Highly active N, S Co-Doped Ultramicroporous Carbon for High-Performance Supercapacitor Electrodes
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Synthesis of NSUC-x
2.2. Characterization of NSUC-x
2.3. Electrochemical Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Ionic Liquids for Supercapacitive Energy Storage: A Mini-Review. Energy Fuel 2021, 35, 8443–8455. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhang, H.; Shao, L.M.; Lü, F.; He, P.J. Preparation and Application of Hierarchical Porous Carbon Materials from Waste and Biomass: A Review. Waste Biomass Valoriz. 2021, 12, 1699–1724. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Ali, F.Z.; Raza, N.; Luo, Y.W.; Kim, K.H.; Yang, J.H.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef]
- Zhu, D.Z.; Wang, Y.W.; Lu, W.J.; Zhang, H.; Song, Z.Y.; Luo, D.; Gan, L.H.; Liu, M.X.; Sun, D.M. A novel synthesis of hierarchical porous carbons from interpenetrating polymer networks for high performance supercapacitor electrodes. Carbon 2017, 111, 667–674. [Google Scholar] [CrossRef]
- Miao, L.; Song, Z.Y.; Zhu, D.Z.; Li, L.C.; Gan, L.H.; Liu, M.X. Recent advances in carbon-based supercapacitors. Mater. Adv. 2020, 1, 945–966. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Ghosh, M.; Ramos-Ramon, J.A.; Saha, S.; Pal, U.; Bhattacharya, S.K.; Das, S. Study on charge storage mechanism in working electrodes fabricated by sol-gel derived spinel NiMn2O4 nanoparticles for supercapacitor application. Appl. Surf. Sci. 2019, 463, 513–525. [Google Scholar] [CrossRef]
- Song, Z.; Miao, L.; Li, L.; Zhu, D.; Gan, L.; Liu, M. A robust strategy of solvent choice to synthesize optimal nanostructured carbon for efficient energy storage. Carbon 2021, 180, 135–145. [Google Scholar] [CrossRef]
- Lei, C.C.; Ji, C.C.; Mi, H.Y.; Yang, C.C.; Zhang, Q.; He, S.X.; Bai, Z.Y.; Qiu, J.S. Engineering kinetics-favorable carbon sheets with an intrinsic network for a superior supercapacitor containing a dual cross-linked hydrogel electrolyte. ACS Appl. Mater. Inter. 2020, 12, 53164–53173. [Google Scholar] [CrossRef]
- Liang, T.T.; Hou, R.L.; Dou, Q.Y.; Zhang, H.Z.; Yan, X.B. The applications of water-in-salt electrolytes in electrochemical energy storage devices. Adv. Funct. Mater. 2021, 31, 202006749. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, L.; Zeng, Y.; Cao, X.; Huang, H.; Liu, J.; Chen, X.; Wei, S.; Gan, L.; Yang, P.; et al. Three-dimensional hierarchical porous carbon derived from resorcinol formaldehyde-zinc tatrate/poly(styrene-maleic anhydride) for high performance supercapacitor electrode. J. Alloys Compd. 2021, 886, 161176. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Miao, L.; Yin, Y.; Liu, M.X.; Gan, L.H.; Li, L.C. Highly N/O co-doped ultramicroporous carbons derived from nonporous metal-organic framework for high performance supercapacitors. Chin. Chem. Lett. 2021, 32, 1491–1496. [Google Scholar] [CrossRef]
- Miao, L.; Duan, H.; Liu, M.X.; Lu, W.J.; Zhu, D.Z.; Chen, T.; Li, L.C.; Gan, L.H. Poly(ionic liquid)-derived, N, S-codoped ultramicroporous carbon nanoparticles for supercapacitors. Chem. Eng. J. 2017, 317, 651–659. [Google Scholar] [CrossRef]
- Nightingale Jr, E. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Guardia, L.; Suarez, L.; Querejeta, N.; Vretenar, V.; Kotrusz, P.; Skakalova, V.; Centeno, T.A. Biomass waste-carbon/reduced graphene oxide composite electrodes for enhanced supercapacitors. Electrochim. Acta 2019, 298, 910–917. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.S.; Duan, H.; Cao, G.P.; Chu, M.; Yang, Y.S. Ultramicroporous carbon as electrode material for supercapacitors. J. Power Sources 2013, 228, 193–197. [Google Scholar] [CrossRef]
- Barczak, M.; Elsayed, Y.; Jagiello, J.; Bandosz, T.J. Exploring the effect of ultramicropore distribution on gravimetric capacitance of nanoporous carbons. Electrochim. Acta 2018, 275, 236–247. [Google Scholar] [CrossRef]
- Ping, Y.J.; Yang, S.J.; Han, J.Z.; Li, X.; Zhang, H.L.; Xiong, B.Y.; Fang, P.F.; He, C.Q. N-self-doped graphitic carbon aerogels derived from metal-organic frameworks as supercapacitor electrode materials with high-performance. Electrochim. Acta 2021, 380, 138237. [Google Scholar] [CrossRef]
- Miao, L.; Zhu, D.Z.; Liu, M.X.; Duan, H.; Wang, Z.W.; Lv, Y.K.; Xiong, W.; Zhu, Q.J.; Li, L.C.; Chai, X.L.; et al. N, S Co-doped hierarchical porous carbon rods derived from protic salt: Facile synthesis for high energy density supercapacitors. Electrochim. Acta 2018, 274, 378–388. [Google Scholar] [CrossRef]
- Liu, M.Q.; Huo, S.L.; Xu, M.; Wu, L.L.; Liu, M.J.; Xue, Y.F.; Yan, Y.M. Structural engineering of N/S co-doped carbon material as high-performance electrode for supercapacitors. Electrochim. Acta 2018, 274, 389–399. [Google Scholar] [CrossRef]
- Laheaar, A.; Delpeux-Ouldriane, S.; Lust, E.; Beguin, F. Ammonia Treatment of Activated Carbon Powders for Supercapacitor Electrode Application. J. Electrochem. Soc. 2014, 161, A568–A575. [Google Scholar] [CrossRef]
- Feng, S.S.; Li, W.; Shi, Q.; Li, Y.H.; Chen, J.C.; Ling, Y.; Asiri, A.M.; Zhao, D.Y. Synthesis of nitrogen-doped hollow carbon nanospheres for CO2 capture. Chem. Commun. 2014, 50, 329–331. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, B.; Park, S.W.; Kim, W.; Shim, J.H. Atomic layer deposition of ruthenium on plasma-treated vertically aligned carbon nanotubes for high-performance ultracapacitors. Nanotechnology 2014, 25, 435404. [Google Scholar] [CrossRef]
- Biener, J.; Stadermann, M.; Suss, M.; Worsley, M.A.; Biener, M.M.; Rose, K.A.; Baumann, T.F. Advanced carbon aerogels for energy applications. Energy Environ. Sci. 2011, 4, 656–667. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.Y.; Zhao, H.; Wang, J.; Zhao, Y.P.; Ge, F.Y.; Cai, Z.S. Combined effect of nitrogen and oxygen heteroatoms and micropores of porous carbon frameworks from Schiff-base networks on their high supercapacitance. J. Mater. Chem. A 2018, 6, 1621–1629. [Google Scholar] [CrossRef]
- Ni, L.; Wang, R.W.; Wang, H.B.; Sun, C.Y.; Sun, B.; Guo, X.; Jiang, S.; Shi, Z.Q.; Jing, W.D.; Zhu, L.K.; et al. Designing nanographitic domains in N-doped porous carbon foam for high performance supercapacitors. Carbon 2018, 139, 1152–1159. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qiao, S.Z.; Liu, H.; Chen, J.; Orpe, A.; Zhao, D.Y.; Lu, G.Q. Extension of The Stober Method to the Preparation of Monodisperse Resorcinol-Formaldehyde Resin Polymer and Carbon Spheres. Angew. Chem. Int. Edit. 2011, 50, 5947–5951. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.K.; Yu, M.; Liu, J.H.; Li, S.M.; Zhang, X.L. sp3-Defect and pore engineered carbon framework for high energy density supercapacitors. J. Power Sources 2020, 464, 228203. [Google Scholar] [CrossRef]
- Qian, X.Y.; Miao, L.; Jiang, J.X.; Ping, G.C.; Xiong, W.; Lv, Y.K.; Liu, Y.F.; Gan, L.H.; Zhua, D.Z.; Liu, M.X. Hydrangea-like N/O codoped porous carbons for high-energy supercapacitors. Chem. Eng. J. 2020, 388, 124208. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.; Wu, S.M.; Li, Z.G.; Fan, B.B.; Li, Y.H.; Zhou, Y.M. Facile synthesis of N/B co-doped hierarchically porous carbon materials based on threonine protic ionic liquids for supercapacitor. Electrochim. Acta 2021, 380, 138230. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.X.; Li, Y.; Deng, B.W.; Yin, B.; Yang, M.B. Design of compressible and elastic N-doped porous carbon nanofiber aerogels as binder-free supercapacitor electrodes. J. Mater. Chem. A 2020, 8, 17257–17265. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Xu, J.T.; Wang, M.; Zhu, L.; Dai, L.M.; Jaroniec, M. Nitrogen Enriched Porous Carbon Spheres: Attractive Materials for Supercapacitor Electrodes and CO2 Adsorption. Chem. Mater. 2014, 26, 2820–2828. [Google Scholar] [CrossRef]
- Lv, H.; Wang, S.F.; Xiao, Z.Y.; Qin, C.R.; Zhai, S.R.; Wang, G.X.; Zhao, Z.Y.; An, Q.D. N-doped hollow carbon spheres with controllable shell numbers for high-performance electrical double-layer capacitors. J. Power Sources 2021, 493, 229679. [Google Scholar] [CrossRef]
- Zhou, J.; Shen, H.L.; Li, Z.H.; Zhang, S.; Zhao, Y.T.; Bi, X.; Wang, Y.S.; Cui, H.Y.; Zhuo, S.P. Porous carbon materials with dual N, S-doping and uniform ultra-microporosity for high performance supercapacitors. Electrochim. Acta 2016, 209, 557–564. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Miao, L.; Duan, H.; Wang, Z.W.; Lv, Y.K.; Xiong, W.; Zhu, D.Z.; Li, L.C.; Liu, M.X.; Gan, L.H. Highly active N, O-doped hierarchical porous carbons for high-energy supercapacitors. Chin. Chem. Lett. 2020, 31, 1226–1230. [Google Scholar] [CrossRef]
- Song, Z.Y.; Miao, L.; Ruhlmann, L.; Lv, Y.K.; Zhu, D.Z.; Li, L.C.; Gan, L.H.; Liu, M.X. Self-assembled carbon superstructures achieving ultra-stable and fast proton-coupled charge storage kinetics. Adv. Mater. 2021, 33, 2104148. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Miao, L.; Zhu, D.; Duan, H.; Lv, Y.; Li, L.; Liu, M.; Gan, L. Adapting a kinetics-enhanced carbon nanostructure to Li/Na hybrid water-in-salt electrolyte for high-energy aqueous supercapacitors. ACS Appl. Energy Mater. 2021, 4, 5727–5737. [Google Scholar] [CrossRef]
- Li, Z.W.; Chen, D.H.; An, Y.F.; Chen, C.L.; Wu, L.Y.; Chen, Z.J.; Sun, Y.; Zhang, X.G. Flexible and anti-freezing quasi-solid-state zinc ion hybrid supercapacitors based on pencil shavings derived porous carbon. Energy Storage Mater. 2020, 28, 307–314. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Tang, Y.B. A novel zinc-ion hybrid supercapacitor for long-life and low-cost energy storage applications. Energy Storage Mater. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Shan, Y.Q.; Xu, Z.X.; Duan, P.G.; Fan, H.L.; Hu, X.; Luque, R. Nitrogen- and Sulfur-Doped Carbon Obtained from Direct Hydrothermal Carbonization of Cellulose and Ammonium Sulfate for Supercapacitor Applications. ACS Sustain. Chem. Eng. 2020, 8, 15809–15814. [Google Scholar] [CrossRef]
- Hamouda, H.A.; Cui, S.Z.; Dai, X.W.; Xiao, L.L.; Xie, X.; Peng, H.; Ma, G.F. Synthesis of porous carbon material based on biomass derived from hibiscus sabdariffa fruits as active electrodes for high-performance symmetric supercapacitors. RSC Adv. 2021, 11, 354–363. [Google Scholar] [CrossRef]
- Kan, Y.F.; Ning, G.Q.; Ma, X.L. Sulfur-decorated nanomesh graphene for high-performance supercapacitors. Chin. Chem. Lett. 2017, 28, 2277–2280. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Y.T.; Yang, Y.L.; Zhao, X.N.; Liu, Y.; Niu, L.Y.; Tichnell, B.; Kong, L.B.; Kang, L.; Liu, Z.; et al. Pomelo peels-derived porous activated carbon microsheets dual-doped with nitrogen and phosphorus for high performance electrochemical capacitors. J. Power Sources 2018, 378, 499–510. [Google Scholar] [CrossRef]
- Song, P.; He, X.M.; Shen, X.P.; Sun, Y.M.; Li, Z.W.; Yuan, A.H.; Zhai, L.Z.; Zhang, D.Y. Dissolution-assistant all-in-one synthesis of N and S dual-doped porous carbon for high-performance supercapacitors. Adv. Powder Technol. 2019, 30, 2211–2217. [Google Scholar] [CrossRef]
- Nie, Z.G.; Wang, Y.; Li, X.Y.; Wang, R.; Zhao, Y.; Song, H.; Wang, H. Heteroatom-doped hierarchical porous carbon from corn straw for high-performance supercapacitor. J. Energy Storage 2021, 44, 103410. [Google Scholar] [CrossRef]
- Liang, X.D.; Liu, R.N.; Wu, X.L. Biomass waste derived functionalized hierarchical porous carbon with high gravimetric and volumetric capacitances for supercapacitors. Micropor. Mesopor. Mater. 2021, 310, 110659. [Google Scholar] [CrossRef]
- Xue, D.F.; Zhu, D.Z.; Xiong, W.; Cao, T.C.; Wang, Z.W.; Lv, Y.K.; Li, L.C.; Liu, M.X.; Gan, L.H. Template-free, self-doped approach to porous carbon spheres with high N/O contents for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 7024–7034. [Google Scholar] [CrossRef]
- Zhu, D.Z.; Wang, Y.W.; Gan, L.H.; Liu, M.X.; Cheng, K.; Zhao, Y.H.; Deng, X.X.; Sun, D.M. Nitrogen-containing carbon microspheres for supercapacitor electrodes. Electrochim. Acta 2015, 158, 166–174. [Google Scholar] [CrossRef]
- Song, Z.Y.; Miao, L.; Li, L.C.; Zhu, D.Z.; Lv, Y.K.; Xiong, W.; Duan, H.; Wang, Z.W.; Gan, L.H.; Liu, M.X. A universal strategy to obtain highly redox-active porous carbons for efficient energy storage. J. Mater. Chem. A 2020, 8, 3717–3725. [Google Scholar] [CrossRef]

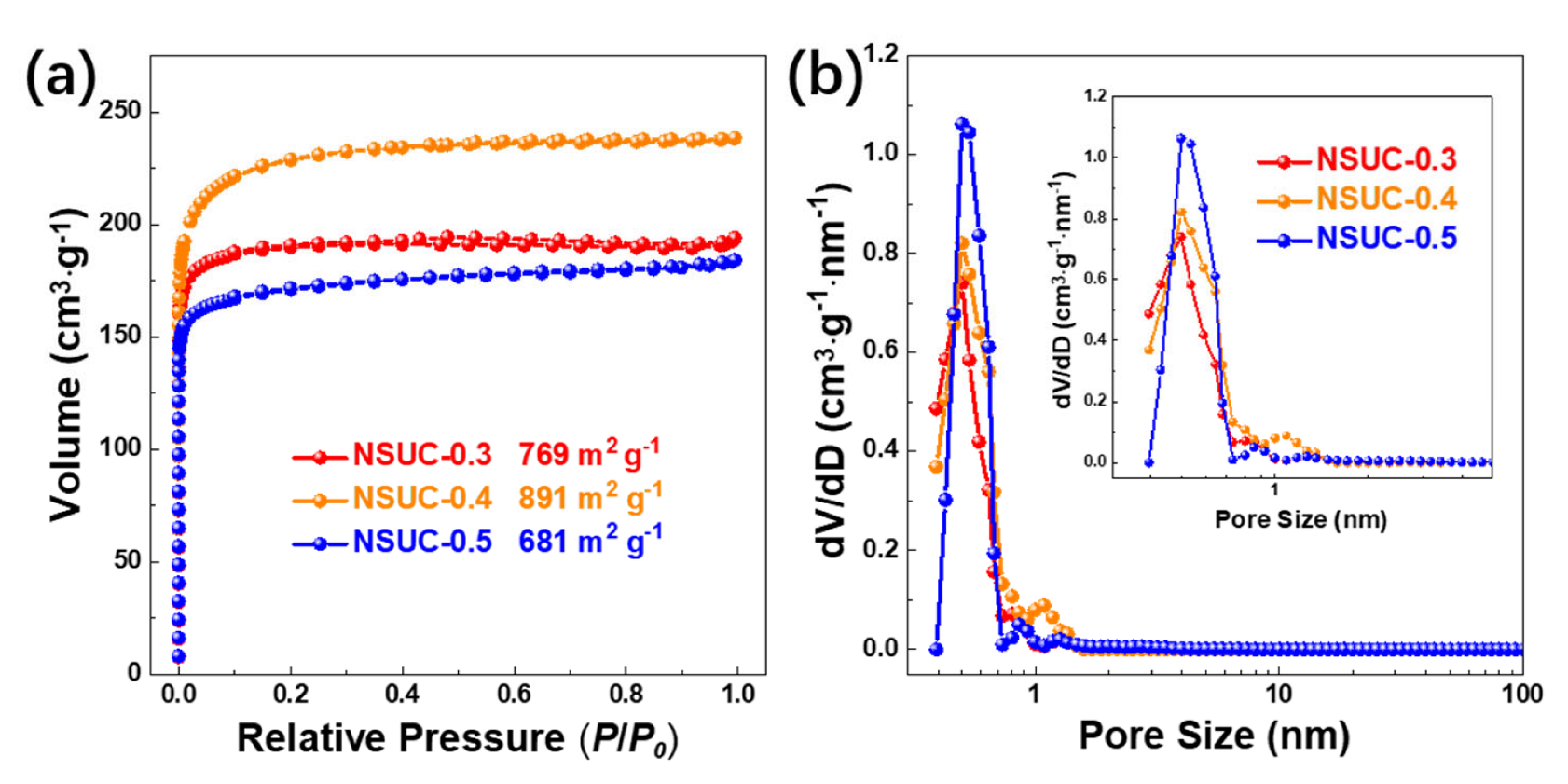
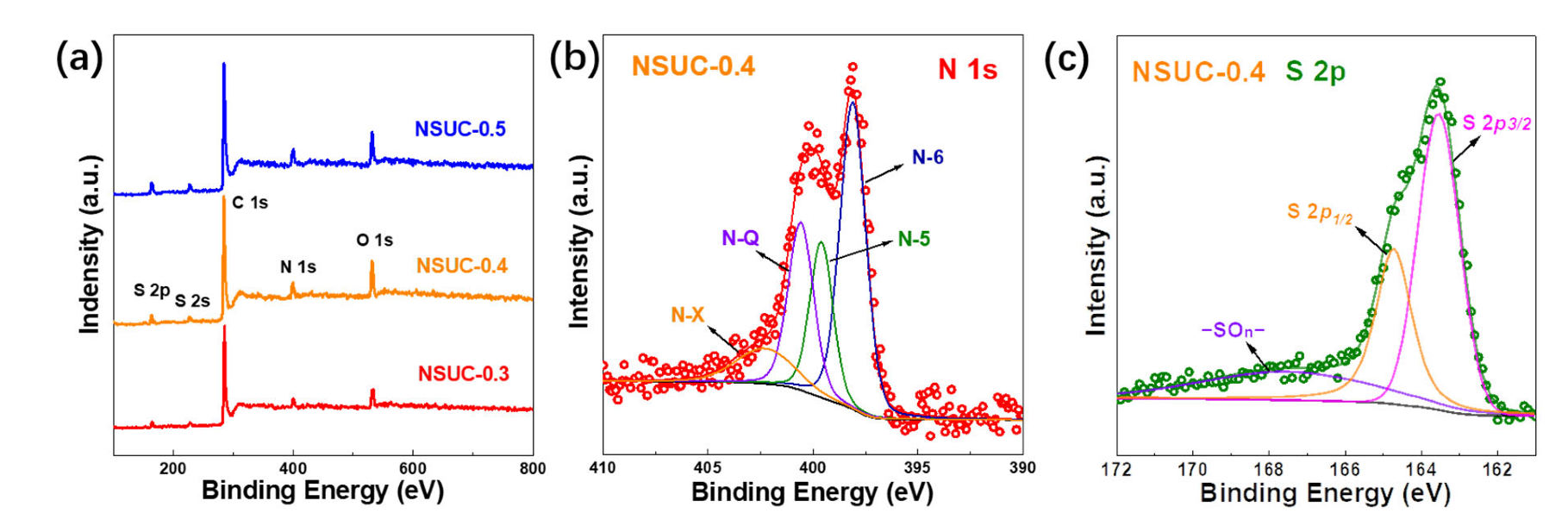




| Samples | Pore Structure Parameters | Element Content (at.%) | |||||
|---|---|---|---|---|---|---|---|
| SBET | SMicro | Vtol | C | N | S | O | |
| NSUC-0.3 | 769 | 701 | 0.30 | 85.85 | 4.91 | 2.14 | 7.11 |
| NSUC-0.4 | 891 | 789 | 0.37 | 80.13 | 7.35 | 2.37 | 10.15 |
| NSUC-0.5 | 681 | 598 | 0.28 | 80.43 | 8.11 | 3.01 | 8.44 |
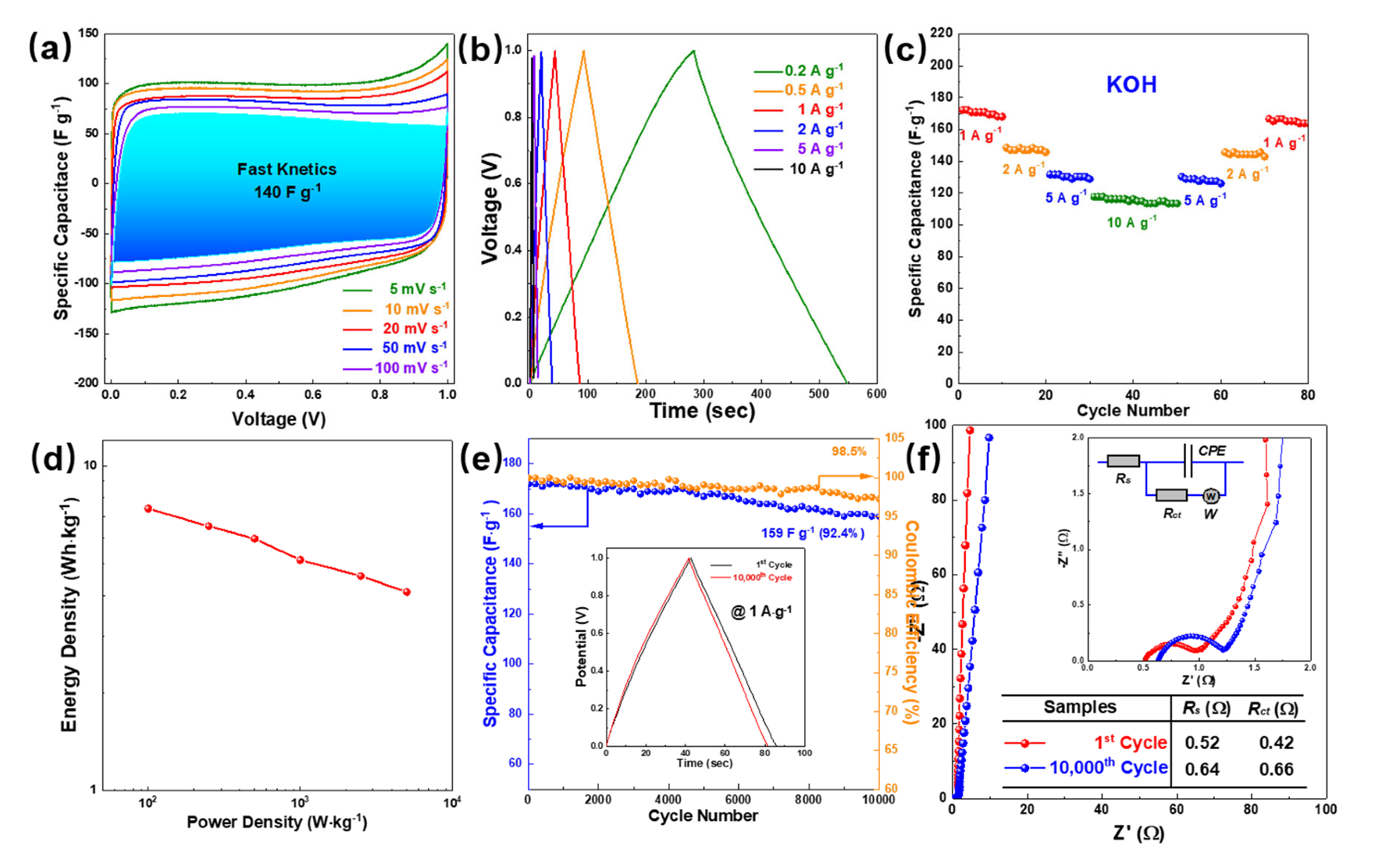
| Sample | Heteroatoms | Electrolyte | Voltage (V) | Capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|
| NSUC-0.4 | N, S | 6 M KOH | −1–0 | 339 (0.5 A g−1) 283 (1 A g−1) | This work |
| Hydrochar | N, S | 6 M KOH | −1–0 | 227.3 (1 A g−1) | [45] |
| HBFC-1 | N, O | 2 M KOH | −1–0 | 194.5 (0.5 A g−1) | [46] |
| Thr-C0.1-B | B, N | 6 M KOH | −1–0 | 242 (1 A g−1) | [35] |
| S@G5 | S | 6 M KOH | −1–0 | 257 (0.25 A g−1) | [47] |
| PPC900-N&P30 | N, P | 2 M KOH | −1–0 | 240 (0.5 A g−1) | [48] |
| NSC | N, S | 6 M KOH | −1–0 | 288 (0.5 A g−1) | [49] |
| N2PC5 | N, O | 6 M KOH | −1–0 | 321.5 (0.5 A g−1) | [50] |
| NSPC-600 | N, S | 6 M KOH | −1–0 | 358 (0.5 A g−1) | [51] |
| NOC700,1:1 | N, O | 1 M H2SO4 | −0.2–0.8 | 311 (1 A g−1) | [40] |
| PSC800 | N, O | 6 M KOH | −1–0 | 344 (1 A g−1) | [52] |
| NCM-700 | N, O | 6 M KOH | −1–0 | 228 (1 A g−1) | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.; Hao, L.; Wang, Y. Highly active N, S Co-Doped Ultramicroporous Carbon for High-Performance Supercapacitor Electrodes. Micromachines 2022, 13, 905. https://doi.org/10.3390/mi13060905
Lu W, Hao L, Wang Y. Highly active N, S Co-Doped Ultramicroporous Carbon for High-Performance Supercapacitor Electrodes. Micromachines. 2022; 13(6):905. https://doi.org/10.3390/mi13060905
Chicago/Turabian StyleLu, Wenjing, Lina Hao, and Yawei Wang. 2022. "Highly active N, S Co-Doped Ultramicroporous Carbon for High-Performance Supercapacitor Electrodes" Micromachines 13, no. 6: 905. https://doi.org/10.3390/mi13060905
APA StyleLu, W., Hao, L., & Wang, Y. (2022). Highly active N, S Co-Doped Ultramicroporous Carbon for High-Performance Supercapacitor Electrodes. Micromachines, 13(6), 905. https://doi.org/10.3390/mi13060905






