Study of the Electron-Phonon Coupling in PbS/MnTe Quantum Dots Based on Temperature-Dependent Photoluminescence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Quantum Dots
2.3. Sample Characterisation
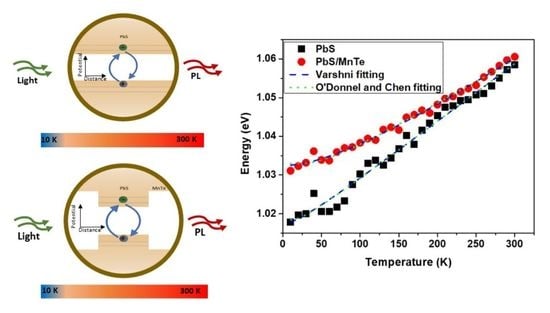
3. Results and Discussion
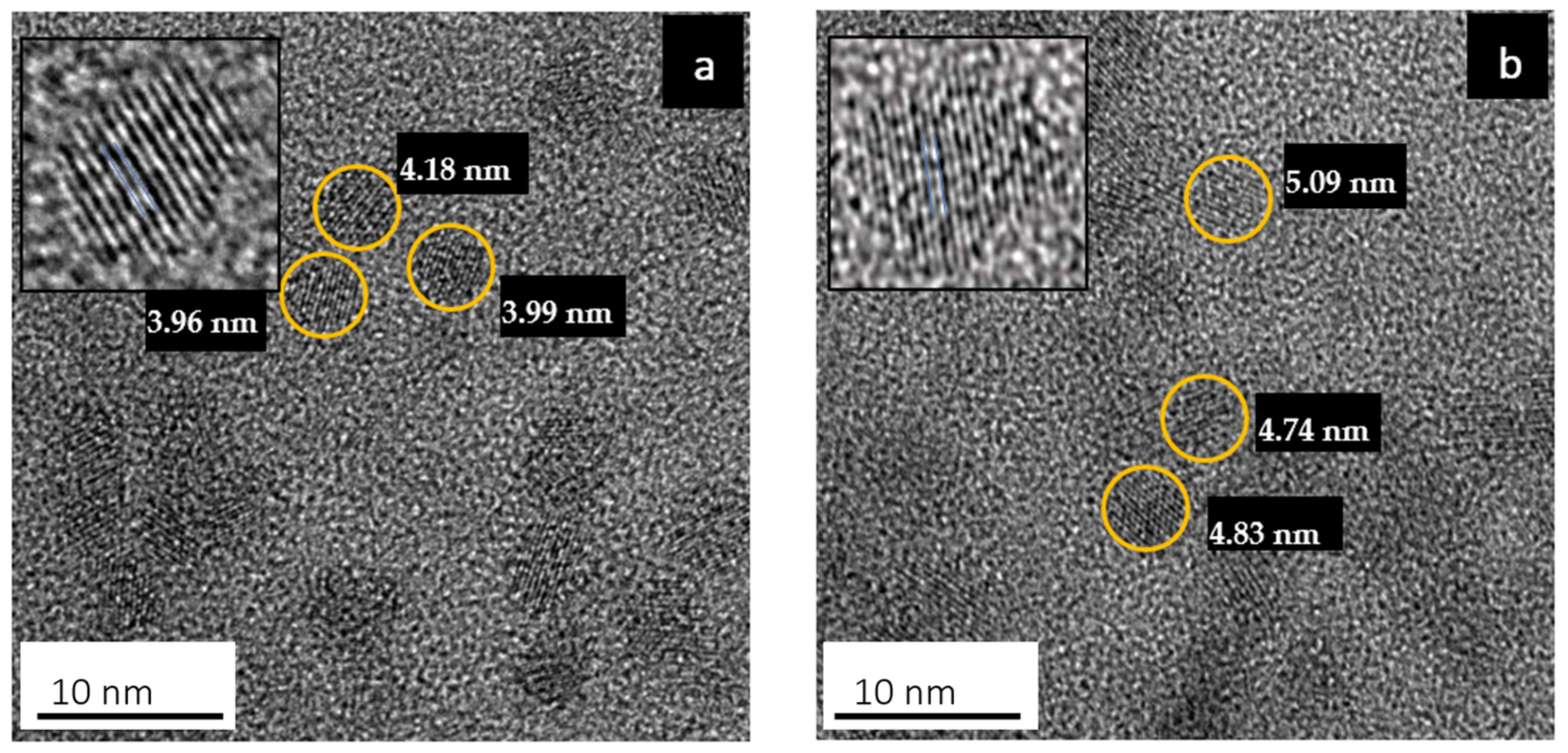
3.1. High-Resolution Transmission Electron Microscopy (HRTEM)
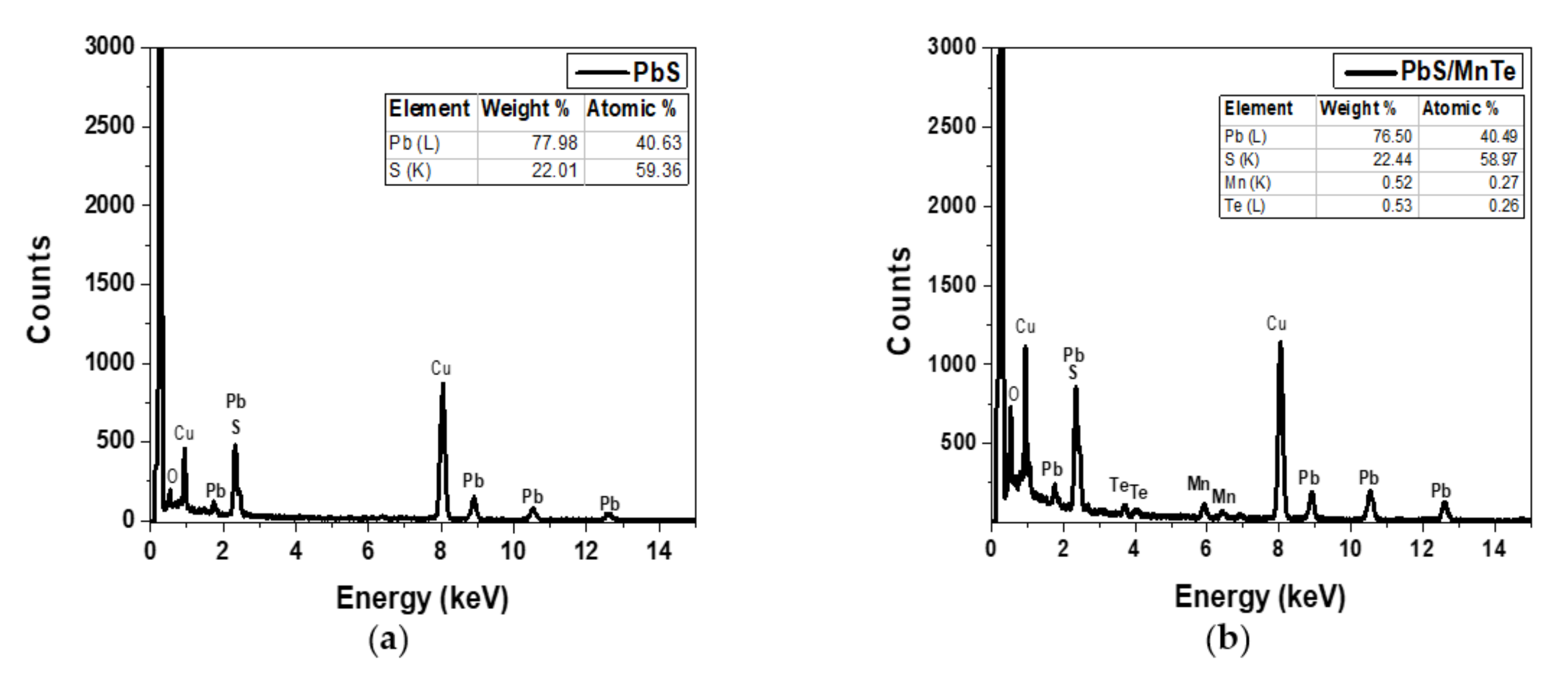
3.2. Energy Dispersive X-ray (EDX)
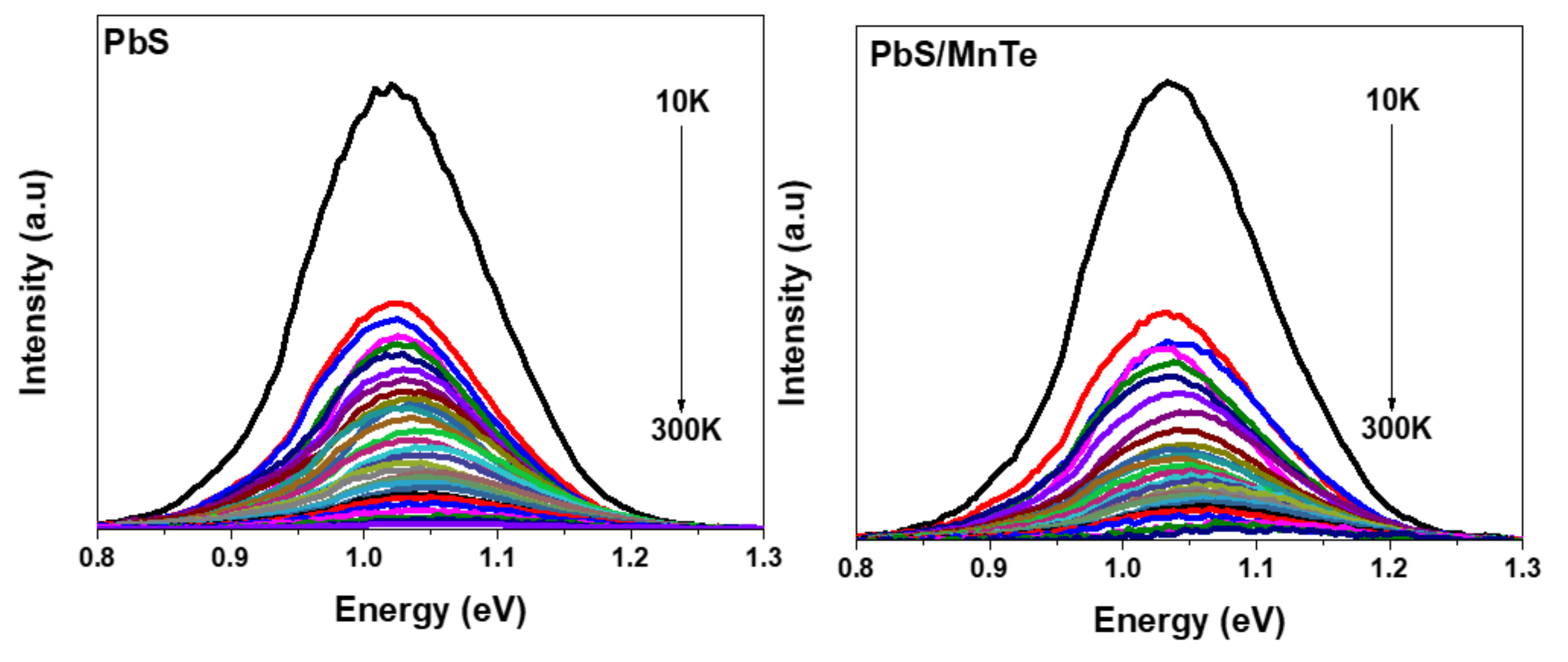
3.3. Photoluminescence (PL)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Su, G.; Liu, C.; Deng, Z.; Zhao, X.; Zhou, X. Size-Dependent Photoluminescence of PbS QDs Embedded in Silicate Glasses. Opt. Mater. Express 2017, 7, 2194. [Google Scholar] [CrossRef]
- Qian, H.; Dong, C.; Peng, J.; Qiu, X.; Xu, Y.; Ren, J. High-Quality and Water-Soluble Near-Infrared Photoluminescent CdHgTe/CdS Quantum Dots Prepared by Adjusting Size and Composition. J. Phys. Chem. C 2007, 111, 16852–16857. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, H.; Deng, D.; Xue, B.; Tang, L.; Mahounga, D.; Qian, Z.; Gu, Y. In Vivo NIR Imaging with PbS Quantum Dots Entrapped in Biodegradable Micelles. J. Biomed. Mater. Res. Part A 2012, 100, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shih, W.; Shih, W. Non-Heavy-Metal ZnS Quantum Dots with Bright Blue Photoluminescence by a One-Step Aqueous Synthesis. Nanotechnology 2007, 18, 205604. [Google Scholar] [CrossRef]
- Hu, R.; Law, W.; Lin, G.; Ye, L.; Liu, J.; Liu, J.; Reynolds, J.L.; Yong, K.-T. PEGylated Phospholipid Micelle-Encapsulated Near-Infrared PbS Quantum Dots for in vitro and in vivo Bioimaging. Theranostics 2012, 2, 723–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, S.; Kim, H.; Kim, S.; Kim, S.; Seok, S. All Solid State Multiply Layered Pbs Colloidal Quantum-Dot-Sensitized Photovoltaic Cells. Energy Environ. Sci. 2011, 4, 4181. [Google Scholar] [CrossRef]
- Wang, P.; Cao, L.; Chen, Y.; Wu, Y.; Di, J. Photoelectrochemical Biosensor Based on Co3O4 Nanoenzyme Coupled with PbS Quantum Dots for Hydrogen Peroxide Detection. ACS Appl. Nano Mater. 2019, 2, 2204–2211. [Google Scholar] [CrossRef]
- Kwon, J.; Han, M.; Jung, D.; Kong, S.; Jung, D. High Sensitivity Shortwave Infrared Photodetector Based on PbS QDs Using P3HT. Nanomaterials 2021, 11, 2683. [Google Scholar] [CrossRef]
- Yang, T.; Lu, M.; Mao, X.; Liu, W.; Wan, L.; Miao, S.; Xu, J. Synthesis of CdS Quantum Dots (QDs) via a Hot-Bubbling Route and Co-Sensitized Solar Cells Assembly. Chem. Eng. J. 2013, 225, 776–783. [Google Scholar] [CrossRef]
- Guerreiro, P.; Ten, S.; Borrelli, N.; Butty, J.; Jabbour, G.; Peyghambarian, N. PbS Quantum-Dot Doped Glasses as Saturable Absorbers for Mode Locking of a Cr:Forsterite Laser. Appl. Phys. Lett. 1997, 71, 1595–1597. [Google Scholar] [CrossRef]
- Wang, D.; Qian, J.; Cai, F.; He, S.; Han, S.; Mu, Y. ‘Green’-Synthesized Near-Infrared PbS Quantum Dots with Silica–PEG Dual-Layer Coating: Ultrastable and Biocompatible Optical Probes Forin Vivoanimal Imaging. Nanotechnology 2012, 23, 245701. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhou, Y.; Ren, F.; Benetti, D.; Yang, F.; Zhao, H.; Rosei, F.; Chaker, M.; Ma, D. Ultrasmall PbS Quantum Dots: A Facile and Greener Synthetic Route and Their High Performance in Luminescent Solar Concentrators. J. Mater. Chem. A 2017, 5, 10250–10260. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, C.; Zhang, G.; Pan, Z.; Huang, Z.; Xu, S.; Rao, H.; Liu, H.; Wu, S.; Wu, X.; et al. All-Inorganic CsPbI3 Quantum Dot Solar Cells with Efficiency over 16% by Defect Control. Adv. Funct. Mater. 2020, 31, 2005930. [Google Scholar] [CrossRef]
- Linkov, P.; Krivenkov, V.; Nabiev, I.; Samokhvalov, P. High Quantum Yield CdSe/ZnS/CdS/ZnS Multishell Quantum Dots for Biosensing and Optoelectronic Applications. Mater. Today Proc. 2016, 3, 104–108. [Google Scholar] [CrossRef]
- Debellis, D.; Gigli, G.; ten Brinck, S.; Infante, I.; Giansante, C. Quantum-Confined and Enhanced Optical Absorption of Colloidal PbS Quantum Dots at Wavelengths with Expected Bulk Behavior. Nano Lett. 2017, 17, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Huang, S.; Su, W.; Chan, W.; Liu, Y. Facile Synthesis and Characterization of Highly Fluorescent and Biocompatiblen-Acetyl-L-Cysteine Capped CdTe/CdS/ZnS Core/Shell/Shell Quantum Dots in Aqueous Phase. Nanotechnology 2012, 23, 495717. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wan, D.; Gong, J. Pegylated Liposome Coated QDs/Mesoporous Silica Core-Shell Nanoparticles for Molecular Imaging. Chem. Commun. 2011, 47, 3442. [Google Scholar] [CrossRef]
- Samokhvalov, P.; Linkov, P.; Michel, J.; Molinari, M.; Nabiev, I. Photoluminescence quantum yield of CdSe-ZnS/CdS/ZnS core-multishell quantum dots approaches 100% due to enhancement of charge carrier confinement. In Colloidal Nanoparticles for Biomedical Applications IX; International Society for Optics and Photonics: San Diego, CA, USA, 2014; Volume 8955, p. 89550S. [Google Scholar]
- Shelawati, T.; Nurisya, M.S.; Mazliana, A.K. Effects of Step-Potential on Confinement Strength of Strain-Induced Type-I Core–Shell Quantum Dots. Superlattices Microstruct. 2019, 131, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Zhang, L.; Soeller, C.; Travas-Sejdic, J. Preparation of Water-Soluble CdTe/CdS Core/Shell Quantum Dots with Enhanced Photostability. J. Lumin. 2007, 127, 721–726. [Google Scholar] [CrossRef]
- Fitzmorris, B.; Cooper, J.; Edberg, J.; Gul, S.; Guo, J.; Zhang, J. Synthesis and Structural, Optical, and Dynamic Properties of Core/Shell/Shell CdSe/ZnSe/ZnS Quantum Dots. J. Phys. Chem. C 2012, 116, 25065–25073. [Google Scholar] [CrossRef]
- Anno, H.; Koyanagi, T.; Matsubara, K. Epitaxial Growth of Zincblende Mnte Films as a New Magneto-Optical Material. J. Cryst. Growth 1992, 117, 816–819. [Google Scholar] [CrossRef]
- Jasim, K. Lead Sulfide Quantum Dot Sensitized Nanocrystalline Solar Cell. KnE Eng. 2018, 3, 171. [Google Scholar] [CrossRef]
- Tubtimtae, A.; Arthayakul, K.; Teekwang, B.; Hongsith, K.; Choopun, S. Mnte Semiconductor-Sensitized Boron-Doped TiO2 and ZnO Photoelectrodes for Solar Cell Applications. J. Colloid Interface Sci. 2013, 405, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zaini, M.S.; Kamarudin, M.A.; Chyi, J.L.Y.; Alang Ahmad, S.A.; Mohmad, A.R. Temperature and Power Dependence of Photoluminescence in PbS Quantum Dots Nanoparticles. Sains Malays. 2019, 48, 1281–1288. [Google Scholar] [CrossRef]
- Zaini, M.; Liew, J.; Alang Ahmad, S.; Mohmad, A.; Ahmad Kamarudin, M. Photoluminescence Investigation of Carrier Localization in Colloidal PbS and PbS/MnS Quantum Dots. ACS Omega 2020, 5, 30956–30962. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yuan, L.; Patterson, R.; Wen, X.; Tapping, P.; Kee, T.; Veetil, B.P.; Zhang, P.; Zhang, Z.; Zhang, Q.; et al. Difference in Hot Carrier Cooling Rate between Langmuir–Blodgett and Drop Cast PbS QD Films Due to Strong Electron–Phonon Coupling. Nanoscale 2017, 9, 17133–17142. [Google Scholar] [CrossRef]
- Ai, B.; Liu, C.; Deng, Z.; Wang, J.; Han, J.; Zhao, X. Low Temperature Photoluminescence Properties of CsPbBr3 Quantum Dots Embedded in Glasses. Phys. Chem. Chem. Phys. 2017, 19, 17349–17355. [Google Scholar] [CrossRef]
- Chun, J.; Yang, W.; Kim, J. Thermal Stability of CdSe/ZnS Quantum Dot-Based Optical Fiber Temperature Sensor. Mol. Cryst. Liq. Cryst. 2011, 538, 333–340. [Google Scholar] [CrossRef]
- Varshni, Y.P. Temperature Dependence of the Energy Gap in Semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Kim, D.; Kuwabara, T.; Nakayama, M. Photoluminescence Properties Related to Localized States in Colloidal PbS Quantum Dots. J. Lumin. 2006, 119, 214–218. [Google Scholar] [CrossRef]
- Lewis, J.; Wu, S.; Jiang, X. Unconventional Gap State of Trapped Exciton in Lead Sulfide Quantum Dots. Nanotechnology 2010, 21, 455402. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Heo, J.; Zhang, X.; Adam, J. Photoluminescence of PbS Quantum Dots Embedded in Glasses. J. Non-Cryst. Solids 2008, 354, 618–623. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, H.; Vidal, F.; Rosei, F.; Vomiero, A.; Ma, D. Size Dependence of Temperature-Related Optical Properties of PbS and PbS/CdS Core/Shell Quantum Dots. J. Phys. Chem. C 2014, 118, 20585–20593. [Google Scholar] [CrossRef]
- LitLitvyak, V.; Cherbunin, R.; Onushchenko, A. Temperature Dependence of the Optical Transitions of PbS Quantum Dots in Silicate Glasses. Bull. Russ. Acad. Sci. Phys. 2017, 81, 1490–1492. [Google Scholar] [CrossRef]
- Devreese, J.; Fomin, V.; Gladilin, V.; Klimin, S. Photoluminescence Spectra of Quantum Dots: Enhanced Efficiency of the Electron-Phonon Interaction. Phys. Status Solidi (B) 2001, 224, 609–612. [Google Scholar] [CrossRef]
- Magaryan, K.; Karimullin, K.; Vasil’eva, I.; Naumov, A. Analysis of the Temperature Dependence of the Exciton Luminescence Spectra of Cadmium Selenide Quantum Dots Grown in a Liquid Crystal Matrix. Opt. Spectrosc. 2019, 126, 41–43. [Google Scholar] [CrossRef]
- Narayanaswamy, A.; Feiner, L.; Meijerink, A.; van der Zaag, P.J. The Effect of Temperature and Dot Size on the Spectral Properties of Colloidal InP/ZnS Core−Shell Quantum Dots. ACS Nano 2009, 3, 2539–2546. [Google Scholar] [CrossRef]
- Karimullin, K.; Arzhanov, A.; Eremchev, I.; Kulnitskiy, B.; Surovtsev, N.; Naumov, A. Combined Photon-Echo, Luminescence and Raman Spectroscopies of Layered Ensembles of Colloidal Quantum Dots. Laser Phys. 2019, 29, 124009. [Google Scholar] [CrossRef]
- Ullrich, B.; Wang, J. Impact of Laser Excitation Variations on the Photoluminescence of Pbs Quantum Dots on GaAs. J. Lumin. 2013, 143, 645–648. [Google Scholar] [CrossRef]
- Olkhovets, A.; Hsu, R.; Lipovskii, A.; Wise, F. Size-Dependent Temperature Variation of the Energy Gap in Lead-Salt Quantum Dots. Phys. Rev. Lett. 1998, 81, 3539–3542. [Google Scholar] [CrossRef]
- Turyanska, L.; Patanè, A.; Henini, M.; Hennequin, B.; Thomas, N. Temperature Dependence of the Photoluminescence Emission from Thiol-Capped PbS Quantum Dots. Appl. Phys. Lett. 2007, 90, 101913. [Google Scholar] [CrossRef] [Green Version]
- Jing, P.; Zheng, J.; Ikezawa, M.; Liu, X.; Lv, S.; Kong, X.; Zhao, J.; Masumoto, Y. Temperature-Dependent Photoluminescence of CdSe-Core CdS/CdZnS/ZnS-Multishell Quantum Dots. J. Phys. Chem. C 2009, 113, 13545–13550. [Google Scholar] [CrossRef]
- Xu, S.J.; Li, G.Q.; Wang, Y.J.; Zhao, Y.; Chen, G.H.; Zhao, D.G.; Zhu, J.J.; Yang, H.; Yu, D.P.; Wang, J.N. Quantum Dissipation and Broadening Mechanisms Due to Electron-Phonon Interactions in Self-Formed InGaN Quantum Dots. Appl. Phys. Lett. 2006, 88, 083123. [Google Scholar] [CrossRef] [Green Version]
- Leifer, K.; Pelucchi, E.; Watanabe, S.; Michelini, F.; Dwir, B.; Kapon, E. Narrow (≈4 meV) Inhomogeneous Broadening and Its Correlation with Confinement Potential of Pyramidal Quantum Dot Arrays. Appl. Phys. Lett. 2007, 91, 081106. [Google Scholar] [CrossRef]
- Gaponenko, M.; Lutich, A.; Tolstik, N.; Onushchenko, A.; Malyarevich, A.; Petrov, E.; Yumashev, K. Temperature-Dependent Photoluminescence of PbS Quantum Dots in Glass: Evidence of Exciton State Splitting and Carrier Trapping. Phys. Rev. B 2010, 82, 125320. [Google Scholar] [CrossRef]
- Gomes, P.F.; Godoy, M.P.F.; Veloso, A.B.; Nakaema, M.K.K.; Iikawa, F.; Brasil, M.J.S.P.; Bortoleto, J.R.R.; Cotta, M.A.; Madureira, J.R. Exciton Binding Energy in Type II Quantum Dots. Phys. Status Solidi (C) 2007, 4, 385–388. [Google Scholar] [CrossRef]
- Zhang, J.; Tolentino, J.; Smith, E.R.; Zhang, J.; Beard, M.C.; Nozik, A.J.; Law, M.; Johnson, J.C. Carrier Transport in PbS and PbSe QD Films Measured by Photoluminescence Quenching. J. Phys. Chem. C 2014, 118, 16228–16235. [Google Scholar] [CrossRef]
- Morello, G.; De Giorgi, M.; Kudera, S.; Manna, L.; Cingolani, R.; Anni, M. Temperature and Size Dependence of Nonradiative Relaxation and Exciton−Phonon Coupling in Colloidal CdTe Quantum Dots. J. Phys. Chem. C 2007, 111, 5846–5849. [Google Scholar] [CrossRef]
- Jagtap, A.; Khatei, J.; Koteswara Rao, K. Exciton–Phonon Scattering and Nonradiative Relaxation of Excited Carriers in Hydrothermally Synthesized CdTe Quantum Dots. Phys. Chem. Chem. Phys. 2015, 17, 27579–27587. [Google Scholar] [CrossRef]
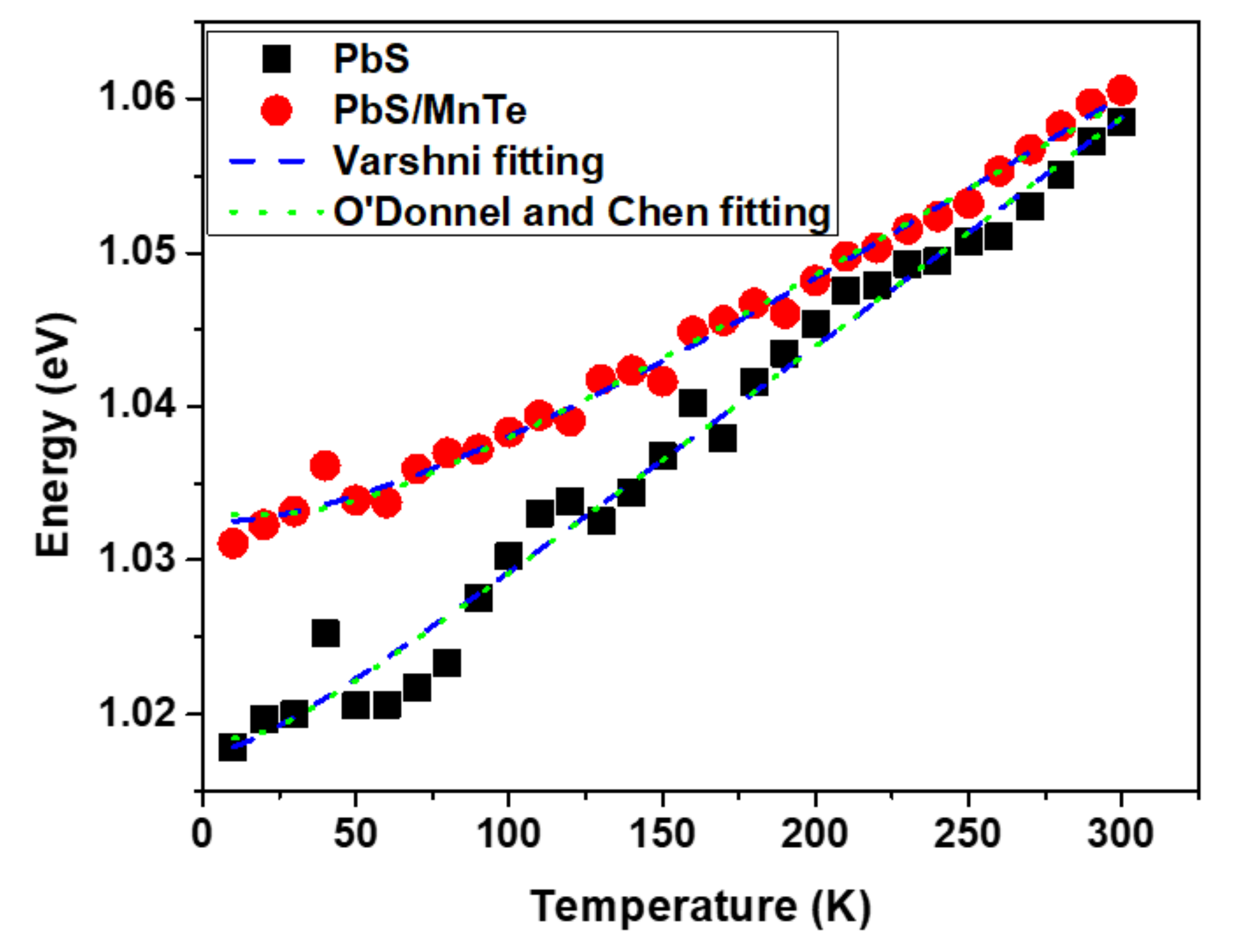
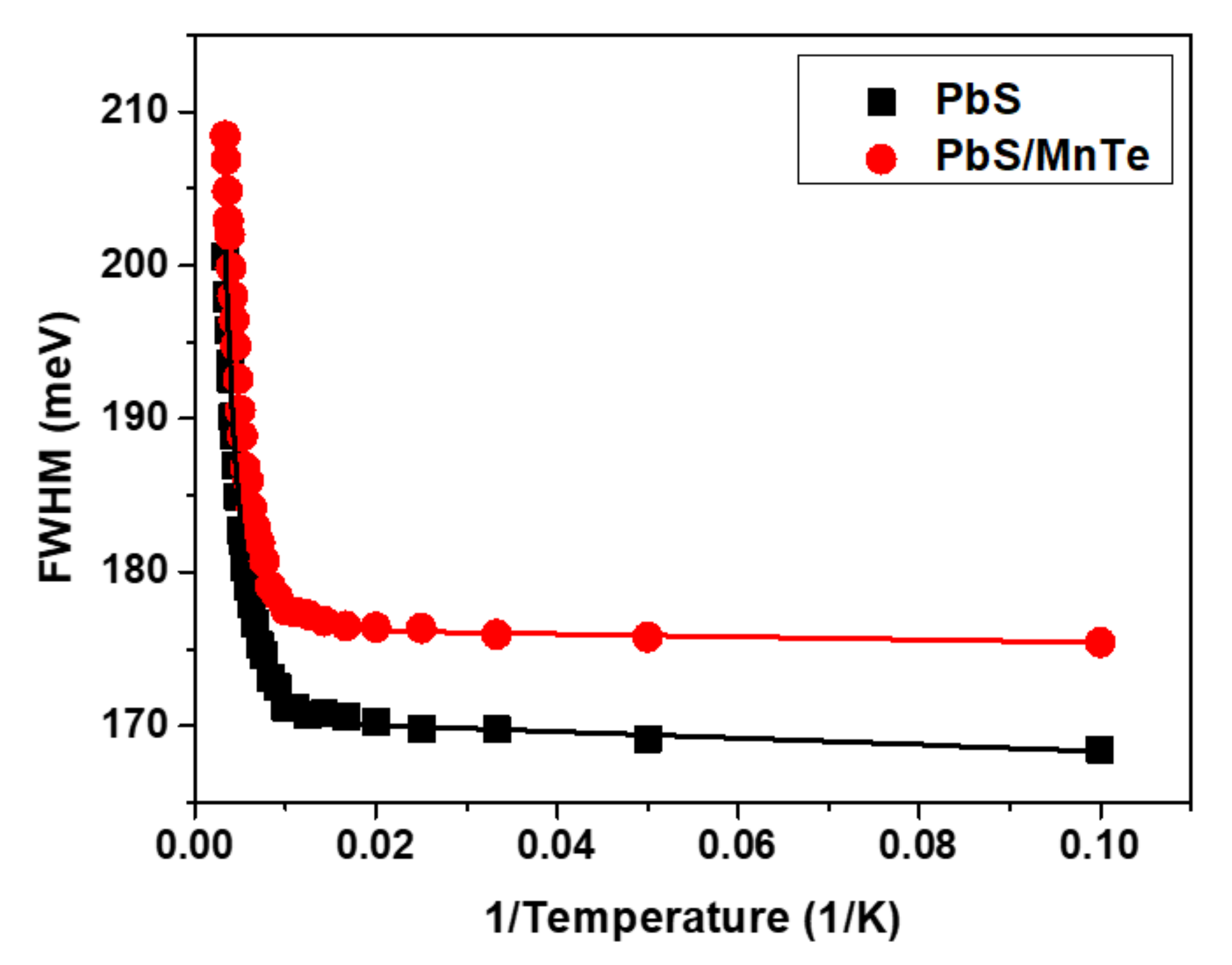
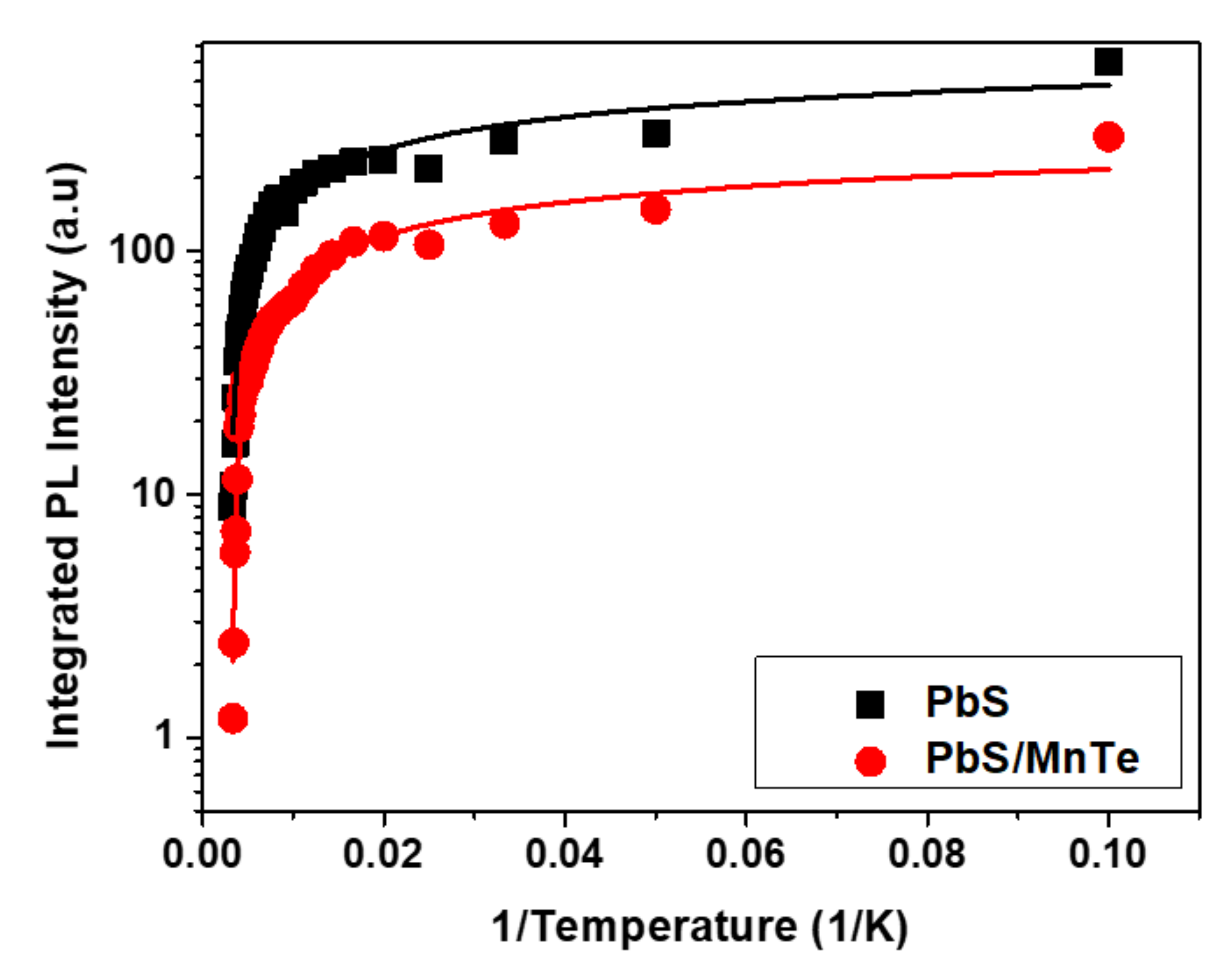
| Samples | |||
|---|---|---|---|
| PbS | 168.94 ± 0.64 | 113.93 ± 1.54 | 89.33 ± 1.06 |
| PbS/MnTe | 174.86 ± 0.58 | 117.53 ± 1.63 | 91.16 ± 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halim, N.D.; Zaini, M.S.; Talib, Z.A.; Liew, J.Y.C.; Kamarudin, M.A. Study of the Electron-Phonon Coupling in PbS/MnTe Quantum Dots Based on Temperature-Dependent Photoluminescence. Micromachines 2022, 13, 443. https://doi.org/10.3390/mi13030443
Halim ND, Zaini MS, Talib ZA, Liew JYC, Kamarudin MA. Study of the Electron-Phonon Coupling in PbS/MnTe Quantum Dots Based on Temperature-Dependent Photoluminescence. Micromachines. 2022; 13(3):443. https://doi.org/10.3390/mi13030443
Chicago/Turabian StyleHalim, Nur Diyana, Muhammad Safwan Zaini, Zainal Abidin Talib, Josephine Ying Chyi Liew, and Mazliana Ahmad Kamarudin. 2022. "Study of the Electron-Phonon Coupling in PbS/MnTe Quantum Dots Based on Temperature-Dependent Photoluminescence" Micromachines 13, no. 3: 443. https://doi.org/10.3390/mi13030443
APA StyleHalim, N. D., Zaini, M. S., Talib, Z. A., Liew, J. Y. C., & Kamarudin, M. A. (2022). Study of the Electron-Phonon Coupling in PbS/MnTe Quantum Dots Based on Temperature-Dependent Photoluminescence. Micromachines, 13(3), 443. https://doi.org/10.3390/mi13030443