Design and Modeling of a Microfluidic Coral Polyps Culture Chip with Concentration and Temperature Gradients
Abstract
:1. Introduction
2. Materials and Methods
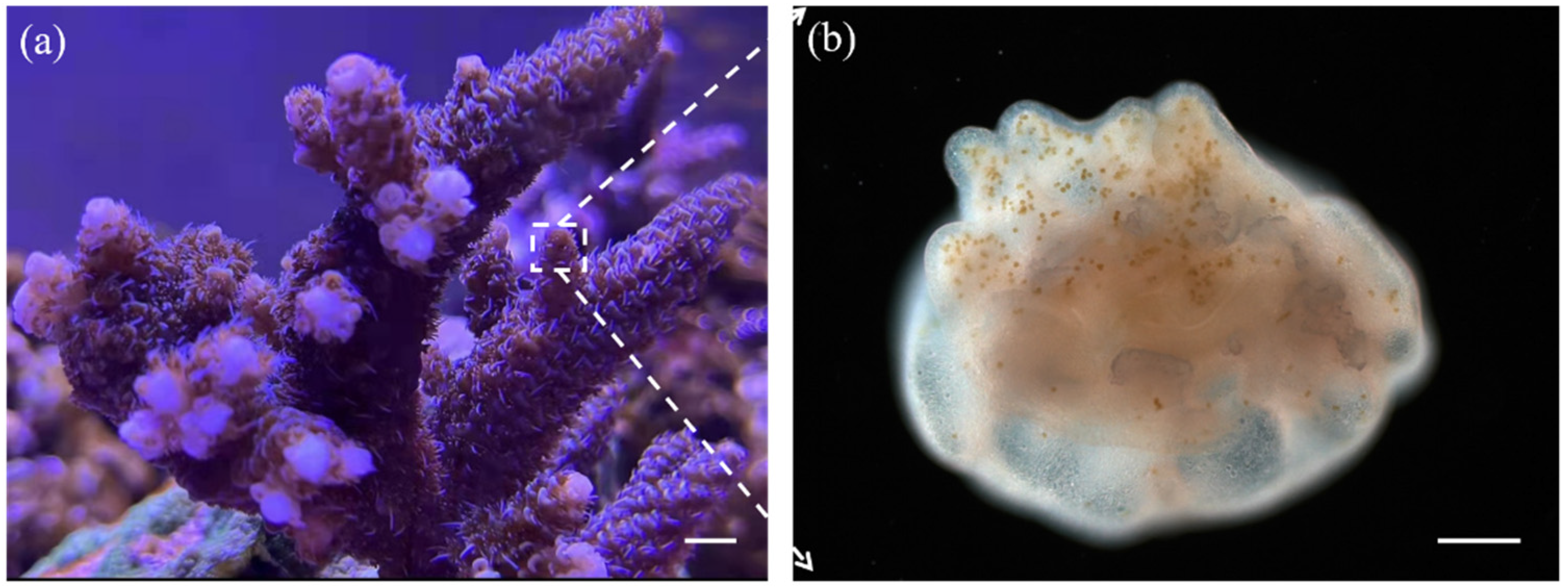
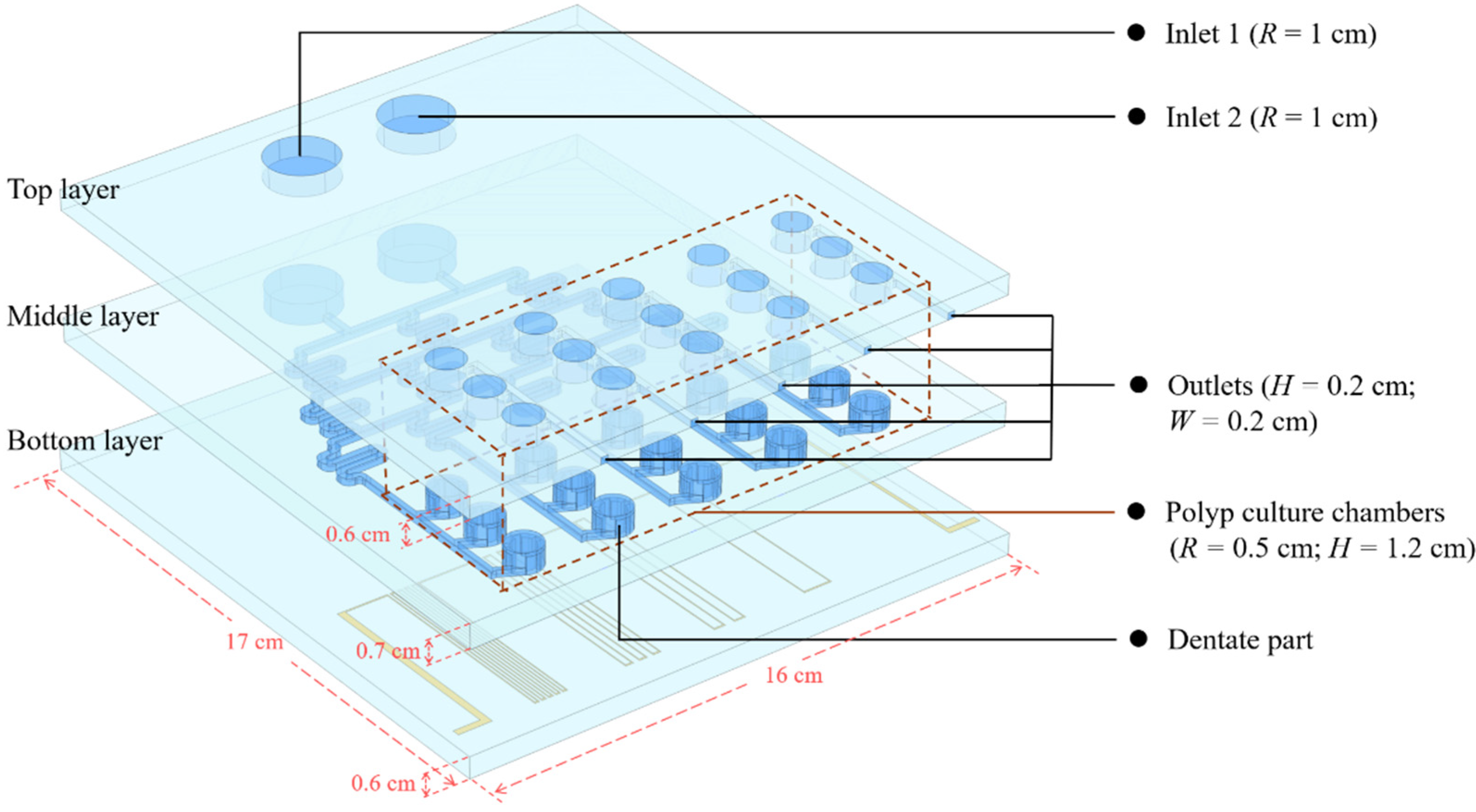
2.1. Design and Modeling of Microfluidic Chip
2.2. Governing Equations
2.2.1. Laminar Flow
2.2.2. Solution Diffusion and Mixing
2.2.3. Joule Heat
2.3. Parameter Setting
2.3.1. Material Property Setting
2.3.2. Boundary Condition Setting
2.3.3. Solver Setting
3. Results and Discussion
3.1. Flow Field Simulation
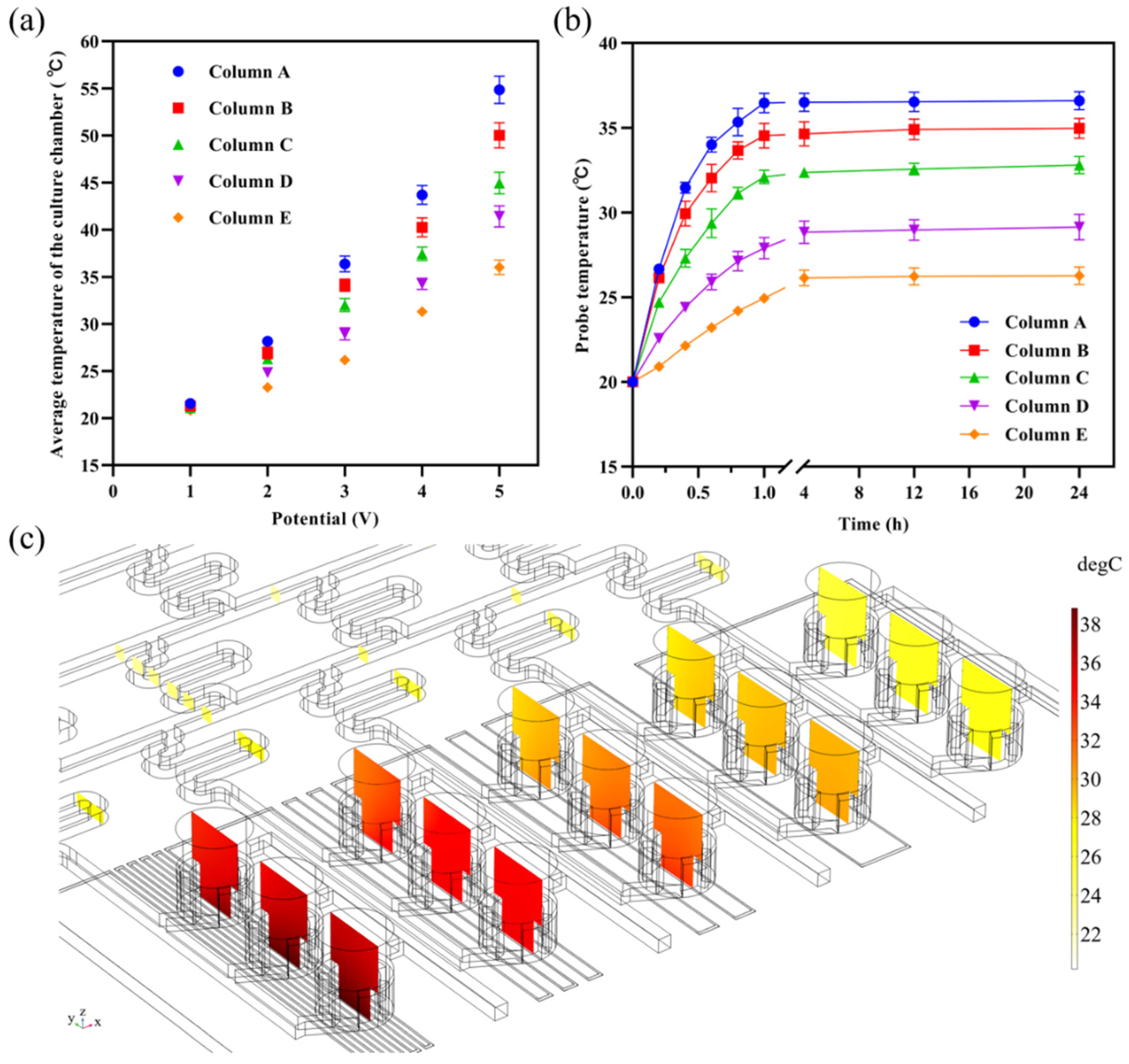
3.2. Temperature Field
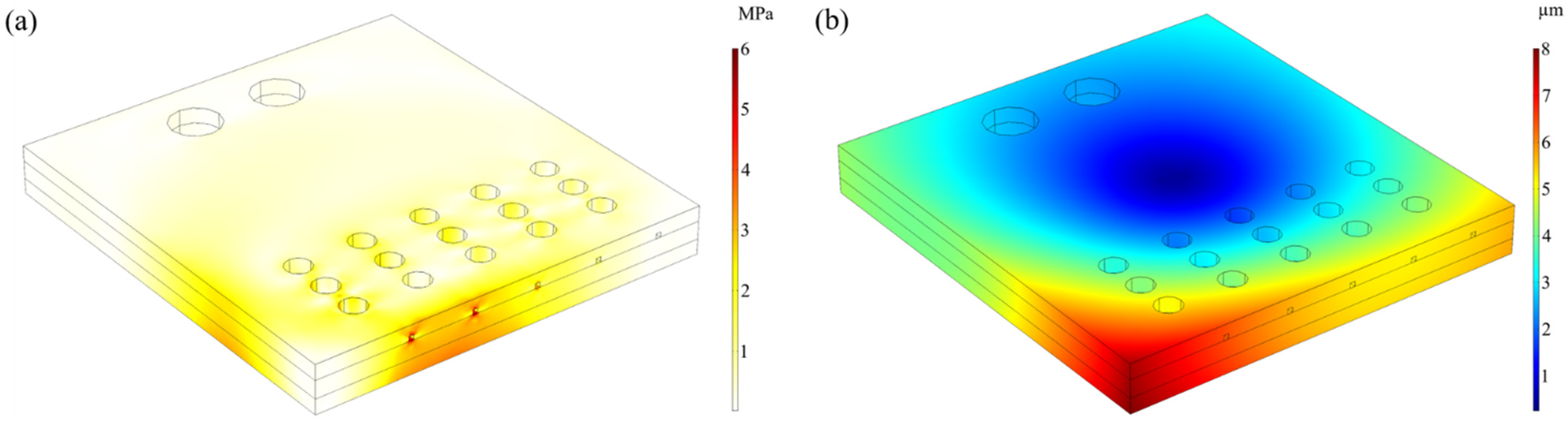
3.3. Thermal Deformation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dellisanti, W.; Chung, J.T.H.; Chow, C.F.Y.; Wu, J.; Wells, M.L.; Chan, L.L. Experimental techniques to assess coral physiology in situ under global and local stressors: Current approaches and novel insights. Front. Physiol. 2021, 12, 656562. [Google Scholar] [CrossRef] [PubMed]
- Del Monaco, C.; Hay, M.E.; Gartrell, P.; Mumby, P.J.; Diaz-Pulido, G. Effects of ocean acidification on the potency of macroalgal allelopathy to a common coral. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicks, L.C.; Hill, R.; Davya, S.K. The influence of irradiance on tolerance to high and low temperature stress exhibited by symbiodinium in the coral, pocillopora damicornis, from the high-latitude reef of lord howe island. Limnol. Oceanogr. 2010, 55, 2476–2486. [Google Scholar] [CrossRef]
- DiPerna, S.; Hoogenboom, M.; Noonan, S.; Fabricius, K. Effects of variability in daily light integrals on the photophysiology of the corals pachyseris speciosa and acropora millepora. PLoS ONE 2018, 13, e0203882. [Google Scholar]
- Bell, T.; Nishida, K.; Ishikawa, K.; Suzuki, A.; Nakamura, T.; Sakai, K.; Ohno, Y.; Iguchi, A.; Yokoyama, Y. Temperature-controlled culture experiments with primary polyps of coral acropora digitifera: Calcification rate variations and skeletal sr/ca, mg/ca, and na/ca ratios. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 484, 129–135. [Google Scholar] [CrossRef]
- Yu, X.P.; Yu, K.F.; Liao, Z.H.; Liang, J.Y.; Deng, C.Q.; Huang, W.; Huang, Y.H. Potential molecular traits underlying environmental tolerance of pavona decussata and acropora pruinosa in weizhou island, northern south china sea. Mar. Pollut. Bull. 2020, 156, 111199. [Google Scholar] [CrossRef]
- Gibbin, E.; Gavish, A.; Domart-Coulon, I.; Kramarsky-Winter, E.; Shapiro, O.; Meibom, A.; Vardi, A. Using nanosims coupled with microfluidics to visualize the early stages of coral infection by vibrio coralliilyticus. BMC Microbiol. 2018, 18, 39. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.S.; Patel, A.K.; Singhania, R.R.; Chen, C.W.; Dong, C.D. Effects of temperature and salinity on growth, metabolism and digestive enzymes synthesis of goniopora columna. Biology 2022, 11, 436. [Google Scholar] [CrossRef]
- Yuyama, I.; Ito, Y.; Watanabe, T.; Hidaka, M.; Suzuki, Y.; Nishida, M. Differential gene expression in juvenile polyps of the coral acropora tenuis exposed to thermal and chemical stresses. J. Exp. Mar. Biol. Ecol. 2012, 430–431, 17–24. [Google Scholar] [CrossRef]
- Chuang, P.-S.; Ishikawa, K.; Mitarai, S. Morphological and genetic recovery of coral polyps after bail-out. Front. Mar. Sci. 2021, 8, 280. [Google Scholar] [CrossRef]
- Van Treuren, W.; Brower, K.K.; Labanieh, L.; Hunt, D.; Lensch, S.; Cruz, B.; Cartwright, H.N.; Tran, C.; Fordyce, P.M. Live imaging of aiptasia larvae, a model system for coral and anemone bleaching, using a simple microfluidic device. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Johansson, S.; Bergman, H.; Xiao, L.; Behrendt, L.; Tenje, M. A microscopy-compatible temperature regulation system for single-cell phenotype analysis—Demonstrated by thermoresponse mapping of microalgae. Lab Chip. 2021, 21, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, L.; Salek, M.M.; Trampe, E.L.; Fernandez, V.I.; Lee, K.S.; Kühl, M.; Stocker, R. Phenochip: A single-cell phenomic platform for high-throughput photophysiological analyses of microalgae. Sci. Adv. 2020, 6, eabb2754. [Google Scholar] [CrossRef] [PubMed]
- Gibbin, E.; Gavish, A.; Krueger, T.; Kramarsky-Winter, E.; Shapiro, O.; Guiet, R.; Jensen, L.; Vardi, A.; Meibom, A. Vibrio coralliilyticus infection triggers a behavioural response and perturbs nutritional exchange and tissue integrity in a symbiotic coral. ISME J. 2018, 13, 989–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garren, M.; Son, K.; Tout, J.; Seymour, J.R.; Stocker, R. Temperature-induced behavioral switches in a bacterial coral pathogen. ISME J. 2015, 10, 1363–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Zhao, J.; He, C.; Lu, Z.; Lu, X. Miniaturized platform for individual coral polyps culture and monitoring. Micromachines 2020, 11, 127. [Google Scholar] [CrossRef] [Green Version]
- Burmeister, A.; Hilgers, F.; Langner, A.; Westerwalbesloh, C.; Kerkhoff, Y.; Tenhaef, N.; Drepper, T.; Kohlheyer, D.; von Lieres, E.; Noack, S.; et al. A microfluidic co-cultivation platform to investigate microbial interactions at defined microenvironments. Lab Chip. 2018, 19, 98–110. [Google Scholar] [CrossRef]
- Shen, Y.; Yalikun, Y.; Tanaka, Y. Recent advances in microfluidic cell sorting systems. Sens. Actuators B Chem. 2019, 282, 268–281. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Wang, Y.; Zhang, T.; Chen, Y.C.; Yoon, E. Label-free estimation of therapeutic efficacy on 3d cancer spheres using convolutional neural network image analysis. Anal. Chem. 2019, 91, 14093–14100. [Google Scholar] [CrossRef]
- Saad, M.G.; Selahi, A.; Zoromba, M.S.; Mekki, L.; El-Bana, M.; Dosoky, N.S.; Nobles, D.; Shafik, H.M. A droplet-based gradient microfluidic to monitor and evaluate the growth of chlorella vulgaris under different levels of nitrogen and temperatures. Algal Res. 2019, 44, 101657. [Google Scholar] [CrossRef]
- Khan, I.; Prabhakar, A.; Delepine, C.; Tsang, H.; Pham, V.; Sur, M. A low-cost 3d printed microfluidic bioreactor and imaging chamber for live-organoid imaging. Biomicrofluidics 2021, 15, 024105. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Xie, D.; Mbogba, M.K.; Chen, Z.; Tian, C.; Xu, L.; Zhao, G. A microfluidic platform with cell-scale precise temperature control for simultaneous investigation of the osmotic responses of multiple oocytes. Lab Chip 2019, 19, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Rodrigues, R.O.; Lima, R.A.; Teixeira, S. Computational simulations in advanced microfluidic devices: A review. Micromachines 2021, 12, 1149. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, X.; Wang, L. Design and fabrication of concentration-gradient generators with two and three inlets in microfluidic chips. Chem. Eng. Technol. 2018, 41, 489–495. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, W.; Luo, P.; Zhang, M.; Wang, Y.; Zhang, H.; Hu, P. A new circular-shape microfluidic network for generating gradients of multiple substances -design, demonstration and application. Sens. Actuators B Chem. 2019, 283, 247–254. [Google Scholar] [CrossRef]
- Si, G.; Sun, L.; Zhang, Z.; Zhang, X. Theoretical thermal-mechanical modelling and experimental validation of a three-dimensional (3d) electrothermal microgripper with three fingers. Micromachines 2021, 12, 1512. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fang, C.; Ren, S.; Pan, J.; Jia, Y.; Shu, Z.; Gao, D. Development of a microfluidic device with precise on-chip temperature control by integrated cooling and heating components for single cell-based analysis. Int. J. Heat Mass Transf. 2019, 130, 660–667. [Google Scholar] [CrossRef]
- Sateesh, J.; Guha, K.; Dutta, A.; Sengupta, P.; Srinivasa Rao, K. Design and analysis of microfluidic kidney-on-chip model: Fluid shear stress based study with temperature effect. Microsyst. Technol. 2018, 25, 2553–2560. [Google Scholar] [CrossRef]
- Liu, G.; Cao, W.; Zhang, G.; Wang, Z.; Tan, H.; Miao, J.; Li, Z.; Zhang, W.; Wang, R. Design and simulation of flexible underwater acoustic sensor based on 3d buckling structure. Micromachines 2021, 12, 1536. [Google Scholar] [CrossRef]

| Material Property | Copper | Silica Glass |
|---|---|---|
| Electrical conductivity (S/m) | 6.0 × 107 | 1.0 × 10−14 |
| Constant-pressure heat capacity (J/(kg·K)) | 385 | 703 |
| Relative dielectric constant | 1 | 3.75 |
| Density (kg/m3) | 8960 | 2203 |
| Thermal conductivity (W/(m2·K)) | 400 | 1.38 |
| Resistivity (Ω·m) | 1.7 × 10−8 | - |
| Column | Concentration (mol/m3) | Inlet Flow Velocity (mm/s) | Average Flow Velocity (mm/s) |
|---|---|---|---|
| A | 0.1 | 0.56 ± 0.04 | 0.33 ± 0.01 |
| B | 0.2 | 0.60 ± 0.05 | 0.34 ± 0.01 |
| C | 0.3 | 0.58 ± 0.07 | 0.34 ± 0.01 |
| D | 0.4 | 0.57 ± 0.08 | 0.34 ± 0.02 |
| E | 0.5 | 0.59 ± 0.04 | 0.34 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Fu, E.S.; Chen, B.; Yan, H. Design and Modeling of a Microfluidic Coral Polyps Culture Chip with Concentration and Temperature Gradients. Micromachines 2022, 13, 832. https://doi.org/10.3390/mi13060832
Zhou S, Fu ES, Chen B, Yan H. Design and Modeling of a Microfluidic Coral Polyps Culture Chip with Concentration and Temperature Gradients. Micromachines. 2022; 13(6):832. https://doi.org/10.3390/mi13060832
Chicago/Turabian StyleZhou, Shizheng, Edgar S. Fu, Bingbing Chen, and Hong Yan. 2022. "Design and Modeling of a Microfluidic Coral Polyps Culture Chip with Concentration and Temperature Gradients" Micromachines 13, no. 6: 832. https://doi.org/10.3390/mi13060832
APA StyleZhou, S., Fu, E. S., Chen, B., & Yan, H. (2022). Design and Modeling of a Microfluidic Coral Polyps Culture Chip with Concentration and Temperature Gradients. Micromachines, 13(6), 832. https://doi.org/10.3390/mi13060832






