Calculation of Effective Thermal Conductivity for Human Skin Using the Fractal Monte Carlo Method
Abstract
:1. Introduction
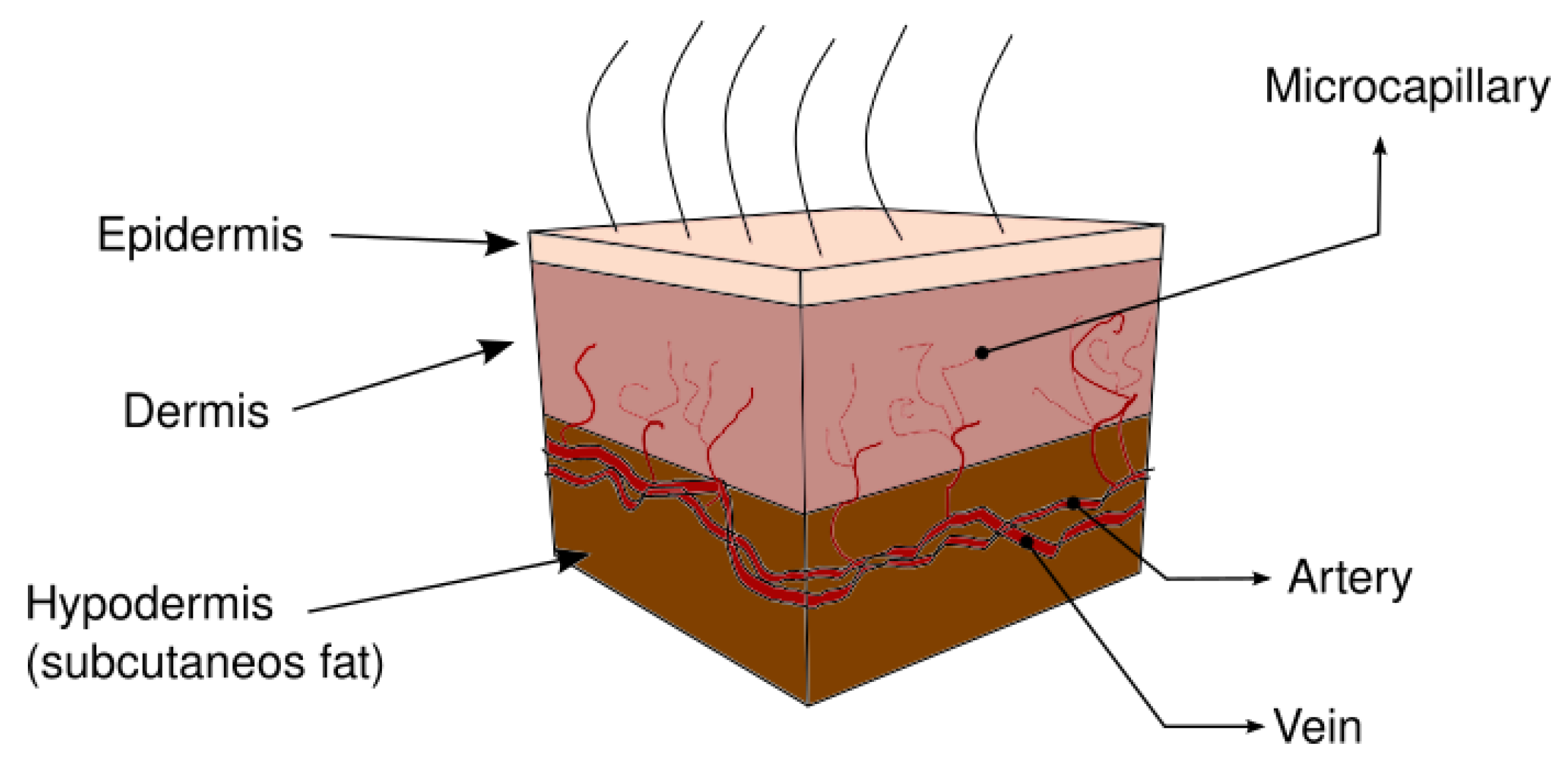
2. Heat Transfer in Human Skin
3. Mathematical Modeling
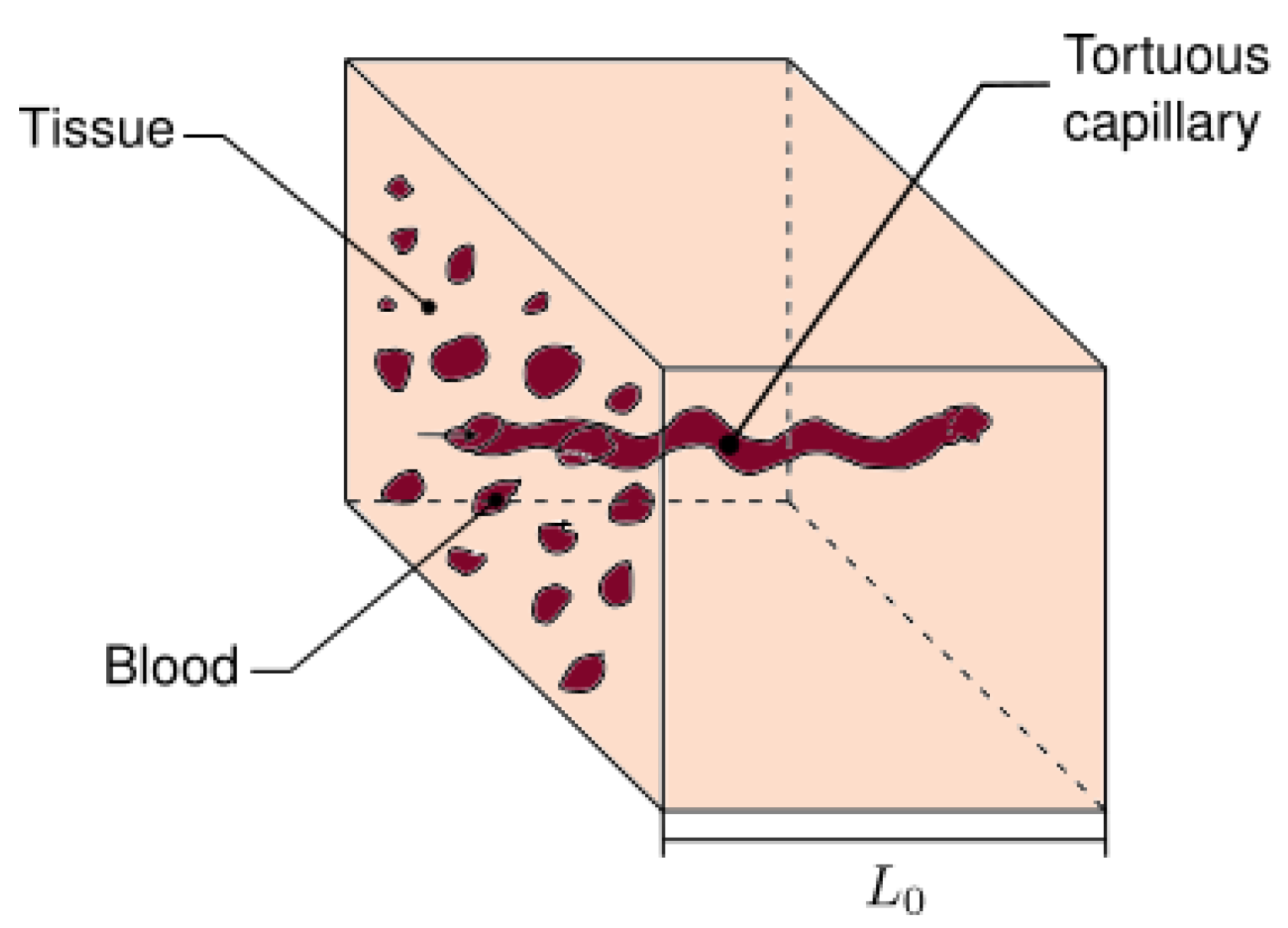
3.1. Fractal Scaling Method
3.2. The Fractal ETC of the REV
3.3. Fractal Dimensions, and
3.4. Non-Newtonian Fractal Velocity
3.5. Monte Carlo Method
3.6. Dimensionless Governing Equation
4. Results
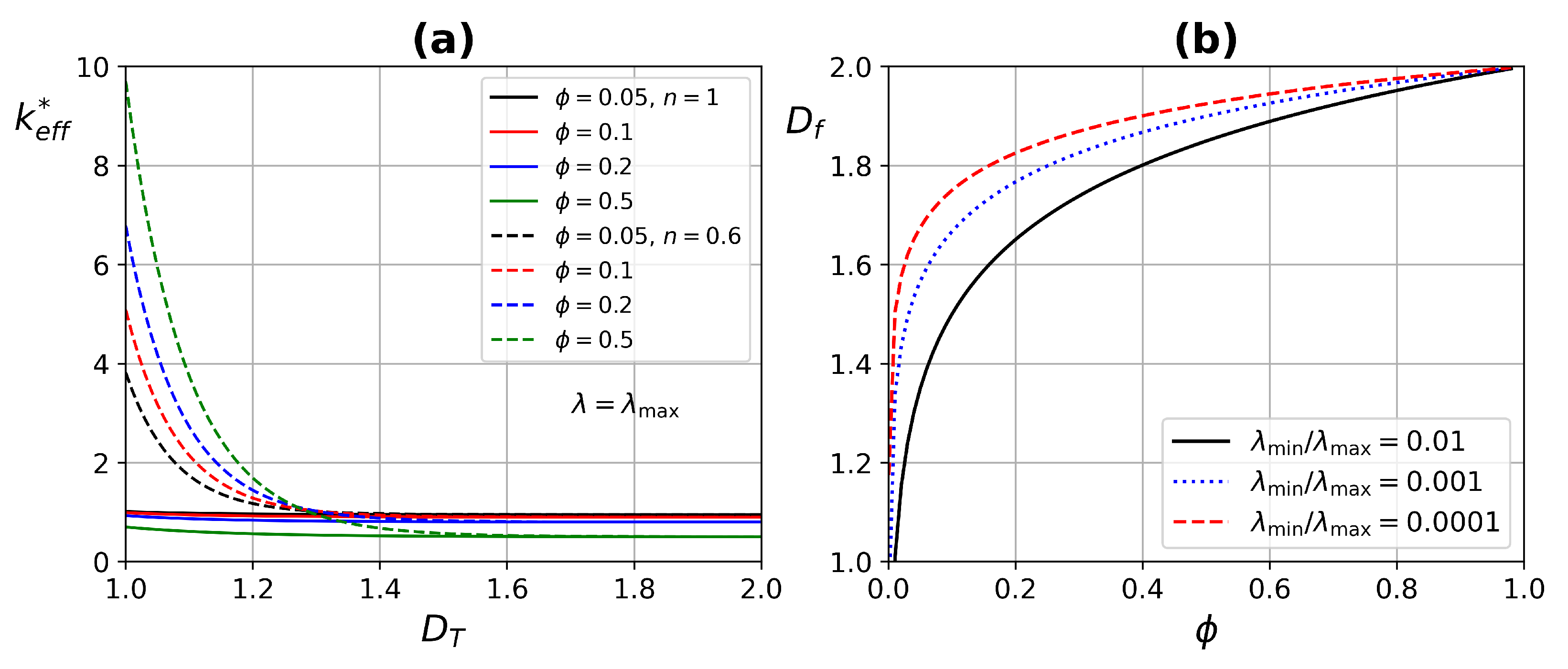
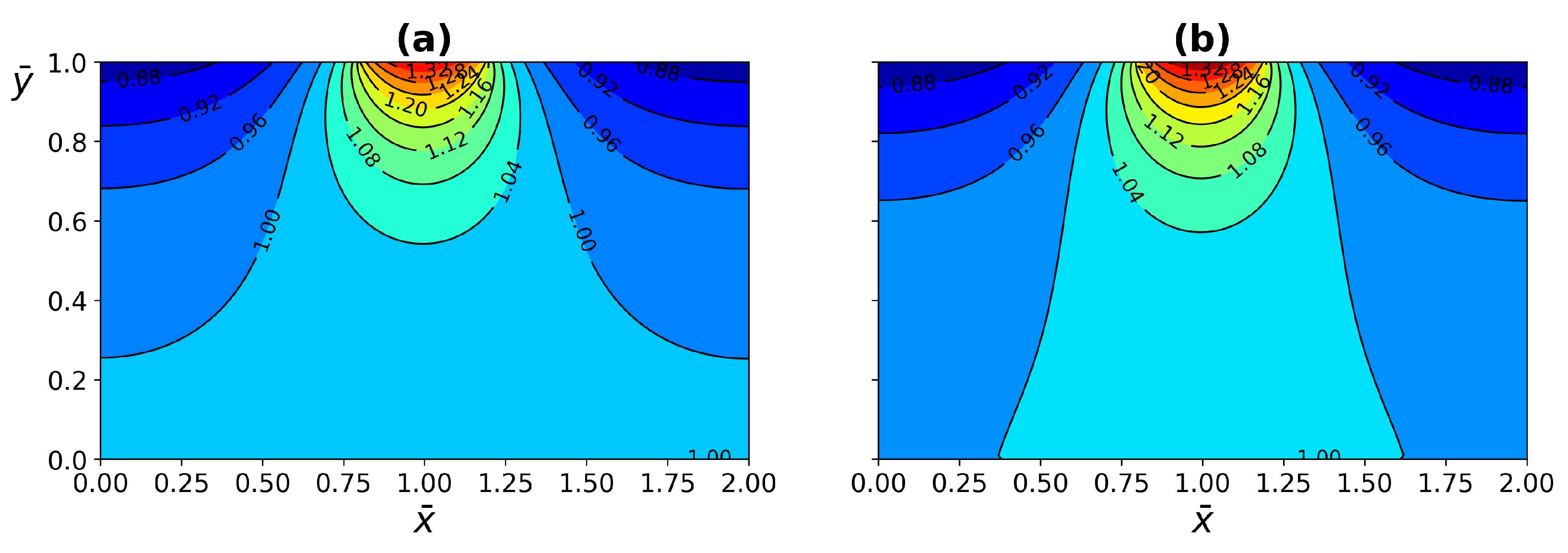
4.1. ETC Analysis
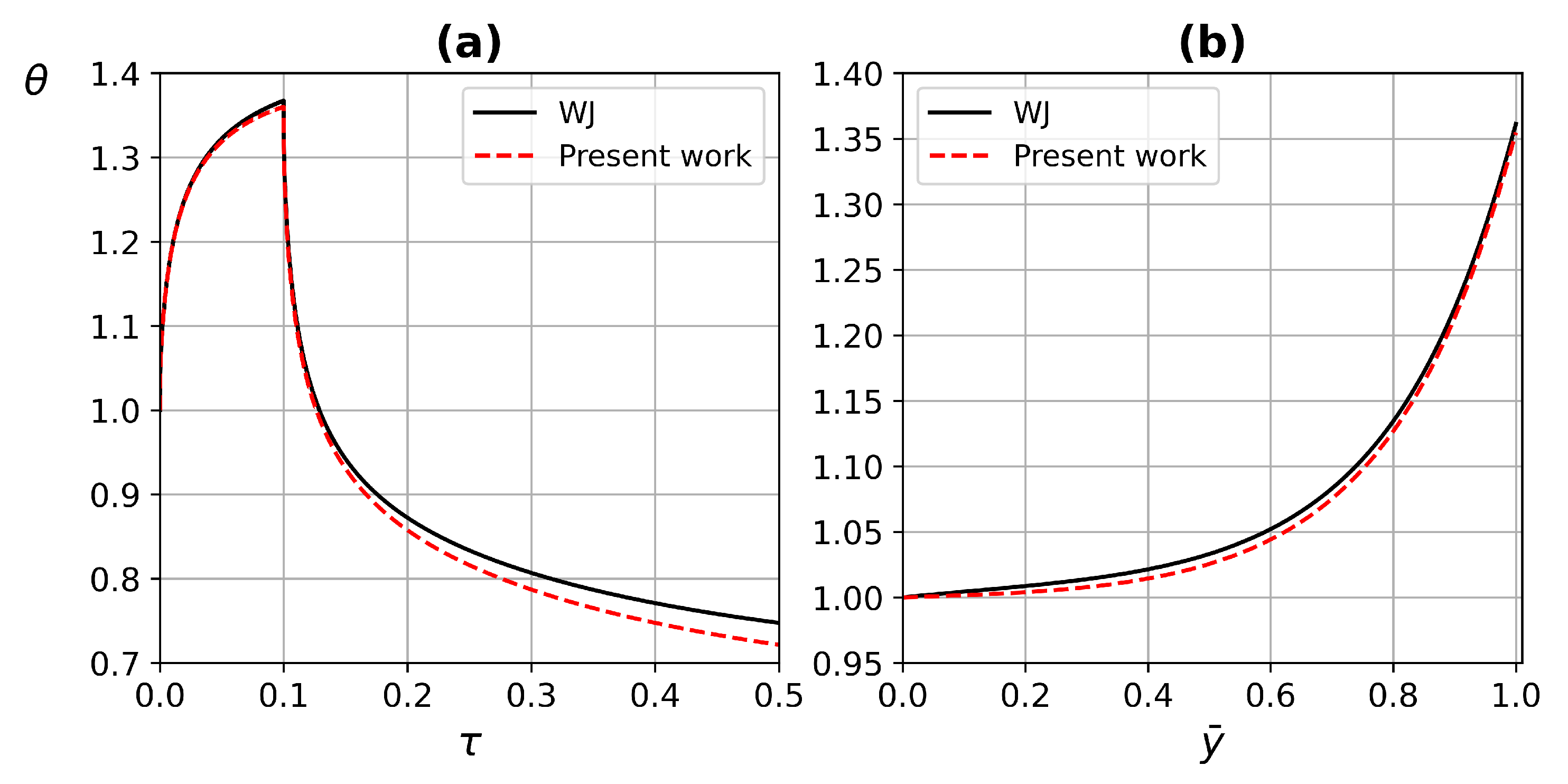
4.2. Code Validation
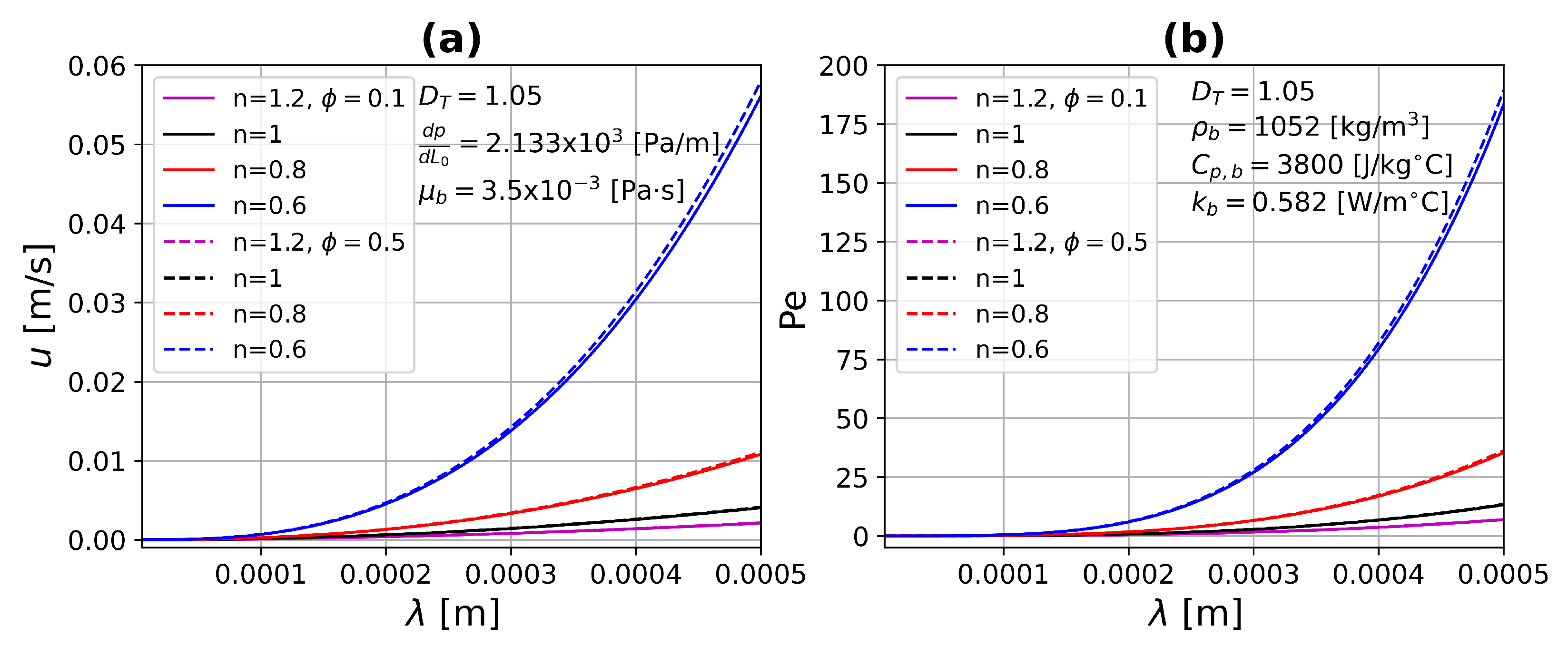
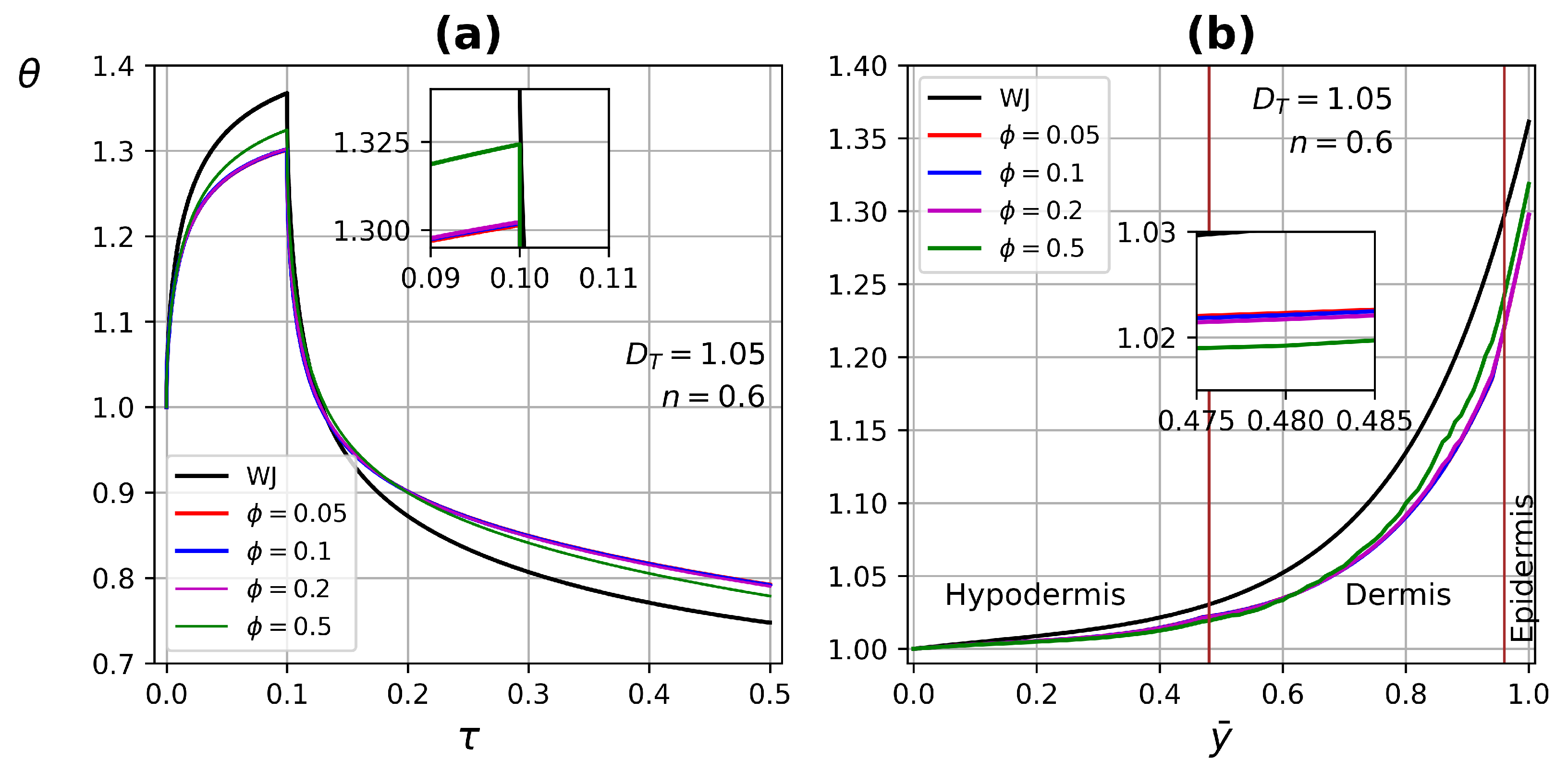
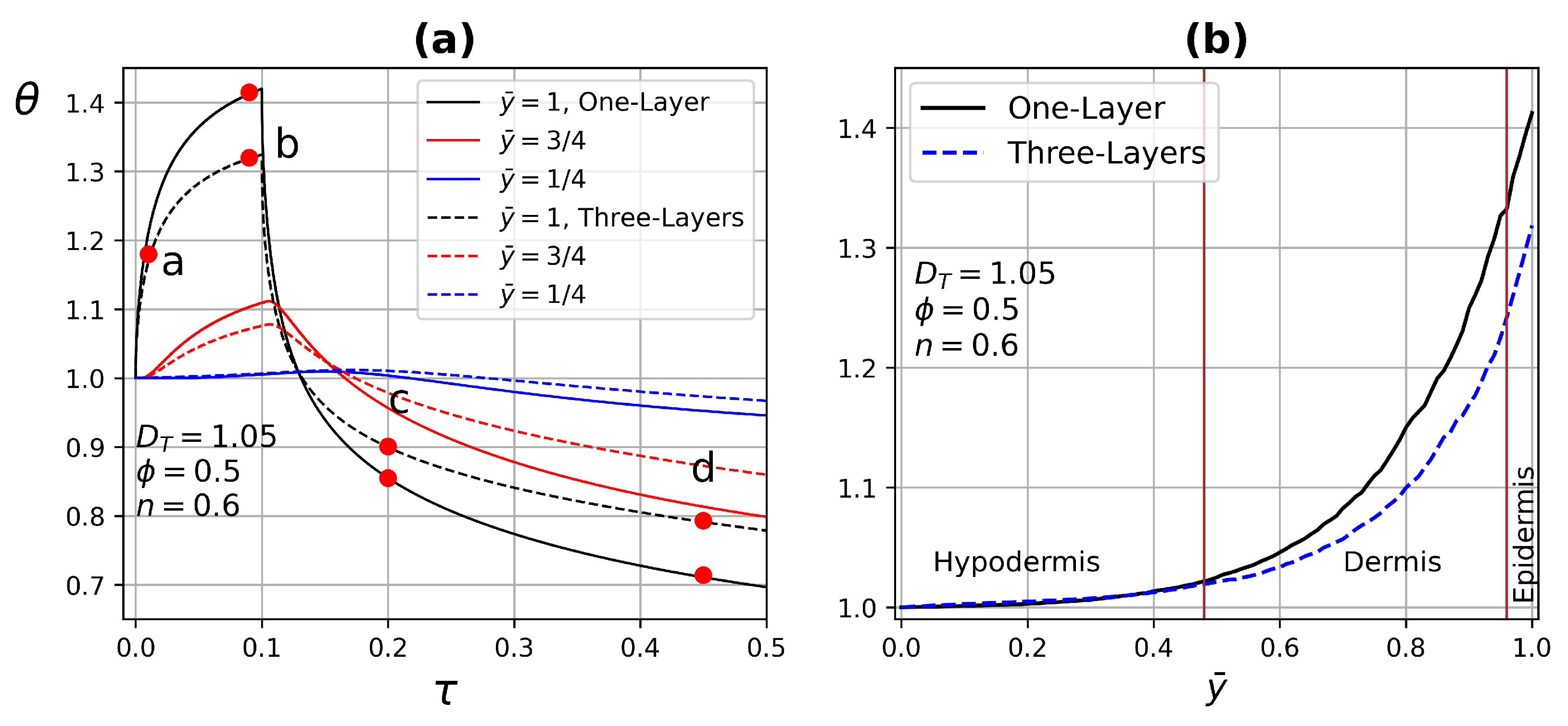
4.3. Dynamical Test Simulations
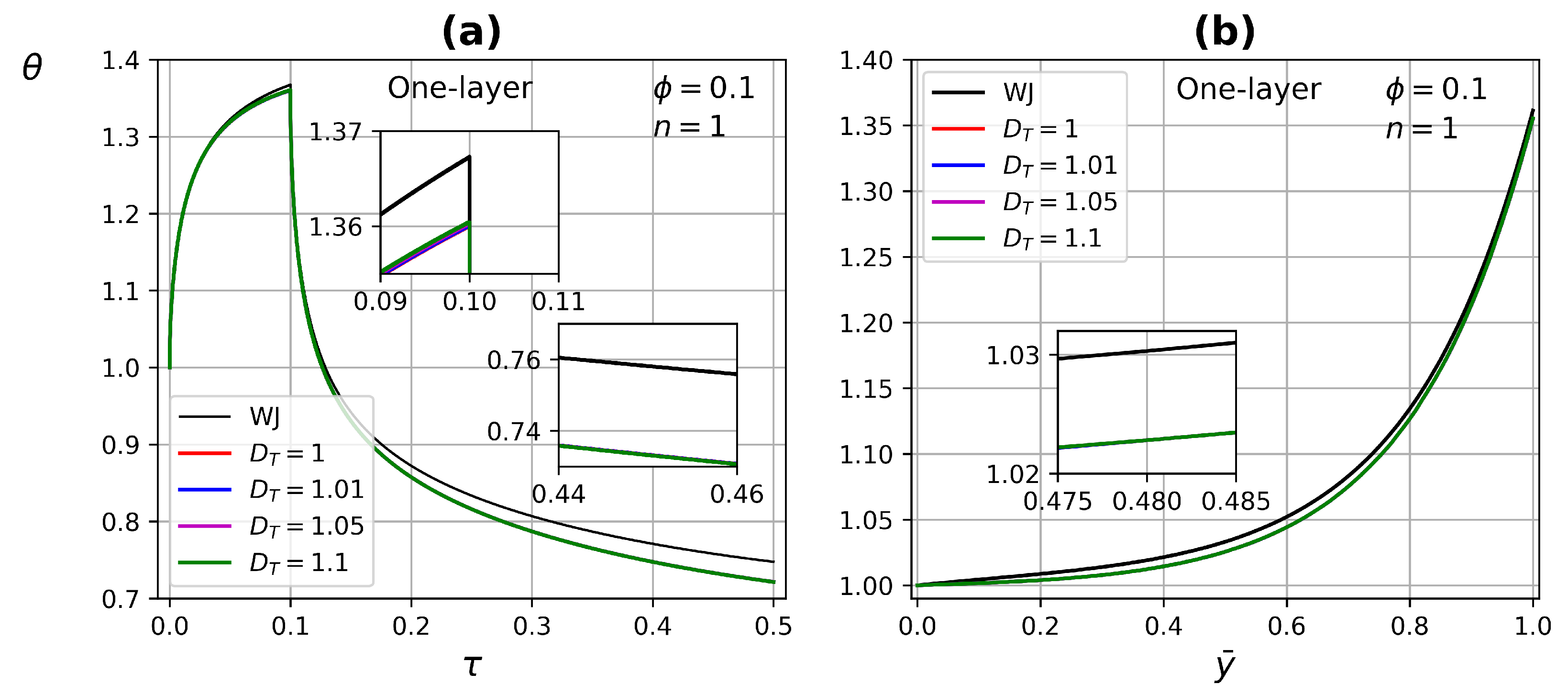
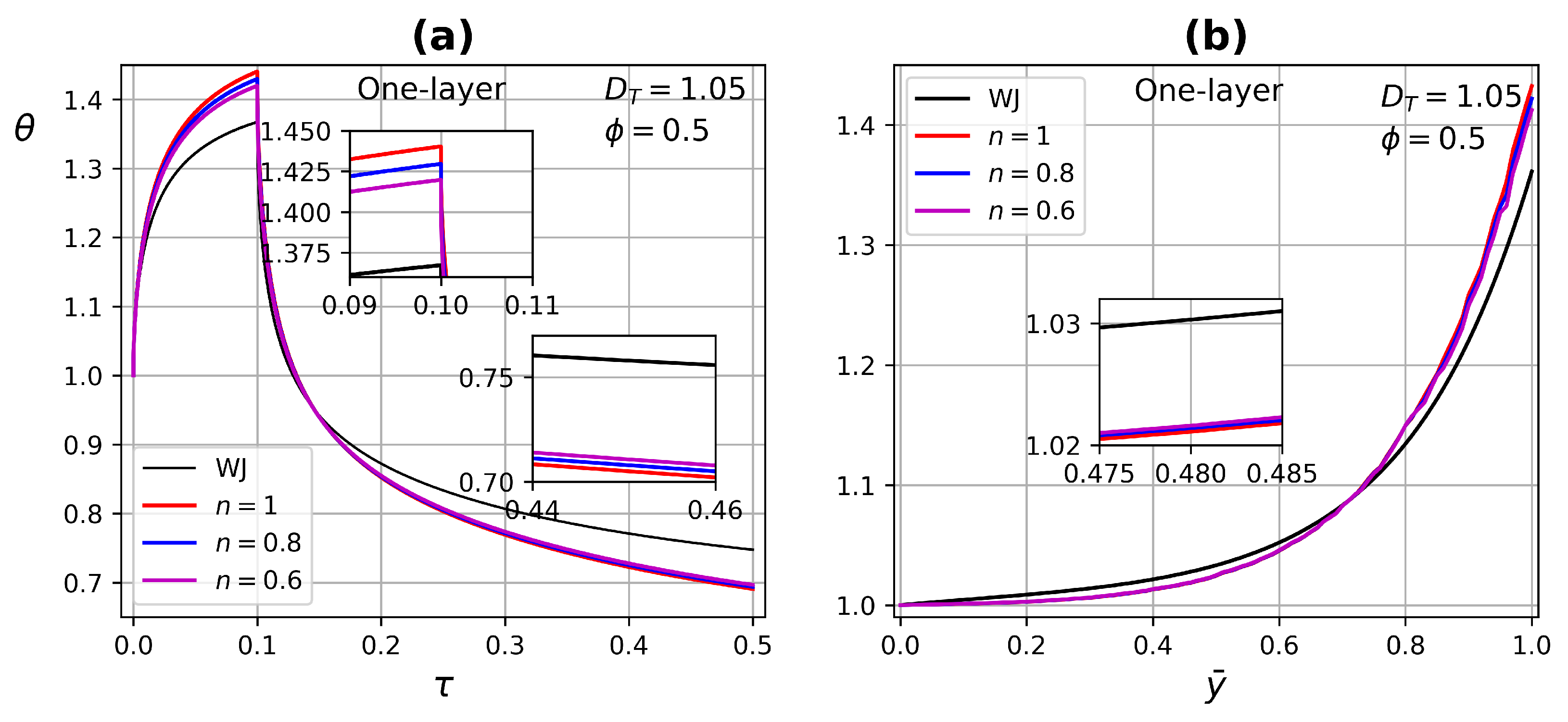
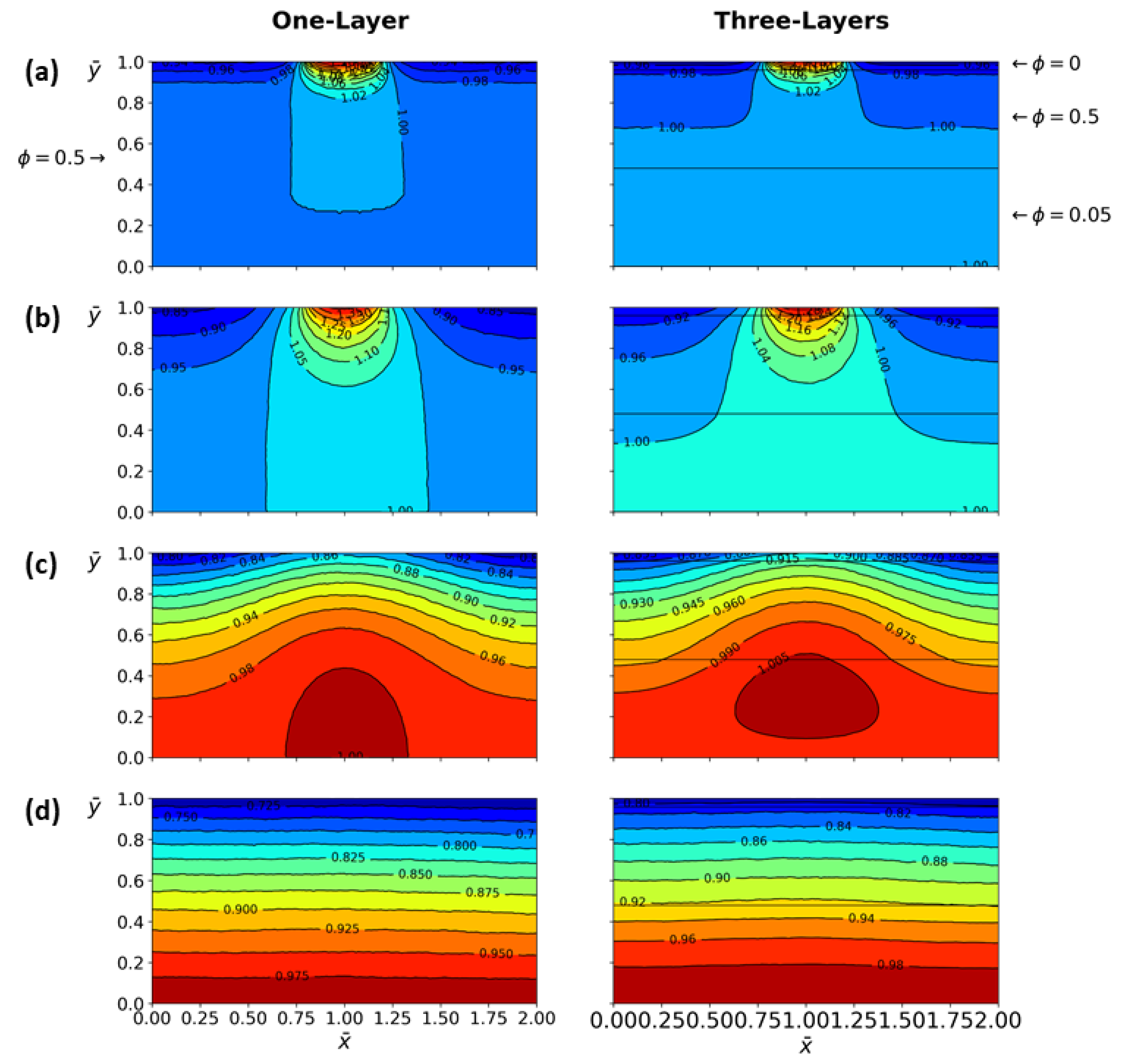
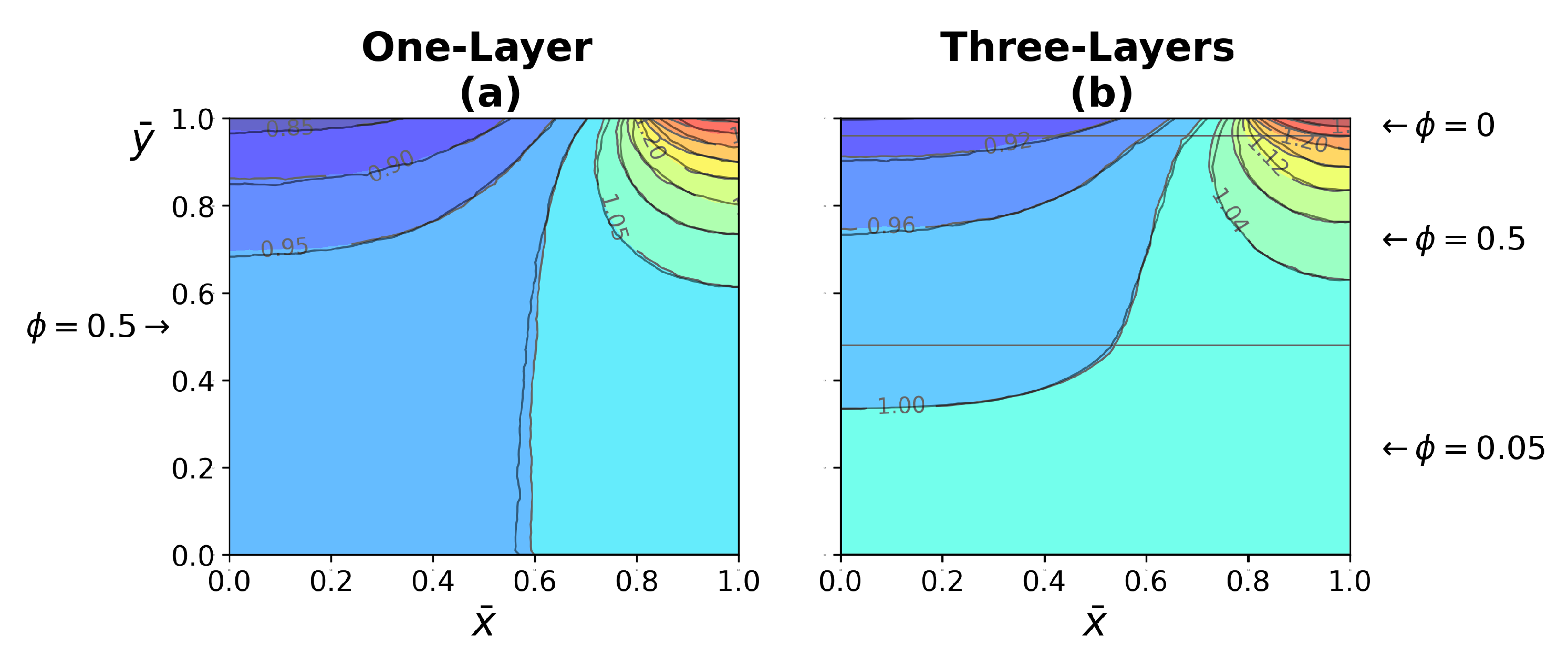
4.3.1. One-Layer Tissue Analysis
4.3.2. Three-Layer Tissue Analysis
5. Conclusions
- The effect of fractal dimension on the ETC was mainly in the range of 1–.
- Higher porosity improves ETC, due to increased blood flow through the tissue, having a higher thermal conductivity.
- In one-layer tissues of low porosity, no significant changes in ETC were found. Increasing porosity, the effect of the power-law index is reflected in both heating and relaxation processes.
- The Peclet number increases substantially due to the combination of large pore diameters and shear thinning fluids.
- In three-layer tissues with different porosities, perfusion with a shear-thinning fluid contributes to the understanding of the heat transfer process in some parts of the human body.
- The ETC involves the main variables of the heat transfer process in human skin; moreover, it is easy to implement for other case studies.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ETC | effective thermal conductivity |
| WJ | Weinbaum and Jiji |
| REV | representative elementary volume |
References
- Xu, F.; Lu, T.J.; Seffen, K.A. Skin thermal pain modeling—A holistic method. J. Therm. Biol. 2008, 33, 223–237. [Google Scholar] [CrossRef]
- Xu, F.; Seffen, K.; Lu, T. Non-Fourier analysis of skin biothermomechanics. Int. J. Heat Mass Transf. 2008, 51, 2237–2259. [Google Scholar] [CrossRef]
- Hristov, J. Bio-heat models revisited: Concepts, derivations, nondimensalization and fractionalization approaches. Front. Phys. 2019, 7, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Pikkula, B.M.; Wang, L.V.; Anvari, B. Comparison of human skin opto-thermal response to near-infrared and visible laser irradiations: A theoretical investigation. Phys. Med. Biol. 2004, 49, 4861–4877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Wang, H.; Jordan, P.M.; Mickens, R.E.; Bejan, A. A mathematical model for skin burn injury induced by radiation heating. Int. J. Heat Mass Transf. 2008, 51, 5497–5510. [Google Scholar] [CrossRef]
- Xu, F.; Wen, T.; Seffen, K.; Lu, T. Modeling of skin thermal pain: A preliminary study. Appl. Math. Comput. 2008, 205, 37–46. [Google Scholar] [CrossRef]
- Xu, F.; Lu, T.J.; Seffen, K.A.; Ng, E.Y.K. Mathematical modeling of skin bioheat transfer. Appl. Mech. Rev. 2009, 62, 1–35. [Google Scholar] [CrossRef]
- Xu, F.; Lu, T. Introduction to Skin Biothermomechanics and Thermal Pain, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Zolfaghari, A.; Maerefat, M. Bioheat transfer. Dev. Heat Transf. 2011, 153–170. [Google Scholar] [CrossRef]
- Liu, K.C.; Wang, Y.N.; Chen, Y.S. Investigation on the bio-heat transfer with the Dual-Phase-Lag effect. Int. J. Therm. Sci. 2012, 58, 29–35. [Google Scholar] [CrossRef]
- Weinbaum, S.; Jiji, L.M. A new simplified bioheat equation for the effect of blood flow on local average tissue temperature. J. Biomech. Eng. 1985, 107, 131–139. [Google Scholar] [CrossRef]
- Nakayama, A.; Sano, Y.; Yoshikawa, K. A rigorous derivation of the bioheat equation for local tissue heat transfer based on a volume averaging theory. Heat Mass Transf. 2010, 46, 739–746. [Google Scholar] [CrossRef]
- Charny, C.K. Mathematical models of bioheat transfer. Adv. Heat Transf. 1992, 22, 19–155. [Google Scholar]
- Jiji, L.M. Heat Conduction, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Wulff, W. The energy conservation equation for living tissues. IEEE Trans. Biomed. Eng. 1974, 21, 494–495. [Google Scholar] [CrossRef]
- Klinger, H.G. Heat transfer in perfused biological tissue I: General theory. Bull. Math. Biol. 1974, 36, 403–415. [Google Scholar] [PubMed]
- Chen, M.M.; Holmes, K.R. Microvascular contributions in tissue heat transfer. Ann. N. Y. Acad. Sci. 1980, 335, 137–150. [Google Scholar] [CrossRef]
- Weinbaum, S.; Jiji, L.M.; Lemons, D.E. Theory and experiment for the effect of vascular microstructure on surface tissue heat transfer: Part I: Anatomical foundation and model conceptualization. J. Biomech. Eng. 1984, 106, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Jiji, L.M.; Lemons, D.E. Theory and experiment for the effect of vascular microstructure on surface tissue heat transfer: Part II: Anatomical foundation and model conceptualization. J. Biomech. Eng. 1984, 106, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H. Thermal (heat) shock biothermomechanical viewpoint. J. Biomech. Eng. 1993, 115, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Xu, L.X. New thermal wave aspects on burn evaluation of skin subjected to instantaneous heating. IEEE Trans. Biomed. Eng. 1999, 46, 420–428. [Google Scholar]
- Tzou, D.Y. Macro- to Microscale Heat Transfer: The Lagging Behaviour, 1st ed., Taylor and Francis: Abingdon, UK, 1997.
- Hobiny, A.D.; Abbas, I.A. Theoretical analysis of thermal damages in skin tissue induced by intense moving heat source. Int. J. Heat Mass Transf. 2018, 124, 1011–1014. [Google Scholar] [CrossRef]
- Alzahrani, F.S.; Abbas, I.A. Analytical estimations of temperature in a living tissue generated by laser irradiation using experimental data. J. Therm. Biol. 2019, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hobiny, A.; Abbas, I. Thermal response of cylindrical tissue induced by laser irradiation with experimental study. Int. J. Numer. Methods Heat Fluid Flow 2019, 30, 4013–4023. [Google Scholar] [CrossRef]
- Hobiny, A.; Alzahrani, F.; Abbas, I.; Marin, M. The effect of fractional time derivative of bioheat model in skin tissue induced to laser irradiation. Symmetry 2020, 12, 602. [Google Scholar] [CrossRef]
- Hobiny, A.; Alzahrani, F.; Abbas, I. Analytical estimation of temperature in living tissues using the tpl bioheat model with experimental verification. Mathematics 2020, 8, 1188. [Google Scholar] [CrossRef]
- Kumari, T.; Singh, S.K. A numerical study of space-fractional three-phase-lag bioheat transfer model during thermal therapy. Heat Transf. 2022, 51, 470–489. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Liu, D. Generalized bio-heat transfer model combining with the relaxation mechanism and nonequilibrium heat transfer. J. Heat Transf. 2021, 144, 031209. [Google Scholar] [CrossRef]
- Arkin, H.; Xu, L.X.; Holmes, K.R. Recent developments in modeling heat transfer in blood perfused tissues. IEEE Trans. Biomed. Eng. 1994, 41, 97–107. [Google Scholar] [CrossRef]
- Khaled, A.R.; Vafai, K. The role of porous media in modeling flow and heat transfer in biological tissues. Int. J. Heat Mass Transf. 2003, 46, 4989–5003. [Google Scholar] [CrossRef]
- Goldstein, R.; Ibele, W.; Patankar, S.; Simon, T.; Kuehn, T.; Strykowski, P.; Tamma, K.; Heberlein, J.; Davidson, J.; Bischof, J.; et al. Heat transfer—A review of 2003 literature. Int. J. Heat Mass Transf. 2006, 49, 451–534. [Google Scholar] [CrossRef]
- Goldstein, R.J.; Ibele, W.E.; Patankar, S.V.; Simon, T.W.; Kuehn, T.H.; Strykowski, P.J.; Tamma, K.K.; Heberlein, J.V.R.; Davidson, J.H.; Bischof, J.; et al. Heat transfer—A review of 2005 literature. Int. J. Heat Mass Transf. 2010, 53, 4397–4447. [Google Scholar] [CrossRef]
- Yang, X.; Liang, Y.; Chen, W. A spatial fractional seepage model for the flow of non-Newtonian fluid in fractal porous medium. Commun. Nonlinear Sci. Numer. Simul. 2018, 65, 70–78. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K. Synthesis of mathematical models representing bioheat transport. Adv. Numer. Heat Transf. 2009, 3, 1–28. [Google Scholar]
- Roetzel, W.; Xuan, Y. Bioheat equation of the human thermal system. Chem. Eng. Technol. 1997, 20, 268–276. [Google Scholar]
- Nakayama, A.; Kuwahara, F. A general bioheat transfer model based on the theory of porous media. Int. J. Heat Mass Transf. 2008, 51, 3190–3199. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Xu, P.; Qiu, S.; Rao, B.; Yu, B. A generalized thermal conductivity model for unsaturated porous media with fractal geometry. Int. J. Heat Mass Transf. 2020, 152, 119540. [Google Scholar] [CrossRef]
- Zhao, C. Review on thermal transport in high porosity cellular metal foams with open cells. Int. J. Heat Mass Transf. 2012, 55, 3618–3632. [Google Scholar] [CrossRef]
- Belova, I.V.; Murch, G.E. Monte Carlo simulation of the effective thermal conductivity in two-phase material. J. Mater. Process. Technol. 2004, 153, 741–745. [Google Scholar] [CrossRef]
- Song, Y.; Youn, J. Evaluation of effective thermal conductivity for carbon nanotube/polymer composites using control volume finite element method. J. Carbon 2006, 44, 710–717. [Google Scholar] [CrossRef]
- Mandelbrot, B. The Fractal Geometry of Nature; Freeman: New York, NY, USA, 1982. [Google Scholar]
- Yu, B. Analysis of flow in fractal porous media. Appl. Mech. Rev. 2008, 61, 050801. [Google Scholar] [CrossRef]
- Xu, P.; Mujumdar, A.S.; Sasmito, A.P.; Yu, B.M. Multiscale Modeling of Porous Media, 1st ed.; Taylor and Francis: Abingdon, UK, 2019. [Google Scholar]
- Kou, J.; Liu, Y.; Wu, F.; Fan, J.; Lu, H.; Xu, Y. Fractal analysis of effective thermal conductivity for three-Phase (unsaturated) porous media. J. Appl. Phys. 2009, 106, 054905. [Google Scholar] [CrossRef] [Green Version]
- Xu, P. A discussion on fractal models for transport physics of porous media. Fractals 2015, 23, 1530001. [Google Scholar] [CrossRef]
- Yu, B.; Zou, M.; Feng, Y. Permeability of fractal porous media by Monte Carlo simulations. Int. J. Heat Mass Transf. 2005, 48, 2787–2794. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.; Yu, B.; Feng, Y.; Xu, P. A Monte Carlo method for simulating fractal surfaces. Physica A 2007, 386, 176–186. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, B.; Feng, K.; Xu, P.; Zou, M. Thermal conductivity of nanofluids and size distribution of nanoparticles by Monte Carlo simulations. J. Nanopart. Res. 2008, 10, 1319–1328. [Google Scholar] [CrossRef]
- Xu, P.; Yu, B.; Qiao, X.; Qiu, S.; Jiang, Z. Radial permeability of fractured porous media by Monte Carlo simulations. Int. J. Heat Mass Transf. 2013, 57, 369–374. [Google Scholar] [CrossRef]
- Vadapalli, U.; Srivastava, R.P.; Vedanti, N.; Dimri, V.P. Estimation of permeability of a sandstone reservoir by a fractal and Monte Carlo simulation approach: A case study. Nonlinear Process. Geophys. 2014, 21, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zheng, Y.; Kou, J. Prediction of effective thermal conductivity of porous media with fractal-Monte Carlo simulations. Fractals 2014, 22, 1440004. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, X.; Jiang, G.; Long, G.; Wang, W.; Zhang, Y.; Liu, G. Kozeny–Carman constant for gas flow through fibrous porous media by fractal-Monte Carlo simulations. Fractals 2019, 27, 1950062. [Google Scholar] [CrossRef]
- Yang, J.; Wang, M.; Wu, L.; Liu, Y.; Qiu, S.; Xu, P. A novel Monte Carlo simulation on gas flow in fractal shale reservoir. Energy 2021, 236, 121513. [Google Scholar] [CrossRef]
- Yu, B.; Li, J. Some fractal characters of porous media. Fractals 2001, 9, 365–372. [Google Scholar] [CrossRef]
- Yu, B.; Cheng, P. A fractal permeability model for bi-dispersed porous media. Int. J. Heat Mass Transf. 2002, 45, 2983–2993. [Google Scholar] [CrossRef]
- Wu, J.; Yu, B. A fractal resistance model for flow through porous media. Int. J. Heat Mass Transf. 2007, 50, 3925–3932. [Google Scholar] [CrossRef]
- Shen, H.; Ye, Q.; Meng, G. Anisotropic fractal model for the effective thermal conductivity of random metal fiber porous media with high porosity. Phys. Lett. A 2017, 381, 3193–3196. [Google Scholar] [CrossRef]
- Qin, X.; Cai, J.; Xu, P.; Dai, S.; Gan, Q. A fractal model of effective thermal conductivity for porous media with various liquid saturation. Int. J. Heat Mass Transf. 2019, 128, 1149–1156. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, B.; Zou, M.; Zhang, D. A generalized model for the effective thermal conductivity of porous media based on self-similarity. J. Phys. D Appl. Phys. 2004, 37, 3030–3040. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, B.; Wang, H.; Yun, M. A fractal analysis of permeability for power-law fluids in porous media. Fractals 2006, 14, 171–177. [Google Scholar] [CrossRef]
- Xu, F.; Wen, T.; Lu, T.; Seffen, K.A. Skin biothermomechanics for medical treatments. J. Mech. Behav. Biomed. Mater. 2008, 1, 172–187. [Google Scholar] [CrossRef]
- Kumar, S.; Damor, R.S.; Shukla, A.K. Numerical study on thermal therapy of triple layer skin tissue using fractional bioheat model. Int. J. Biomath. 2018, 11, 1850052. [Google Scholar] [CrossRef]
- Johnston, B.M.; Johnston, P.R.; Corney, S.; Kilpatrick, D. Non–Newtonian blood flow in human right coronary arteries: Steady state simulations. J. Biomech. 2004, 37, 709–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Cai, J.; Zou, M. On the physical properties of apparent two-phase fractal porous media. Vadose Zone J. Fractals 2009, 8, 177–186. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Altamirano, G.; Vargas, R.O.; Escandón, J.P.; Mil-Martínez, R.; Rojas-Montero, A. Calculation of Effective Thermal Conductivity for Human Skin Using the Fractal Monte Carlo Method. Micromachines 2022, 13, 424. https://doi.org/10.3390/mi13030424
Rojas-Altamirano G, Vargas RO, Escandón JP, Mil-Martínez R, Rojas-Montero A. Calculation of Effective Thermal Conductivity for Human Skin Using the Fractal Monte Carlo Method. Micromachines. 2022; 13(3):424. https://doi.org/10.3390/mi13030424
Chicago/Turabian StyleRojas-Altamirano, Guillermo, René O. Vargas, Juan P. Escandón, Rubén Mil-Martínez, and Alan Rojas-Montero. 2022. "Calculation of Effective Thermal Conductivity for Human Skin Using the Fractal Monte Carlo Method" Micromachines 13, no. 3: 424. https://doi.org/10.3390/mi13030424






