Azo-Dye-Functionalized Polycarbonate Membranes for Textile Dye and Nitrate Ion Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gold Nanoparticle Synthesis
2.3. Stamping
2.4. Anionic Direct Azo-Dye-Functionalization Process
2.5. Rejection and Flow Rate Measurements
2.6. Water Flow Rate Characterization of the Unmodified Polycarbonate Filters
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hovers, E.; Shimon, I.; Bar-Yosef, O.; Vandermeersch, B. An Early Case of Color Symbolism: Ochre Use by Modern Humans in Qafzeh Cave. Curr. Anthropol. 2003, 44, 491–522. [Google Scholar] [CrossRef] [Green Version]
- Cardon, D. Natural dyes, our global heritage of colours. In Textiles and Settlement: From Plains Space to Cyber Space, Textile Society of America Symposium, Proceedings of the12th Biennial Symposium, Lincoln, NE, USA; Textile Society of America: Berkeley, CA, USA, 2010. [Google Scholar]
- Kanetkar, V.R. Colour: History and advancements. Resonance 2010, 15, 794–803. [Google Scholar] [CrossRef]
- Henshilwood, C.S.; D’Errico, F.; van Niekerk, K.L.; Coquinot, Y.; Jacobs, Z.; Lauritzen, S.-E.; Menu, M.; García-Moreno, R. A 100,000-Year-Old Ochre-Processing Workshop at Blombos Cave, South Africa. Science 2011, 334, 219–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.-C. A cross-cultural analysis of symbolic meanings of color. Chang Gung J. Humanit. Soc. Sci. 2014, 7, 49–74. [Google Scholar]
- Chequer, F.M.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Cardoso, J.C.; Zanoni, M.V.B.; de Oliveira, D.P. Textile Dyes: Dyeing Process and Environmental Impact. In Eco-Friendly Textile Dyeing and Finishing; Gunay, M., Ed.; IntechOpen—Open Access Books: London, UK, 2013. [Google Scholar]
- Christie, R.M. Colour Chemistry, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S.; Kumar, N.A. Azodyes as markers for tumor hypoxia imaging and therapy: An up-to-date review. Chem. Interact. 2019, 307, 91–104. [Google Scholar] [CrossRef]
- Nasim, K.; Digvijaysinh, P.K.; Debasis, D. Recent Applications of Azo Dyes: A Paradigm Shift from Medicinal Chemistry to Biomedical Sciences. Mini-Rev. Med. Chem. 2021, 21, 1071–1084. [Google Scholar]
- Germinario, G.; D’Ambrosio, G.S.V.; van der Werf, I.D.; Sabbatini, L. Chemical composition of felt-tip pen inks. Anal. Bioanal. Chem. 2018, 410, 1079–1094. [Google Scholar] [CrossRef]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Analysis of Dyes in Cosmetics: Challenges and Recent Developments. Cosmetics 2018, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Manayi, A.; Saeidnia, S. Cosmetics and Personal Care Products. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 1043–1049. [Google Scholar]
- Li, Y.; Yang, Y.; Yin, S.; Zhou, C.; Ren, D. Inedible azo dyes and their analytical methods in foodstuffs and beverages. J. AOAC Int. 2018, 101, 1314–1327. [Google Scholar] [CrossRef] [Green Version]
- Wall, R.; Crawley, H. Artificial Food Colours and Children: Why We Want to Limit and Label Foods Containing the ‘Southampton Six’ Food Colours on the UK Market Post-Brexit; First Steps Nutrition Trust: London, UK, 2020. [Google Scholar]
- Hunger, K. (Ed.) Cationic Dyes for Paper, Leather, and Other Substrates. In Industrial Dyes: Chemistry, Properties, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 53–55. [Google Scholar]
- Gičević, A.; Hindija, L.; Karačić, A. Toxicity of azo dyes in pharmaceutical industry. In International Conference on Medical and Biological Engineering; IFMBE Proceedings; Springer: Cham, Switzerland, 2019; pp. 581–587. [Google Scholar]
- Brown, D. Effects of colorants in the aquatic environment. Ecotoxicol. Environ. Saf. 1987, 13, 139–147. [Google Scholar] [CrossRef]
- Growther, L.; Meenakshi, M. Biotechnological Approaches to Combat Textile Effluents. Internet J. Microbiol. 2008, 7, 1–7. [Google Scholar]
- Lewis, D.M. Coloration in the next century. Rev. Prog. Coloration Relat. Top. 1999, 23, 23–28. [Google Scholar] [CrossRef]
- Laing, I.G. The impact of effluent regulations on the dyeing industry. Rev. Prog. Coloration Relat. Top. 1991, 21, 56–71. [Google Scholar] [CrossRef]
- Maas, R.; Chaudhari, S. Adsorption and biological decolourization of azo dye Reactive Red 2 in semicontinuous anaerobic reactors. Process Biochem. 2005, 40, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Muthu, S.S. Dye Biodegradation, Mechanisms and Techniques: Recent Advances; Springer: Singapore, 2021; pp. 1–50. [Google Scholar]
- Garcia, V.S.; Rosa, J.M.; Borrely, S.I. Toxicity and color reduction of a textile effluent containing reactive red 239 dye by electron beam irradiation. Radiat. Phys. Chem. 2020, 172, 108765. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Environmental Impacts of the Textile Industry and Its Assessment through Life Cycle Assessment. In Roadmap to Sustainable Textiles and Clothing—Environmental and Social Aspects of Textiles and Clothing Supply Chain; Springer: Singapore, 2014; pp. 1–9. [Google Scholar]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–371. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; Elharfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Rajaguru, P.; Fairbairn, L.; Ashby, J.; Willington, M.; Turner, S.; Woolford, L.; Chinnasamy, N.; Rafferty, J. Genotoxicity studies on the azo dye Direct Red 2 using the in vivo mouse bone marrow micronucleus test. Mutat. Res. Toxicol. Environ. Mutagen. 1999, 444, 175–180. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cerniglia, C.E.; Chen, H. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front. Biosci. (Elite Ed.) 2012, 4, 568–586. [Google Scholar] [CrossRef]
- Chequer, F.M.; Dorta, D.J.; de Oliveira, D.P. Azo dyes and their metabolites: Does the discharge of the azo dye into water bodies represent human and ecological risks. Adv. Treat. Text. Effl. 2011, 48, 28–48. [Google Scholar]
- Bae, J.-S.; Freeman, H.S.; Kim, S.D. Influences of new azo dyes to the aquatic ecosystem. Fibers Polym. 2006, 7, 30–35. [Google Scholar] [CrossRef]
- Oliveira, D.P.; Carneiro, P.A.; Rech, C.M.; Zanoni, M.V.; Claxton, L.D.; Umbuzeiro, G.A. Mutagenic compounds generated from the chlorination of disperse azo-dyes and their presence in drinking water. Environ. Sci. Technol. 2006, 40, 6682–6689. [Google Scholar] [CrossRef]
- Tang, A.Y.L.; Lo, C.K.Y.; Kan, C.W. Textile dyes and human health: A systematic and citation network analysis review. Coloration Technol. 2018, 134, 245–257. [Google Scholar] [CrossRef]
- Hessel, C.; Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Guidelines and legislation for dye house effluents. J. Environ. Manag. 2007, 83, 171–180. [Google Scholar] [CrossRef]
- Pal, H. Environmental Issues in Textiles: Global Regulations, Restrictions and Research. In Detox Fashion; Textile Science and Clothing, Technology; Muthu, S.S., Ed.; Springer: Singapore, 2017; pp. 27–45. [Google Scholar]
- Saratale, R.; Saratale, G.D.; Chang, J.; Govindwar, S. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Kunz, A.; Peralta-Zamora, P.; de Moraes, S.G.; Durán, N. New trends in the treatment of textile effluents. Química Nova 2002, 25, 78–82. [Google Scholar] [CrossRef]
- Rápó, E.; Tonk, S. Factors Affecting Synthetic Dye Adsorption; Desorption Studies: A Review of Results from the Last Five Years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef] [PubMed]
- Orts, F.; del Río, A.I.; Molina, J.; Bonastre, J.; Cases, F. Electrochemical treatment of real textile wastewater: Trichromy Procion HEXL®. J. Electroanal. Chem. 2018, 808, 387–394. [Google Scholar] [CrossRef]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.; Marchant, R.; Smyth, W.F. Microbial decolourisation and degradation of textile dyes. Appl. Microbiol. Biotechnol. 2001, 56, 81–87. [Google Scholar] [CrossRef]
- Kikuchi, T.; Moriwaki, H.; Nakanishi, H.; Kondo, H.; Sasaki, T.; Imada, G.; Harada, N. Reaction of congo red in water after irradiation by pulsed intense relativistic electron beam. Plasma Fusion Res. 2011, 6, 1206021. [Google Scholar] [CrossRef] [Green Version]
- Anjaneyulu, Y.; Chary, N.S.; Raj, D.S.S. Decolourization of industrial effluents—Available methods and emerging. Rev. Environ. Sci. Bio/Technol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Slama, H.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Alghasi, A.; Porkar, G.; Jaafari, J. Using Medium Pressure Ultraviolet Reactor for Removing Azo Dyes in Textile Wastewater Treatment Plant. World Appl. Sci. J. 2011, 12, 797–802. [Google Scholar]
- Covi, M.A., Jr.; dos Santos, E.A.G.; de Paulo Silva, G.F.; Maimoni, H.N.; Martins, M.L.; de Moura Assis, C.A.; Camargo, C.M.; Camargo, M.A.F. Advances in the Treatment of Textile Effluents: A Review. Open Access Libr. J. 2019, 6, e5549. [Google Scholar]
- Benkhaya, S.; Lgaz, H.; Alrashdi, A.; M’rabet, S.; EL Bachiri, A.; Assouag, M.; Chung, I.; EL Harfi, A. Upgrading the performances of polysulfone/polyetherimide ultrafiltration composite membranes for dyes removal: Experimental and molecular dynamics studies. J. Mol. Liq. 2021, 331, 115743. [Google Scholar] [CrossRef]
- Said, B.; M’Rabet, S.; Hsissou, R.; El Harfi, A. Synthesis of new low-cost organic ultrafiltration membrane made from Polysulfone/Polyetherimide blends and its application for soluble azoic dyes removal. J. Mater. Res. Technol. 2020, 9, 4763–4772. [Google Scholar] [CrossRef]
- Benkhaya, S.; Achiou, B.; Ouammou, M.; Bennazha, J.; Younssi, S.A.; M’Rabet, S.; Elharfi, A. Preparation of low-cost composite membrane made of polysulfone/polyetherimide ultrafiltration layer and ceramic pozzolan support for dyes removal. Mater. Today Commun. 2019, 19, 212–219. [Google Scholar] [CrossRef]
- Benkhaya, S.; Lgaz, H.; Chraibi, S.; Alrashdi, A.; Rafik, M.; Lee, H.; EL Harfi, A. Polysulfone/Polyetherimide Ultrafiltration composite membranes constructed on a three-component Nylon-fiberglass-Nylon support for azo dyes removal: Experimental and molecular dynamics simulations. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126941. [Google Scholar] [CrossRef]
- Kamaz, M.; Rocha, P.; Sengupta, A.; Qian, X.; Wickramasinghe, R.S. Efficient removal of chemically toxic dyes using microorganism from activated sludge: Understanding sorption mechanism, kinetics, and associated thermodynamics. Sep. Sci. Technol. 2018, 53, 1760–1776. [Google Scholar] [CrossRef]
- Paździor, K.; Klepacz-Smółka, A.; Ledakowicz, S.; Sójka-Ledakowicz, J.; Mrozińska, Z.; Żyłła, R. Integration of nanofiltration and biological degradation of textile. Chemosphere 2009, 75, 250–255. [Google Scholar] [CrossRef]
- Sarasa, J.; Roche, M.; Ormad, M.; Gimeno, E.; Puig, A.; Ovelleiro, J. Treatment of a wastewater resulting from dyes manufacturing with ozone and chemical coagulation. Water Res. 1998, 32, 2721–2727. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M. Novel trends in AOPs for textile wastewater treatment. Enhanced dye by-products removal by catalytic and synergistic actions. Water Resour. Ind. 2021, 26, 100160. [Google Scholar] [CrossRef]
- Benkhaya, S.; el Harfi, S.; el Harfi, A. Classifications, properties and applications of textile dyes: A review. Appl. J. Environ. Eng. Sci. 2017, 3, 311–320. [Google Scholar]
- Gregory, P. Classification of Dyes by Chemical Structure. In The Chemistry and Application of Dyes; Waring, D.R., Hallas, G., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 17–47. [Google Scholar]
- Benkhaya, S.; M’Rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [Green Version]
- Shaul, G.M.; Holdsworth, T.J.; Dempsey, C.R.; Dostal, K.A. Fate of water soluble azo dyes in the activated sludge process. Chemosphere 1991, 22, 107–119. [Google Scholar] [CrossRef]
- Mussak, R.A.M.; Bechtold, T. Natural Colorants in Textile Dyeing. In Handbook of Natural Colorants; Bechtold, T., Mussak, R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 315–335. [Google Scholar]
- O’Neill, C.; Hawkes, F.R.; Hawkes, D.L.; Lourenco, N.D.; Pinheiro, H.M.H.M. Colour in textile effluents—Sources, measurement, discharge consents and simulation: A review. J. Chem. Technol. Biotechnol. 1999, 74, 1009–1018. [Google Scholar] [CrossRef]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Bick, R.; Halsey, E.; Ekenga, C.C. The global environmental injustice of fast fashion. Environ. Health 2018, 17, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgerding, A.J.; Hites, R.A. Identification and Measurement of Food and Cosmetic Dyes in a Municipal Wastewater Treatment Plant. Environ. Sci. Technol. 1994, 28, 1278–1284. [Google Scholar] [CrossRef]
- Gebrati, L.; EL Achaby, M.; Chatoui, H.; Laqbaqbi, M.; El Kharraz, J.; Aziz, F. Inhibiting effect of textile wastewater on the activity of sludge from the biological treatment process of the activated sludge plant. Saudi J. Biol. Sci. 2019, 26, 1753–1757. [Google Scholar] [CrossRef]
- Oliveira, G.A.R.; Ferraz, E.R.A.; Chequer, F.M.D.; Grando, M.D.; Angeli, J.P.F.; Tsuboy, M.S.; Marcarini, J.C.; Mantovani, M.S.; Osugi, M.E.; Lizier, T.M.; et al. Chlorination treatment of aqueous samples reduces, but does not eliminate, the mutagenic effect of the azo dyes Disperse Red 1, Disperse Red 13 and Disperse Orange 1. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010, 703, 200–208. [Google Scholar] [CrossRef]
- Vacchi, F.I.; Albuquerque, A.F.; Vendemiatti, J.A.; Morales, D.A.; Ormond, A.B.; Freeman, H.S.; Zocolo, G.J.; Zanoni, M.V.B.; Umbuzeiro, G. Chlorine disinfection of dye wastewater: Implications for a commercial azo dye mixture. Sci. Total Environ. 2012, 442, 302–309. [Google Scholar] [CrossRef]
- Abidin, C.Z.; Fahmi, M.R.; Soon-An, O.; Makhtar, S.N.; Rahmat, N.R. Decolourization of an azo dye in aqueous solution by ozonation in a semi-batch bubble column reactor. Sci. Asia 2015, 41, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.-G.; Gu, J.-D. Physiology and biochemistry of reduction of azo compounds by Shewanella strains relevant to electron transport chain. Appl. Microbiol. Biotechnol. 2010, 83, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Jonstrup, M.; Kumar, N.; Murto, M.; Mattiasson, B. Sequential anaerobic–aerobic treatment of azo dyes: Decolourisation and amine degradability. Desalination 2011, 280, 339–346. [Google Scholar] [CrossRef]
- Pinheiro, H.; Touraud, E.; Thomas, O. Aromatic amines from azo dye reduction: Status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dye. Pigment. 2004, 61, 121–139. [Google Scholar] [CrossRef]
- World Population Prospects 2019: Highlights; United Nations, Department of Economic and Social Affairs: New York, NY, USA, 2019.
- Oki, T.; Kanae, S. Global Hydrological Cycles and World Water Resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiklomanov, I.A. World fresh water resources. In Water in Crisis: A Guide to the World’s Freash Water Resources; Gleick, P.H., Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 1993; pp. 13–24. [Google Scholar]
- Jury, W.A.; Vaux, H.J. The Emerging Global Water Crisis: Managing Scarcity and Conflict between Water Users. Adv. Agron. 2007, 95, 1–76. [Google Scholar]
- du Plessis, A. Current and Future Water Scarcity and Stress. In Water as an Inescapable Risk; In Springer Water; Springer: Cham, Switzerland, 2019. [Google Scholar]
- He, J.; Lin, X.-M.; Chan, H.; Vuković, L.; Král, P.; Jaeger, H.M. Diffusion and Filtration Properties of Self-Assembled Gold Nanocrystal Membranes. Nano Lett. 2011, 11, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Mcbride, S.P.; Jaeger, H.M.; Lin, X.-M. Ion transport controlled by nanoparticle functionalized membranes. Nat. Commun. 2014, 5, 5847. [Google Scholar] [CrossRef] [Green Version]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Kidambi, P.R.; Mariappan, D.D.; Dee, N.T.; Vyatskikh, A.; Zhang, S.; Karnik, R.; Hart, A.J. A scalable route to nanoporous large-area atomically thin graphene membranes by roll-to-roll chemical vapor deposition and polymer support casting. ACS Appl. Mater. Interfaces 2018, 10, 10369–10378. [Google Scholar] [CrossRef] [Green Version]
- Thebo, K.H.; Qian, X.; Zhang, Q.; Chen, L.; Cheng, H.M.; Ren, W. Highly stable graphene-oxide-based membranes with superior permeability. Nat. Commun. 2018, 9, 1486. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, S.; Hou, D.; Duan, H.; Deng, B.; Zeng, Z.; Liu, B.; Sun, L.; Song, R.; Du, J.; et al. Tunable Pore Size from Sub-Nanometer to a Few Nanometers in Large-Area Graphene Nanoporous Atomically Thin Membranes. ACS Appl. Mater. Interfaces 2021, 13, 29926–29935. [Google Scholar] [CrossRef]
- Jang, D.; Idrobo, J.; Laoui, T.; Karnik, R. Water and solute transport governed by tunable pore size distributions in nanoporous graphene membranes. ACS Nano 2017, 11, 10042–10052. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Y.; Ren, Z.F. Physics and applications of aligned carbon nanotubes. Adv. Phys. 2011, 60, 553–678. [Google Scholar] [CrossRef]
- van Rijn, P.; Tutus, M.; Kathrein, C.; Zhu, L.; Wessling, M.; Schwaneberg, U.; Böker, A. Challenges and advances in the field of self-assembled membranes. Chem. Soc. Rev. 2013, 42, 6578–6592. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, M.S.; Nagendran, A.; Rana, D. Tailored polymer nanocomposite membranes based on carbon, metal oxide and silicon nanomaterials: A review. J. Mater. Chem. A 2019, 7, 8723–8745. [Google Scholar] [CrossRef]
- Punia, P.; Bharti, M.K.; Chalia, S.; Dhar, R.; Ravelo, B.; Thakur, P.; Thakur, A. Recent advances in synthesis, characterization, and applications of nanoparticles for contaminated water treatment—A review. Ceram. Int. 2021, 47, 1526–1550. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, K.; Chen, B. Membranes prepared from graphene-based nanomaterials for sustainable applications: A review. Environ. Sci. Nano 2017, 4, 2267–2285. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Lin, S. Pore model for nanofiltration: History, theoretical framework, key predictions, limitations, and prospects. J. Membr. Sci. 2021, 118809, 620. [Google Scholar] [CrossRef]
- Lin, X.M.; Sorensen, C.M.; Klabunde, K.J. Ligand-Induced Gold Nanocrystal Superlattice Formation in Colloidal Solution. Chem. Mater. 1999, 11, 198–202. [Google Scholar] [CrossRef]
- Lin, X.M.; Sorensen, C.; Klabunde, K.J. Digestive Ripening, Nanophase Segregation and Superlattice Formation in Gold Nanocrystal Colloids. J. Nanopart. Res. 2000, 2, 157–164. [Google Scholar] [CrossRef]
- Lin, M.-L.; Yang, F.; Lee, S. Digestive ripening for self-assembly of thiol-capped gold nanoparticles: The effects of adding dodecanethiol and reflux-heating. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 448, 16–22. [Google Scholar] [CrossRef]
- Motte, L.; Billoudet, F.; Pileni, M.P. Self-Assembled Monolayer of Nanosized Particles Differing by Their Sizes. J. Phys. Chem. 1995, 99, 16425–16429. [Google Scholar] [CrossRef]
- Bigioni, T.P.; Lin, X.M.; Nguyen, T.T.; Corwin, E.I.; Witten, T.A.; Jaeger, H.M. Kinetically driven self assembly of highly ordered nanoparticle monolayers. Nat. Mater. 2006, 5, 265–270. [Google Scholar] [CrossRef]
- Santhanam, V.; Liu, J.; Agarwal, R.; Andres, R.P. Self-Assembly of Uniform Monolayer Arrays of Nanoparticles. Langmuir 2003, 19, 7881–7887. [Google Scholar] [CrossRef]
- Tezcanli-Güyer, G.; Ince, N. Individual and combined effects of ultrasound, ozone and UV irradiation: A case study with textile dyes. Ultrasonics 2004, 42, 603–609. [Google Scholar] [CrossRef]
- Schaep, J.; Van der Bruggen, B.; Vandecasteele, C.; Wilms, D. Influence of ion size and charge in nanofiltration. Sep. Purif. Technol. 1998, 14, 155–162. [Google Scholar] [CrossRef]
- Cirillo, A.; Tomaiuolo, G.; Guido, S. Membrane Fouling Phenomena in Microfluidic Systems: From Technical Challenges to Scientific Opportunities. Micromachines 2021, 12, 820. [Google Scholar] [CrossRef]
- Apel, P. Track etching technique in membrane technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Kaniukov, E.Y.; Shumskaya, E.E.; Yakimchuk, D.V.; Kozlovskiy, A.L.; Ibragimova, M.A.; Zdorovets, M.V. Evolution of the polyethylene terephthalate track membranes parameters at the etching process. J. Contemp. Phys. (Armen. Acad. Sci.) 2017, 52, 155–160. [Google Scholar] [CrossRef]
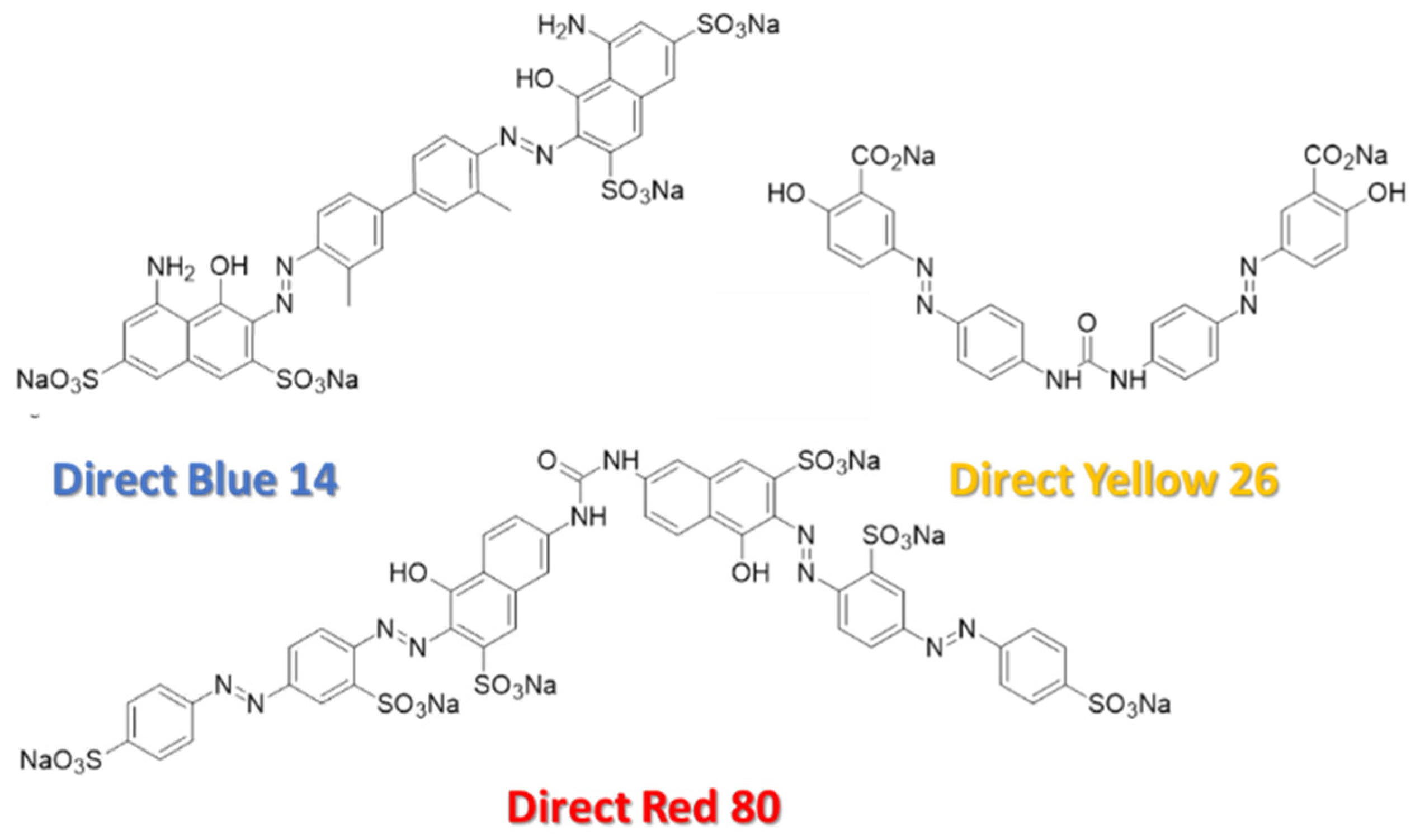
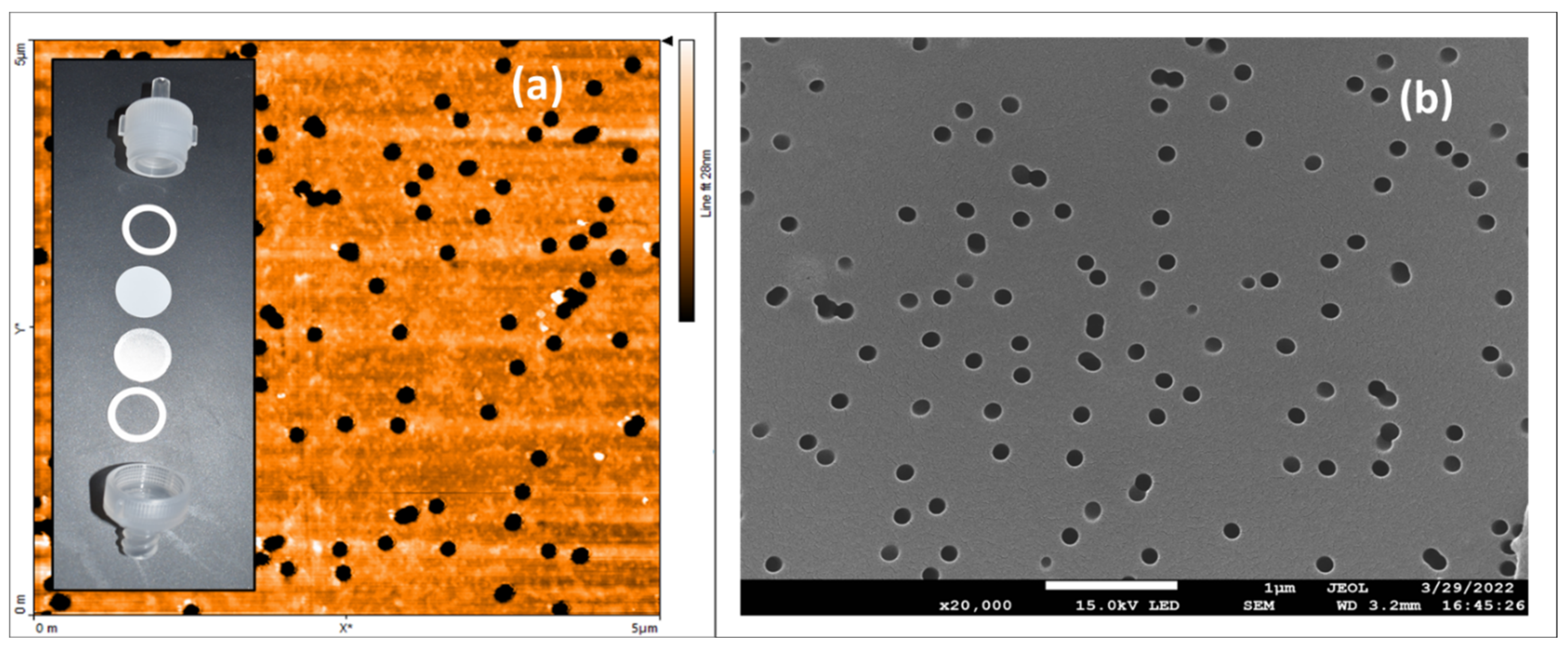
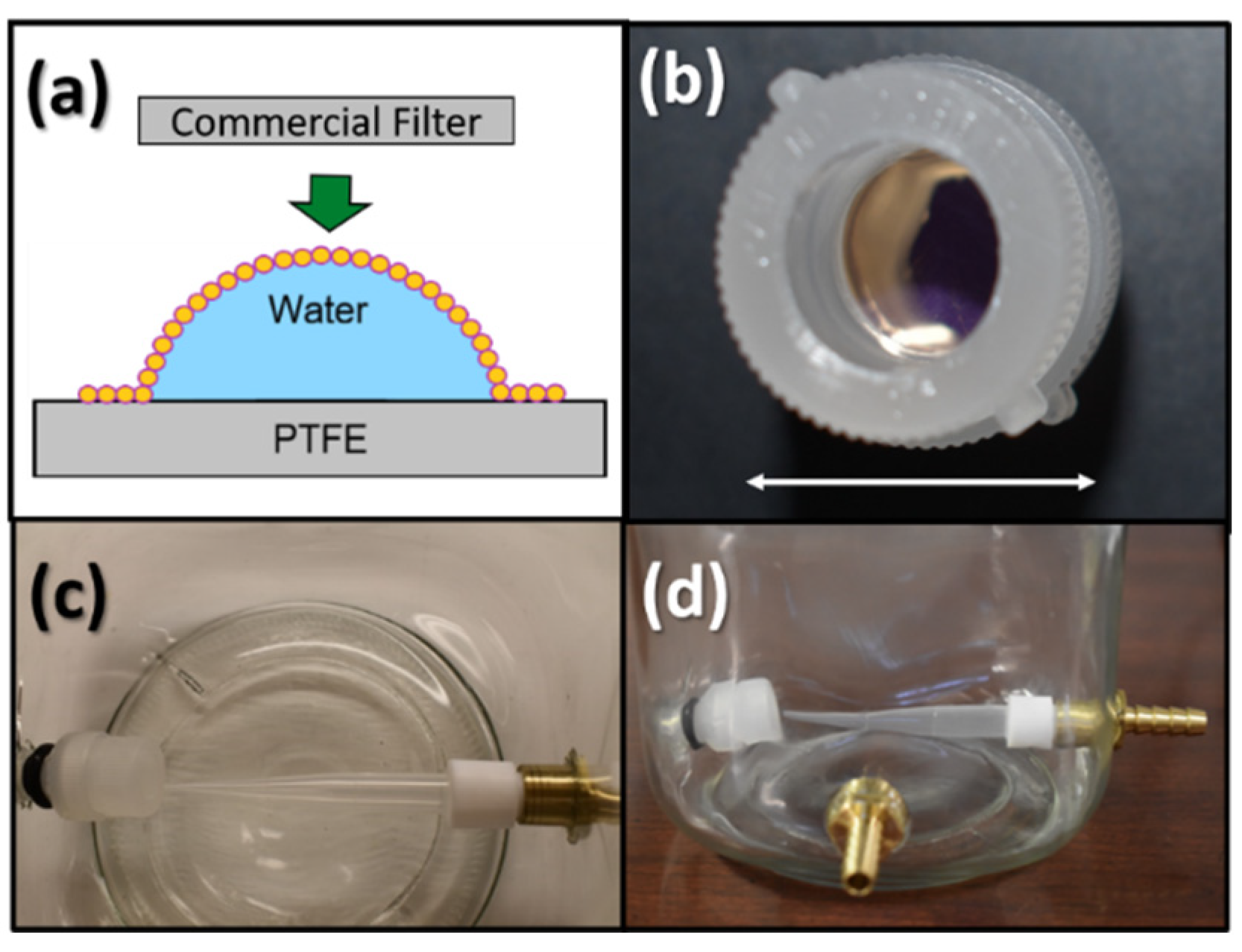
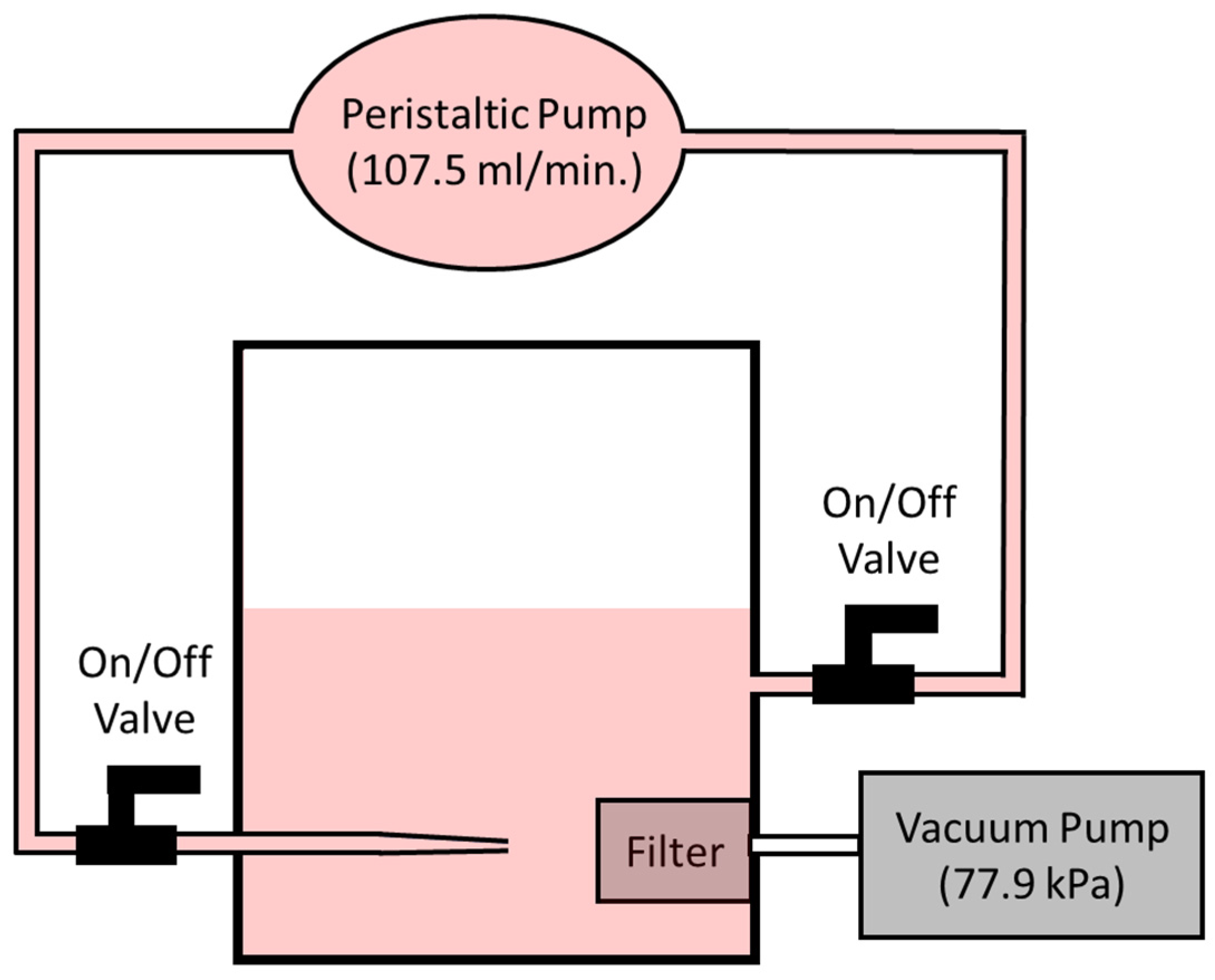
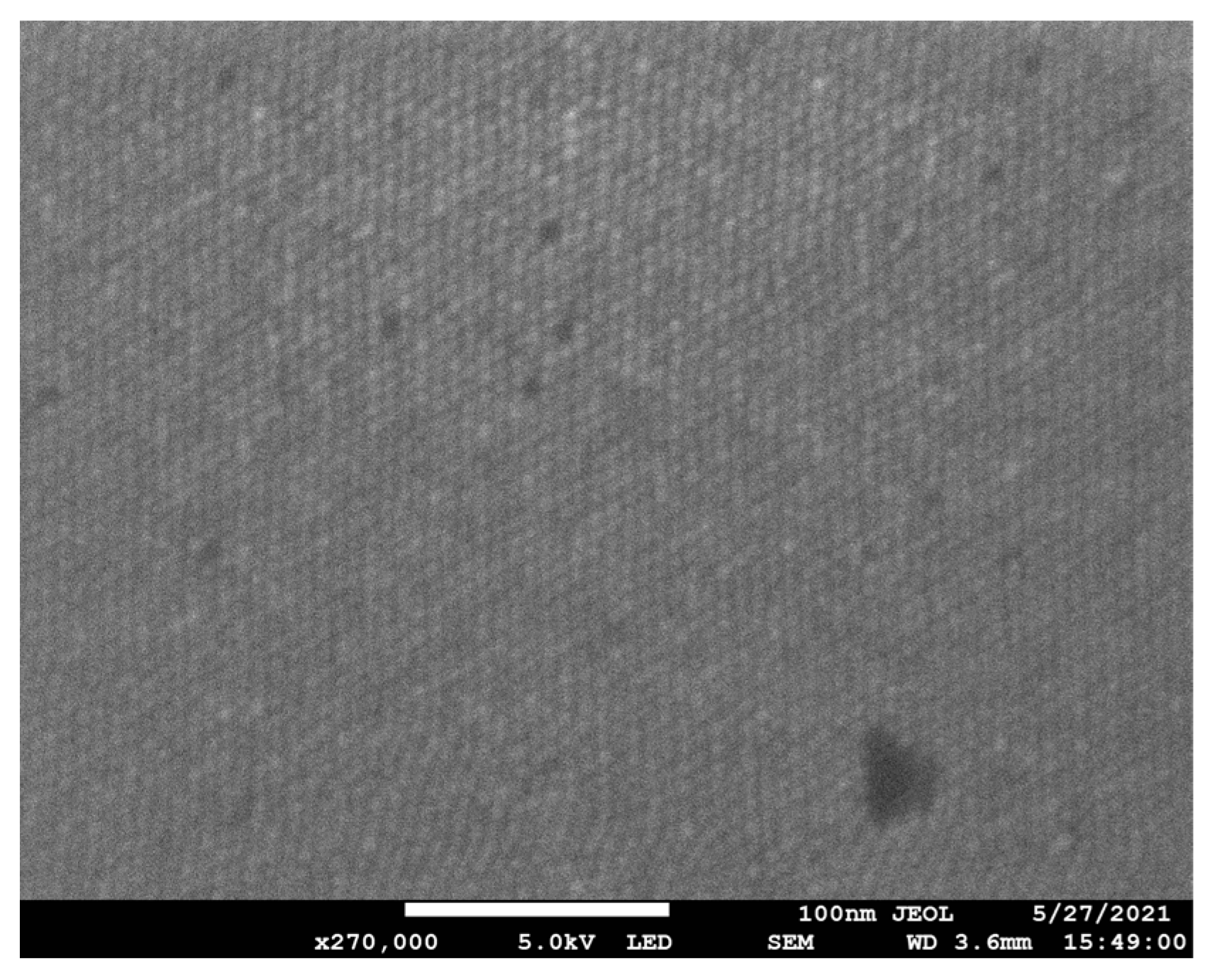
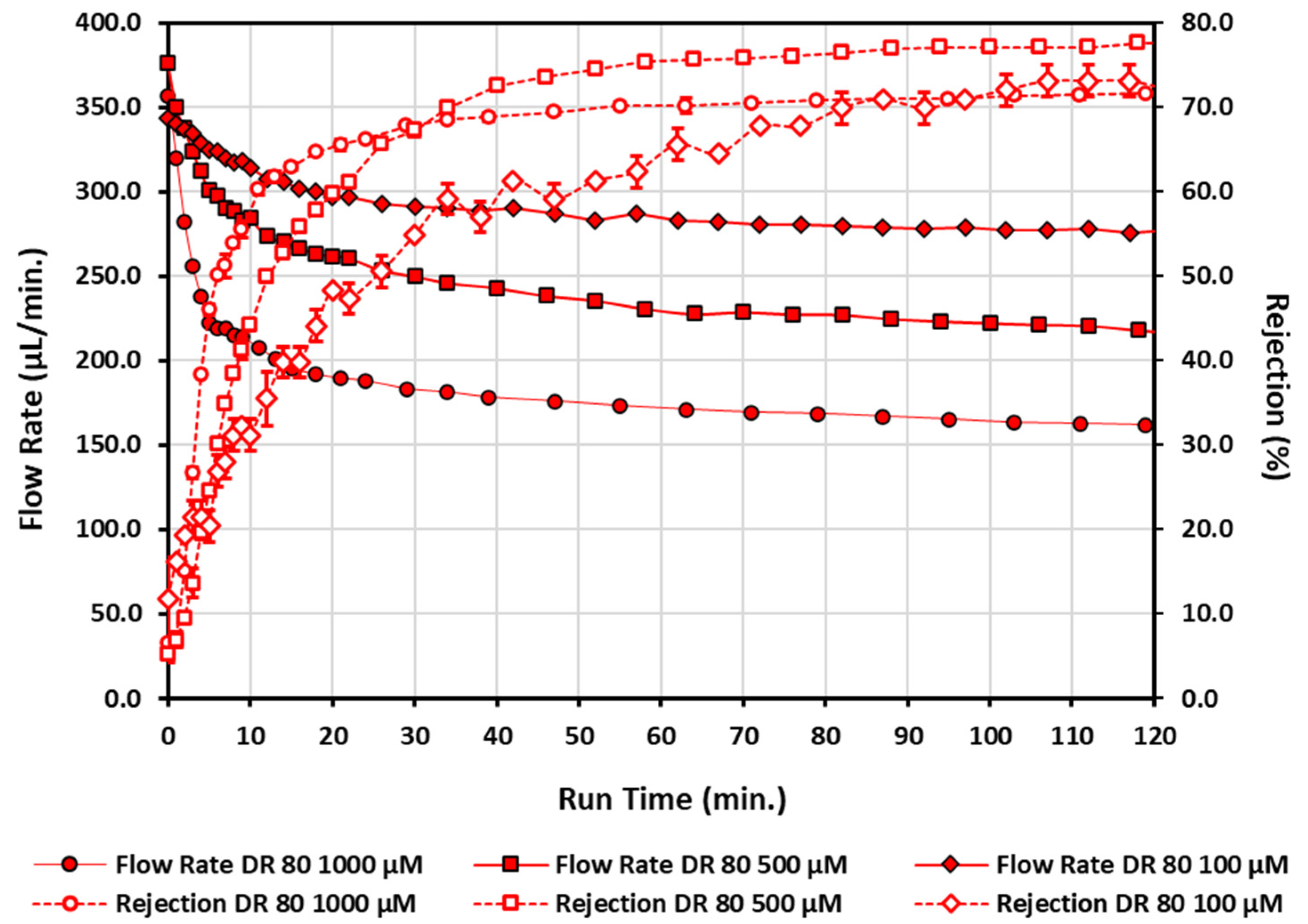
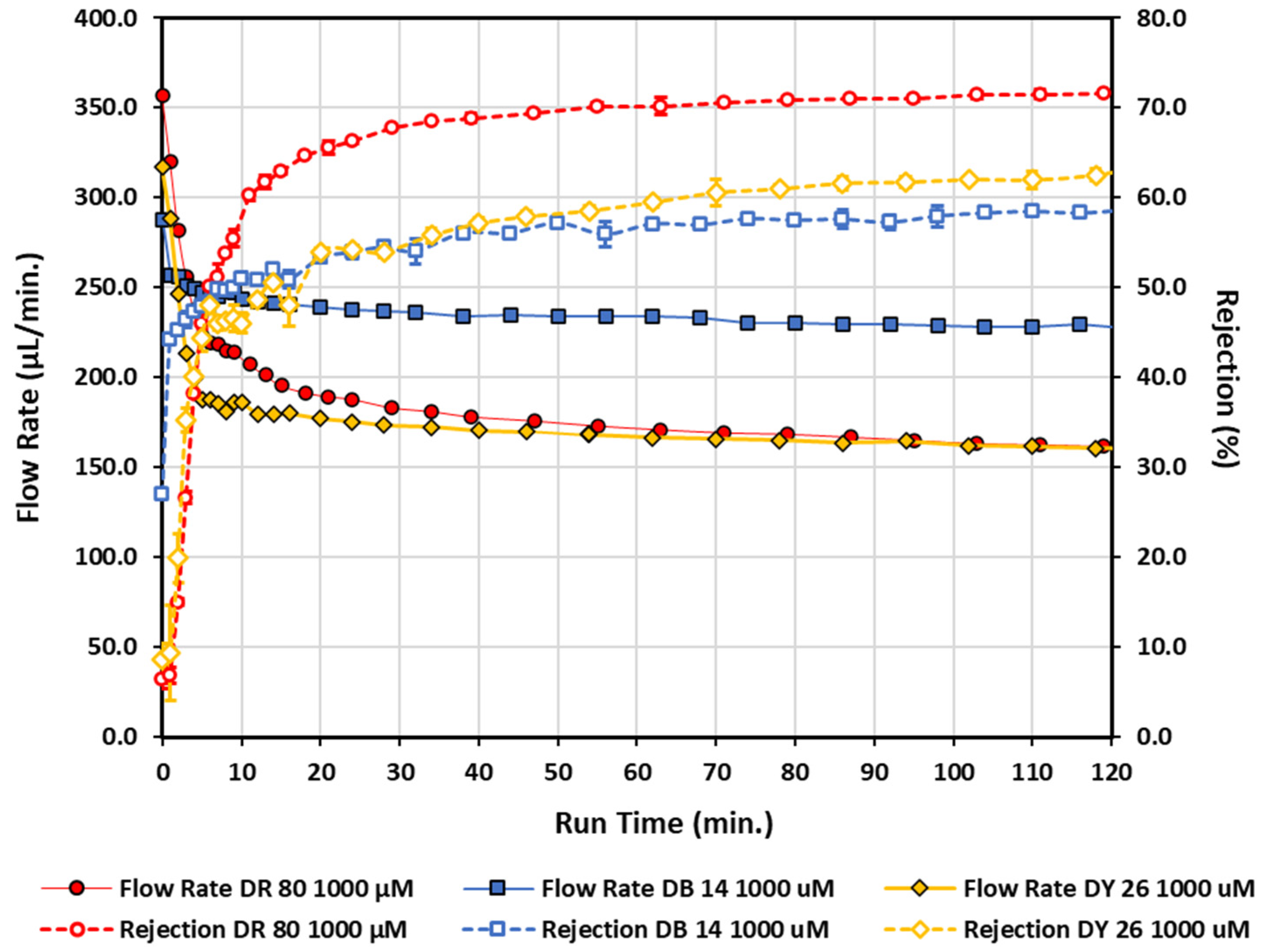


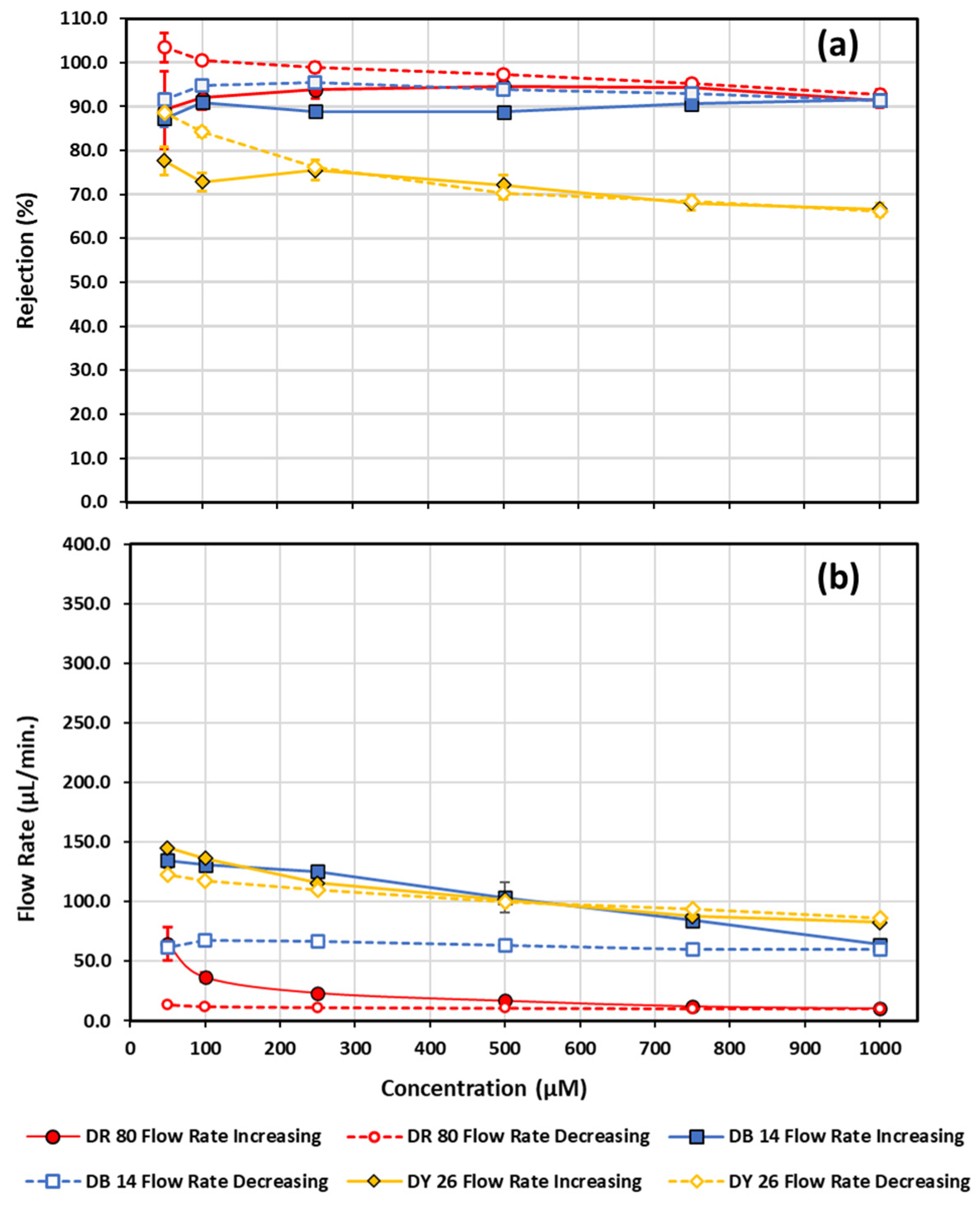
| Conc. (µM) | Flow Rate (µL/min) | Rejection (%) | |
|---|---|---|---|
| DY 26 | 50 | 291.5 ± 7.1 | 53.3 ± 3.7 |
| DB 14 | 50 | 292.7 ± 0.6 | 71.2 ± 1.5 |
| DR 80 | 50 | 280.9 ± 5.0 | 69.5 ± 10.5 |
| 1000 µM DR 80 Functionalization | |||
| DR 80 | 50 | 192.4 ± 1.0 | 87.8 ± 5.8 |
| DB 14 | 50 | 198.9 ± 1.4 | 94.0 ± 1.7 |
| DY26 | 50 | 196.3 ± 1.8 | 81.6 ± 2.1 |
| % Dec. In Flow Rate (%) | % Inc. in Rejection (%) | ||
| DR 80 | 50 | 31.5 | 26.3 |
| DB 14 | 50 | 32.0 | 32.1 |
| DY26 | 50 | 32.7 | 53.2 |
| Conc. (µM) | Flow Rate (µL/min) | Rejection (%) | |
|---|---|---|---|
| DY 26 | 50 | 169.5 ± 3.3 | 77.3 ± 1.3 |
| DB 14 | 50 | 157.1 ± 3.7 | 93.5 ± 1.2 |
| DR 80 | 50 | 125.6 ± 6.3 | 96.9 ± 2.4 |
| 1000 µM DR 80 Functionalization | |||
| DR 80 | 50 | 90.2 ± 3.3 | 98.9 ± 0.9 |
| DB 14 | 50 | 98.9 ± 2.6 | 94.5 ± 0.5 |
| DY26 | 50 | 96.0 ± 2.2 | 93.1 ± 1.4 |
| % Dec. in Flow Rate (%) | % Inc. in Rejection (%) | ||
| DR 80 | 50 | 28.1 | 2.1 |
| DB 14 | 50 | 37.0 | 1.0 |
| DY26 | 50 | 43.4 | 20.5 |
| Conc. (µM) | Na-Rejection (%) | NO3-Rejection (%) |
|---|---|---|
| 250 | 0.0 ± 0.0 | −6.2 ± 4.2 |
| 500 | 2.3 ± 4.5 | 3.8 ± 3.1 |
| 750 | −3.1 ± 3.6 | −0.8 ± 5.3 |
| 1000 µM DR 80 Functionalization | ||
| 750 | 17.6 ± 4.8 | 18.1 ± 7.0 |
| 500 | 25.0 ± 4.5 | 27.0 ± 3.8 |
| 250 | 33.3 ± 0.0 | 28.8 ± 28.2 |
| NaNO3-Flow Rate% Dec. | ||
| 250 | 45.6 | |
| 500 | 47.2 | |
| 750 | 51.3 | |
| Conc. (µM) | Na-Rejection (%) | NO3-Rejection (%) |
|---|---|---|
| 250 | 17.9 ± 7.1 | 16.7 ± 4.5 |
| 500 | 11.1 ± 4.8 | 10.7 ± 6.1 |
| 750 | 4.7 ± 3.1 | 8.8 ± 4.6 |
| 1000 µM DR 80 Functionalization | ||
| 750 | 31.3 ± 0.0 | 35.9 ± 2.6 |
| 500 | 30.0 ± 8.2 | 33.3 ± 6.8 |
| 250 | 50.0 ± 0.0 | 37.5 ± 4.8 |
| NaNO3-Flow Rate% Dec. | ||
| 250 | 53.1 | |
| 500 | 58.1 | |
| 750 | 60.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cockerham, C.; Caruthers, A.; McCloud, J.; Fortner, L.M.; Youn, S.; McBride, S.P. Azo-Dye-Functionalized Polycarbonate Membranes for Textile Dye and Nitrate Ion Removal. Micromachines 2022, 13, 577. https://doi.org/10.3390/mi13040577
Cockerham C, Caruthers A, McCloud J, Fortner LM, Youn S, McBride SP. Azo-Dye-Functionalized Polycarbonate Membranes for Textile Dye and Nitrate Ion Removal. Micromachines. 2022; 13(4):577. https://doi.org/10.3390/mi13040577
Chicago/Turabian StyleCockerham, Carrie, Ashton Caruthers, Jeremy McCloud, Laura M. Fortner, Sungmin Youn, and Sean P. McBride. 2022. "Azo-Dye-Functionalized Polycarbonate Membranes for Textile Dye and Nitrate Ion Removal" Micromachines 13, no. 4: 577. https://doi.org/10.3390/mi13040577






