies. Front. Neural Circuits 2017, 11, 105. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, M. Electro-chemotactic stimuli for cell replacement therapy in neurosensory retina. Neural Regen. Res. 2020, 15, 450–452. [Google Scholar] [CrossRef]
- Mishra, S.; Pena, J.S.; Redenti, S.; Vazquez, M. A novel electro-chemotactic approach to impact the directional migration of transplantable retinal progenitor cells. Exp. Eye Res. 2019, 185, 107688. [Google Scholar] [CrossRef]
- Mishra, S.; Thakur, A.; Redenti, S.; Vazquez, M. A model microfluidics-based system for the human and mouse retina. Biomed. Microdevices 2015, 17, 107. [Google Scholar] [CrossRef] [Green Version]
- Rountree, C.M.; Raghunathan, A.; Troy, J.B.; Saggere, L. Prototype chemical synapse chip for spatially patterned neurotransmitter stimulation of the retina ex vivo. Microsyst. Nanoeng. 2017, 3, 17052. [Google Scholar] [CrossRef]
- Dodson, K.H.; Echevarria, F.D.; Li, D.; Sappington, R.M.; Edd, J.F. Retina-on-a-chip: A microfluidic platform for point access signaling studies. Biomed. Microdevices 2015, 17, 114. [Google Scholar] [CrossRef] [Green Version]
- Seigel, G.M. Review: R28 retinal precursor cells: The first 20 years. Mol. Vis. 2014, 20, 301–306. [Google Scholar]
- Unachukwu, U.J.; Warren, A.; Li, Z.; Mishra, S.; Zhou, J.; Sauane, M.; Lim, H.; Vazquez, M.; Redenti, S. Predicted molecular signaling guiding photoreceptor cell migration following transplantation into damaged retina. Sci. Rep. 2016, 6, 22392. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, S.; Unachukwu, U.; Thakur, A.; Majeska, R.; Redenti, S.; Vazquez, M. In vitro formation of neuroclusters in microfluidic devices and cell migration as a function of stromal-derived growth factor 1 gradients. Cell Adhes. Migr. 2017, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Caine, M.; Bian, S.; Tang, Y.; Garcia, P.; Henman, A.; Dreher, M.; Daly, D.; Carlisle, R.; Stride, E.; Willis, S.L.; et al. In situ evaluation of spatiotemporal distribution of doxorubicin from Drug-eluting Beads in a tissue mimicking phantom. Eur. J. Pharm.Sci. Off. J. Eur. Fed. Pharm. Sci. 2021, 160, 105772. [Google Scholar] [CrossRef]
- Hunt, N.C.; Hallam, D.; Chichagova, V.; Steel, D.H.; Lako, M. The Application of Biomaterials to Tissue Engineering Neural Retina and Retinal Pigment Epithelium. Adv. Healthc. Mater. 2018, 7, e1800226. [Google Scholar] [CrossRef]
- Schulz, A.; Rickmann, A.; Wahl, S.; Germann, A.; Stanzel, B.V.; Januschowski, K.; Szurman, P. Alginate- and Hyaluronic Acid-Based Hydrogels as Vitreous Substitutes: An In Vitro Evaluation. Transl. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Vazquez, M. A Gal-MmicroS Device to Evaluate Cell Migratory Response to Combined Galvano-Chemotactic Fields. Biosensors 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albro, M.B.; Rajan, V.; Li, R.; Hung, C.T.; Ateshian, G.A. Characterization of the Concentration-Dependence of Solute Diffusivity and Partitioning in a Model Dextran-Agarose Transport System. Cell. Mol. Bioeng. 2009, 2, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldkamp, C.T.; Peterson, F.C.; Pelzek, A.J.; Volkman, B.F. The monomer-dimer equilibrium of stromal cell-derived factor-1 (CXCL 12) is altered by pH, phosphate, sulfate, and heparin. Protein Sci. 2005, 14, 1071–1081. [Google Scholar] [CrossRef] [Green Version]
- Jadach, B.; Swietlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-known Polymer. J. Pharm. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Aslani, P.; Kennedy, R.A. Studies on diffusion in alginate gels. I. Effect of cross-linking with calcium or zinc ions on diffusion of acetaminophen. J. Control. Release 1996, 42, 75–82. [Google Scholar] [CrossRef]
- Auriemma, G.; Cerciello, A.; Aquino, R.P.; Gaudio, P.D.; Fusco, B.M.; Russo, P. Pectin and Zinc Alginate: The Right Inner/Outer Polymer Combination for Core-Shell Drug Delivery Systems. Pharmaceutics 2020, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Esch, M.; Sukhorukov, V.L.; Kurschner, M.; Zimmermann, U. Dielectric properties of alginate beads and bound water relaxation studied by electrorotation. Biopolymers 1999, 50, 227–237. [Google Scholar] [CrossRef]
- Kong, Q.; Able, R.A., Jr.; Dudu, V.; Vazquez, M. A microfluidic device to establish concentration gradients using reagent density differences. J. Biomech. Eng. 2010, 132, 121012. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Diaz, E.; Cano-Jorge, M.; Zamarron-Hernandez, D.; Cabriales, L.; Paez-Larios, F.; Cruz-Ramirez, A.; Vazquez-Victorio, G.; Fiordelisio, T.; Hautefeuille, M. Micro-Macro: Selective Integration of Microfeatures Inside Low-Cost Macromolds for PDMS Microfluidics Fabrication. Micromachines 2019, 10, 576. [Google Scholar] [CrossRef] [Green Version]
- Truskett, V.N.; Watts, M.P. Trends in imprint lithography for biological applications. Trends Biotechnol. 2006, 24, 312–317. [Google Scholar] [CrossRef]
- Wylomanska, A.; Iskander, D.R.; Burnecki, K. Omnibus test for normality based on the Edgeworth expansion. PLoS ONE 2020, 15, e0233901. [Google Scholar] [CrossRef] [PubMed]
- Pena, C.D.; Zhang, S.; Markey, M.; Venkatesh, T.; Vazquez, M. Collective behaviors of Drosophila-derived retinal progenitors in controlled microenvironments. PLoS ONE 2019, 14, e0226250. [Google Scholar] [CrossRef] [Green Version]
- Haderspeck, J.C.; Chuchuy, J.; Kustermann, S.; Liebau, S.; Loskill, P. Organ-on-a-chip technologies that can transform ophthalmic drug discovery and disease modeling. Expert Opin. Drug Discov. 2019, 14, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Goncalves, A.; Kustermann, S.; Antonetti, D.A.; Jayagopal, A. Organ-On-A-Chip Technologies for Advanced Blood-Retinal Barrier Models. J. Ocul. Pharmacol. Ther. 2020, 36, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeste, J.; Garcia-Ramirez, M.; Illa, X.; Guimera, A.; Hernandez, C.; Simo, R.; Villa, R. A compartmentalized microfluidic chip with crisscross microgrooves and electrophysiological electrodes for modeling the blood-retinal barrier. Lab Chip 2017, 18, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, P.J.; Liu, Z.; Zhang, K.; Han, X.; Saito, Y.; Xia, X.; Yokoi, K.; Shen, H.; Qin, L. Retinal synaptic regeneration via microfluidic guiding channels. Sci. Rep. 2015, 5, 13591. [Google Scholar] [CrossRef]
- Arik, Y.B.; Buijsman, W.; Loessberg-Zahl, J.; Cuartas-Velez, C.; Veenstra, C.; Logtenberg, S.; Grobbink, A.M.; Bergveld, P.; Gagliardi, G.; den Hollander, A.I.; et al. Microfluidic organ-on-a-chip model of the outer blood-retinal barrier with clinically relevant read-outs for tissue permeability and vascular structure. Lab Chip 2021, 21, 272–283. [Google Scholar] [CrossRef]
- Paek, J.; Park, S.E.; Lu, Q.; Park, K.T.; Cho, M.; Oh, J.M.; Kwon, K.W.; Yi, Y.S.; Song, J.W.; Edelstein, H.I.; et al. Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues. ACS Nano 2019, 13, 7627–7643. [Google Scholar] [CrossRef]
- Achberger, K.; Cipriano, M.; Duchs, M.J.; Schon, C.; Michelfelder, S.; Stierstorfer, B.; Lamla, T.; Kauschke, S.G.; Chuchuy, J.; Roosz, J.; et al. Human stem cell-based retina on chip as new translational model for validation of AAV retinal gene therapy vectors. Stem Cell Rep. 2021, 16, 2242–2256. [Google Scholar] [CrossRef]
- Achberger, K.; Probst, C.; Haderspeck, J.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife 2019, 8, e46188. [Google Scholar] [CrossRef]
- Ragelle, H.; Dernick, K.; Khemais, S.; Keppler, C.; Cousin, L.; Farouz, Y.; Louche, C.; Fauser, S.; Kustermann, S.; Tibbitt, M.W.; et al. Human Retinal Microvasculature-on-a-Chip for Drug Discovery. Adv. Healthc. Mater. 2020, 9, e2001531. [Google Scholar] [CrossRef]
- Chung, M.; Lee, S.; Lee, B.J.; Son, K.; Jeon, N.L.; Kim, J.H. Wet-AMD on a Chip: Modeling Outer Blood-Retinal Barrier In Vitro. Adv. Healthc. Mater. 2018, 7, 1700028. [Google Scholar] [CrossRef]
- Rettinger, C.L.; Wang, H.C. Quantitative Assessment of Retina Explant Viability in a Porcine Ex Vivo Neuroretina Model. J. Ocul. Pharmacol. Ther. 2018, 34, 521–530. [Google Scholar] [CrossRef]
- Mut, S.R.; Vazquez, M. Commentary: Organ Cultures for Retinal Diseases. Front. Neurosci. 2021, 15, 714094. [Google Scholar] [CrossRef]
- Finkel, Z.; Esteban, F.; Rodriguez, B.; Fu, T.; Ai, X.; Cai, L. Diversity of Adult Neural Stem and Progenitor Cells in Physiology and Disease. Cells 2021, 10, 2045. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- German, O.L.; Vallese-Maurizi, H.; Soto, T.B.; Rotstein, N.P.; Politi, L.E. Retina stem cells, hopes and obstacles. World J. Stem Cells 2021, 13, 1446–1479. [Google Scholar] [CrossRef]
- Ferro Desideri, L.; Traverso, C.E.; Nicolo, M. The emerging role of the angiopoietin-Tie pathway as therapeutic target for treating retinal diseases. Expert Opin. Ther. Targets 2022, 26, 145–154. [Google Scholar] [CrossRef]
- Kickova, E.; Sadeghi, A.; Puranen, J.; Tavakoli, S.; Sen, M.; Ranta, V.P.; Arango-Gonzalez, B.; Bolz, S.; Ueffing, M.; Salmaso, S.; et al. Pharmacokinetics of Pullulan-Dexamethasone Conjugates in Retinal Drug Delivery. Pharmaceutics 2021, 14, 12. [Google Scholar] [CrossRef]
- Liu, J.; Tong, K.; Lin, Y.; Lee, V.W.H.; So, K.F.; Shih, K.C.; Lai, J.S.M.; Chiu, K. Effectiveness of Microcurrent Stimulation in Preserving Retinal Function of Blind Leading Retinal Degeneration and Optic Neuropathy: A Systematic Review. Neuromodulation J. Int. Neuromodulation Soc. 2021, 24, 992–1002. [Google Scholar] [CrossRef]
- Zhao, M.; Chalmers, L.; Cao, L.; Vieira, A.C.; Mannis, M.; Reid, B. Electrical signaling in control of ocular cell behaviors. Prog. Retin. Eye Res. 2012, 31, 65–88. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.I.; Poo, M.M. Electrical activity and development of neural circuits. Nat. Neurosci. 2001, 4, 1207–1214. [Google Scholar] [CrossRef]
- Gafarov, F.M. Neural electrical activity and neural network growth. Neural Netw. 2018, 101, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M. Microfluidic and Microscale Assays to Examine Regenerative Strategies in the Neuro Retina. Micromachines 2020, 11, 1089. [Google Scholar] [CrossRef] [PubMed]
- Jamroz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications-Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Zhao, B.; Liu, X.; Li, X.; Zeng, C.; Shi, H.; Xu, X.; Lin, T.; Dai, L.; Liu, Y. Aligned Nanofibers from Polypyrrole/Graphene as Electrodes for Regeneration of Optic Nerve via Electrical Stimulation. ACS Appl. Mater. Interfaces 2016, 8, 6834–6840. [Google Scholar] [CrossRef]
- Spearman, B.S.; Agrawal, N.K.; Rubiano, A.; Simmons, C.S.; Mobini, S.; Schmidt, C.E. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. A 2020, 108, 279–291. [Google Scholar] [CrossRef]
- Acosta, M.L.; Shin, Y.-S.; Ready, S.; Fletcher, E.L.; Christie, D.L.; Kalloniatis, M. Retinal metabolic state of the proline-23-histidine rat model of retinitis pigmentosa. Am. J. Physiol.-Cell Physiol. 2010, 298, C764–C774. [Google Scholar] [CrossRef] [Green Version]
- Allmendinger, A.; Butt, Y.L.; Mueller, C. Intraocular pressure and injection forces during intravitreal injection into enucleated porcine eyes. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. e.V 2021, 166, 87–93. [Google Scholar] [CrossRef]
- Merino, J.J.; Bellver-Landete, V.; Oset-Gasque, M.J.; Cubelos, B. CXCR4/CXCR7 molecular involvement in neuronal and neural progenitor migration: Focus in CNS repair. J. Cell Physiol. 2015, 230, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Lejkowska, R.; Kawa, M.P.; Pius-Sadowska, E.; Roginska, D.; Luczkowska, K.; Machalinski, B.; Machalinska, A. Preclinical Evaluation of Long-Term Neuroprotective Effects of BDNF-Engineered Mesenchymal Stromal Cells as Intravitreal Therapy for Chronic Retinal Degeneration in Rd6 Mutant Mice. Int. J. Mol. Sci. 2019, 20, 777. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Feng, S.; Li, L.; Lu, S.; Zhang, Y.; Long, M. Two Complementary Signaling Pathways Depict Eukaryotic Chemotaxis: A Mechanochemical Coupling Model. Front. Cell Dev. Biol. 2021, 9, 786254. [Google Scholar] [CrossRef]
- Narang, A.; Subramanian, K.K.; Lauffenburger, D.A. A mathematical model for chemoattractant gradient sensing based on receptor-regulated membrane phospholipid signaling dynamics. Ann. Biomed. Eng. 2001, 29, 677–691. [Google Scholar] [CrossRef]
- SenGupta, S.; Parent, C.A.; Bear, J.E. The principles of directed cell migration. Nat. Rev. Mol. Cell Biol. 2021, 22, 529–547. [Google Scholar] [CrossRef]
- Sroka, J.; Zimolag, E.; Lasota, S.; Korohoda, W.; Madeja, Z. Electrotaxis: Cell Directional Movement in Electric Fields. Methods Mol. Biol. 2018, 1749, 325–340. [Google Scholar] [CrossRef] [PubMed]



| SDF | EF | EC | |
|---|---|---|---|
| NT (cells) | 35 ± 2 | 55 ± 4 | 101 ± 4 |
| PDAVG (μm) | 10.1 ± 5.9 | 125 ± 68.5 | 247 ± 123 |
| Range (μm) | (2–20) | (25–225) | (40–440) |
| RT (μm) | 18 | 200 | 400 |
| pNORM | 0.12 | 0.88 | <0.001 |
| SDF | EF | EC | |
|---|---|---|---|
| Number of Motile Cells, NT | 35 ± 2 | 55 ± 4 | 101 ± 4 |
| Low Motility (Q1) | 20.0% | 21.8% | 21.8% |
| Average Motility (Q2, Q3) | 71.4% | 67.3% | 49.5% |
| Elevated Motility (Q4) | 8.6% | 10.9% | 28.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mut, S.R.; Mishra, S.; Vazquez, M. A Microfluidic Eye Facsimile System to Examine the Migration of Stem-like Cells. Micromachines 2022, 13, 406. https://doi.org/10.3390/mi13030406
Mut SR, Mishra S, Vazquez M. A Microfluidic Eye Facsimile System to Examine the Migration of Stem-like Cells. Micromachines. 2022; 13(3):406. https://doi.org/10.3390/mi13030406
Chicago/Turabian StyleMut, Stephen Ryan, Shawn Mishra, and Maribel Vazquez. 2022. "A Microfluidic Eye Facsimile System to Examine the Migration of Stem-like Cells" Micromachines 13, no. 3: 406. https://doi.org/10.3390/mi13030406
APA StyleMut, S. R., Mishra, S., & Vazquez, M. (2022). A Microfluidic Eye Facsimile System to Examine the Migration of Stem-like Cells. Micromachines, 13(3), 406. https://doi.org/10.3390/mi13030406






