CFD Analysis and Life Cycle Assessment of Continuous Synthesis of Magnetite Nanoparticles Using 2D and 3D Micromixers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
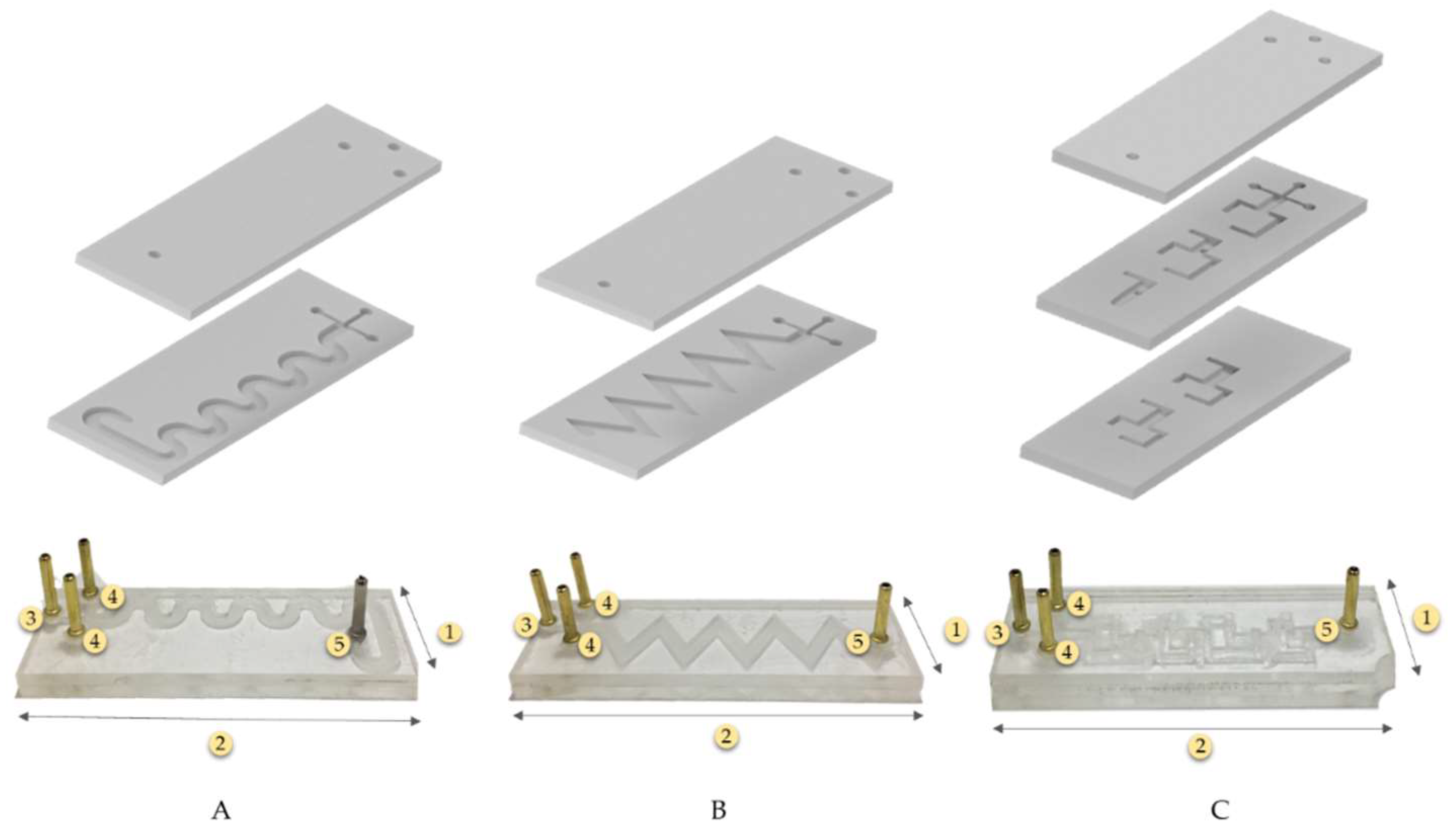
2.2. Microfluidic Geometry Design
2.3. Microfluidic Simulation
2.4. Manufacture of Prototypes
2.5. Synthesis and Characterization of Magnetite Particles
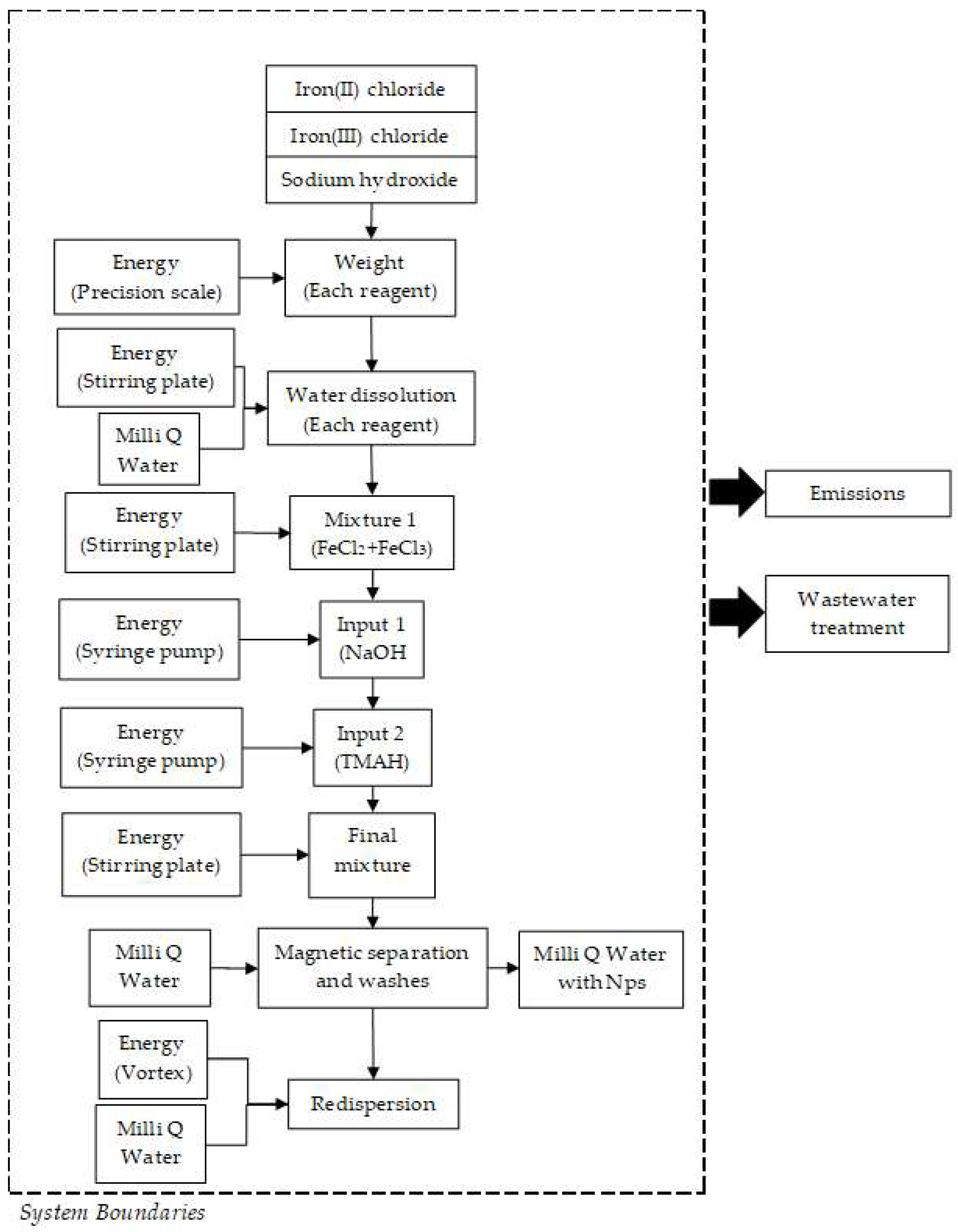
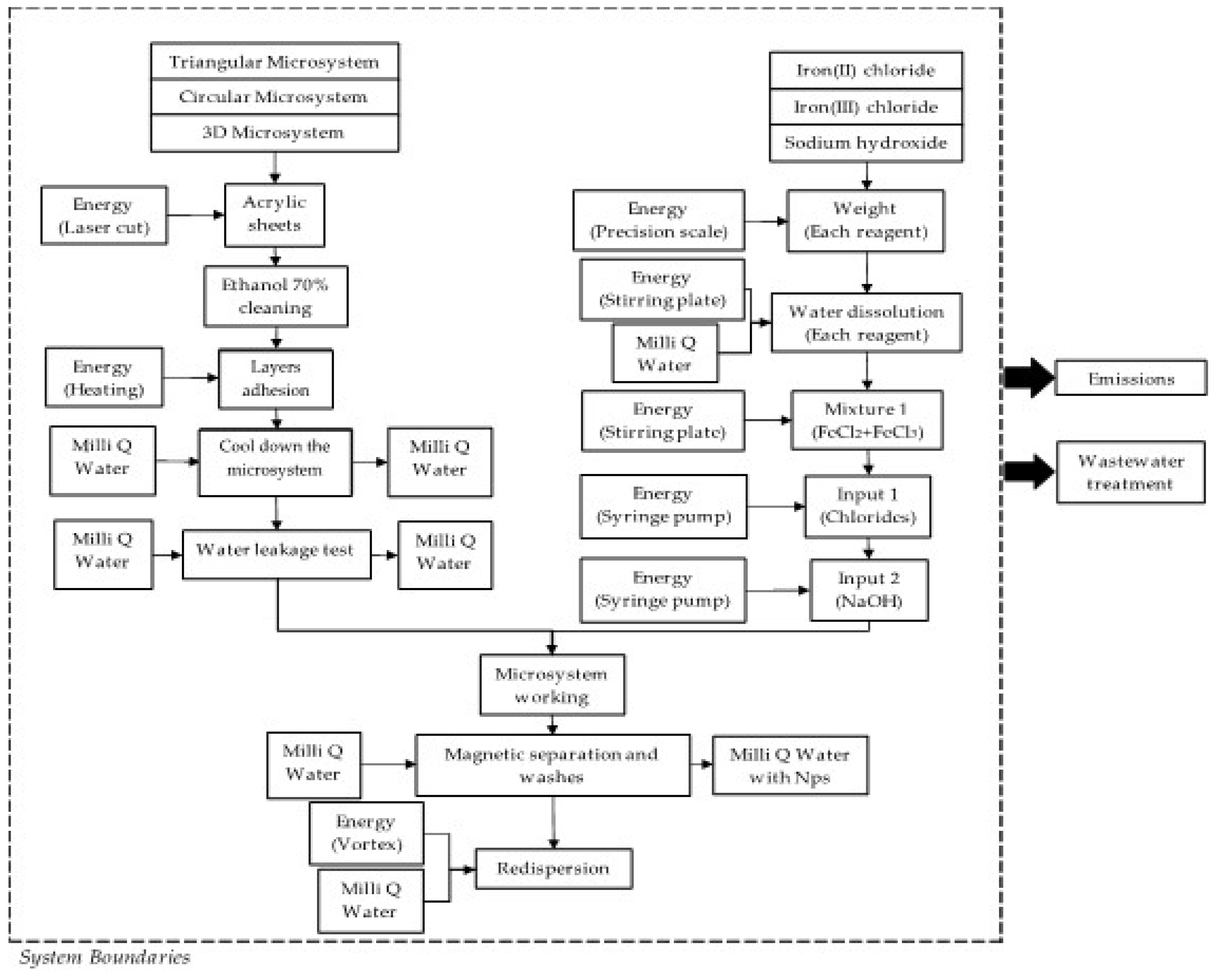
2.6. Life Cycle Assessment (LCA) of the Magnetite (Fe3O4) Synthesis by Co-Precipitation and Micromixers Methods
2.6.1. Goal and Scope Definition
2.6.2. Life Cycle Inventory
3. Results
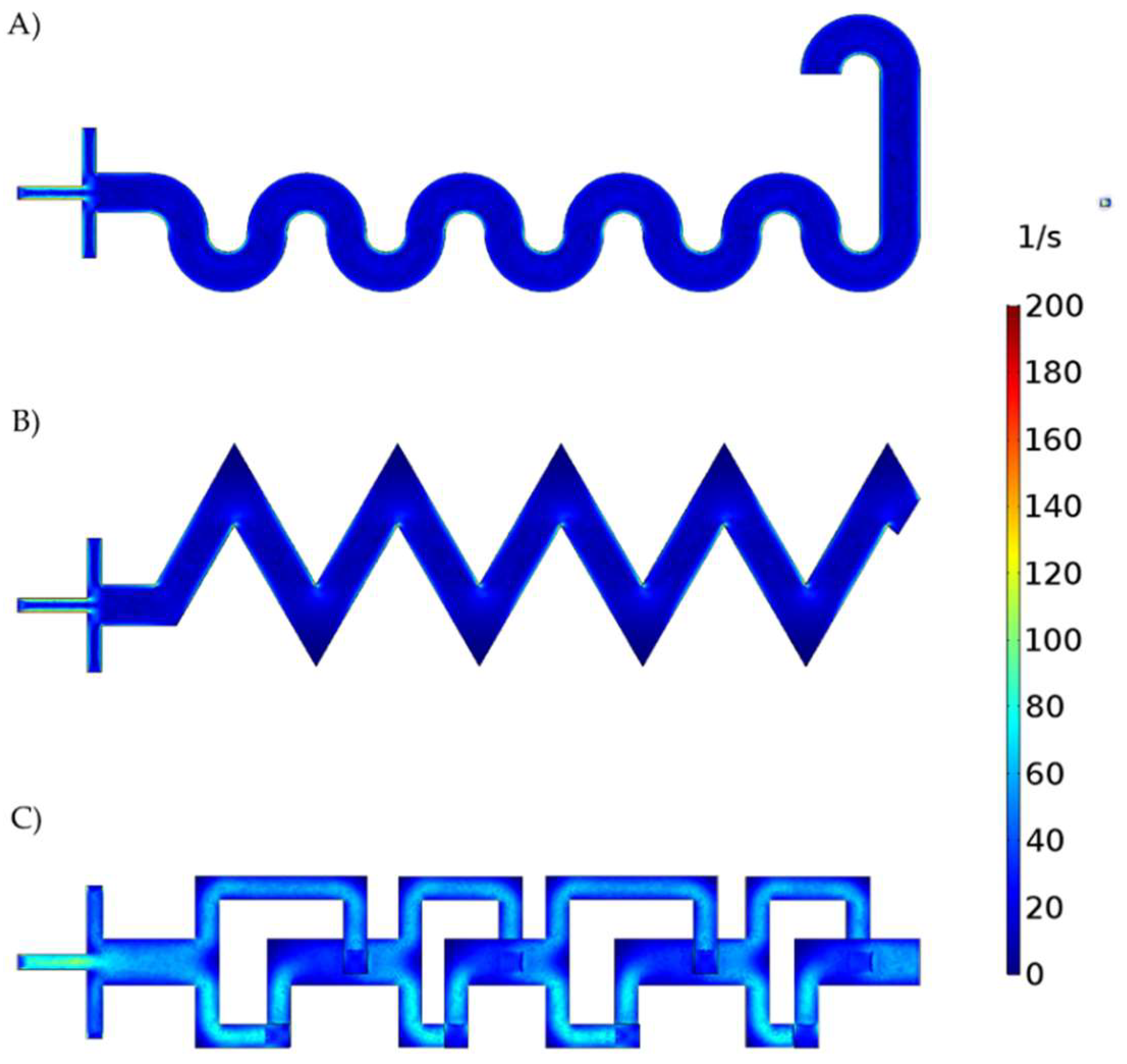
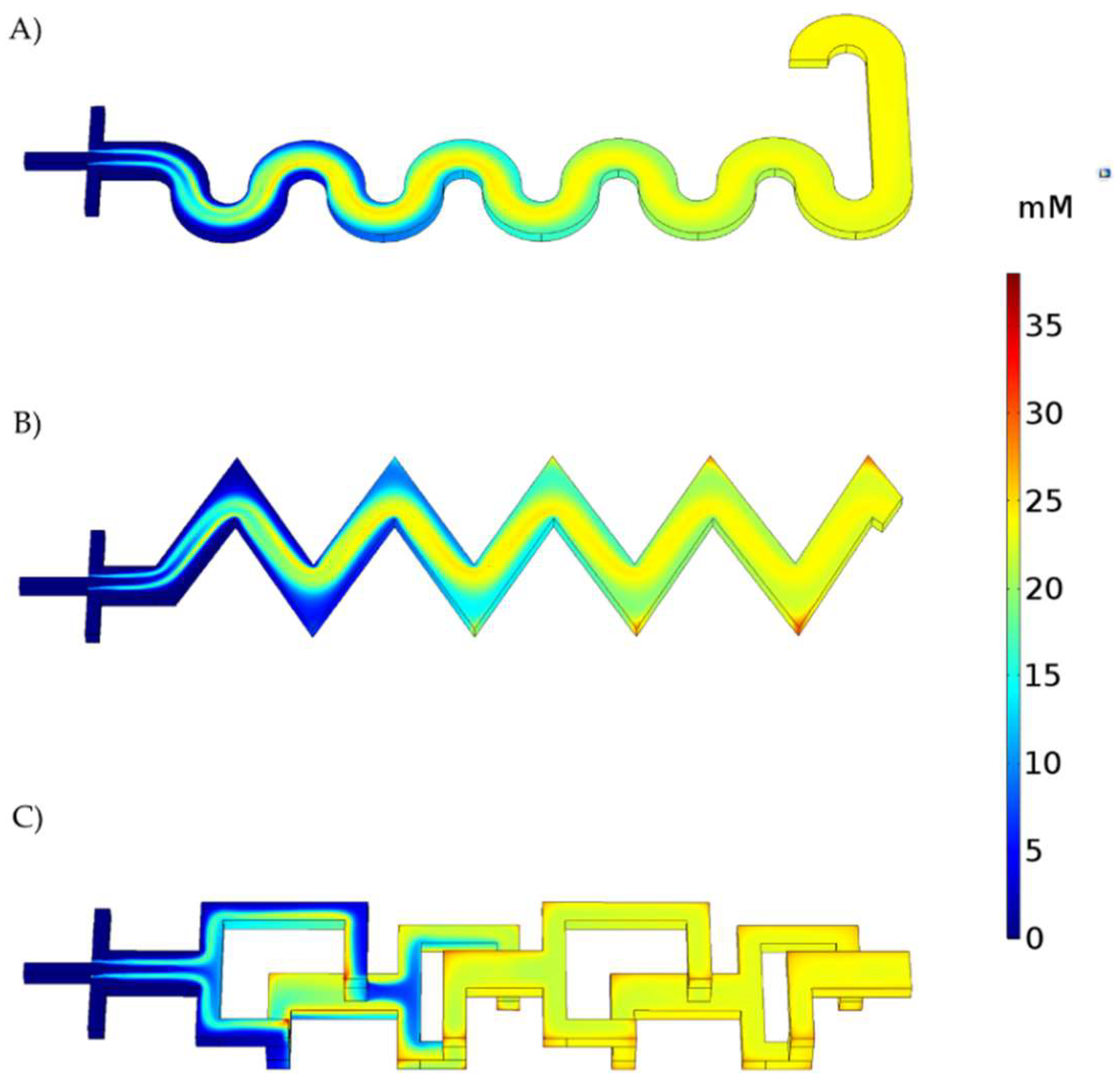
3.1. Microfluidic CFD Simulation
3.2. Reaction Simulation
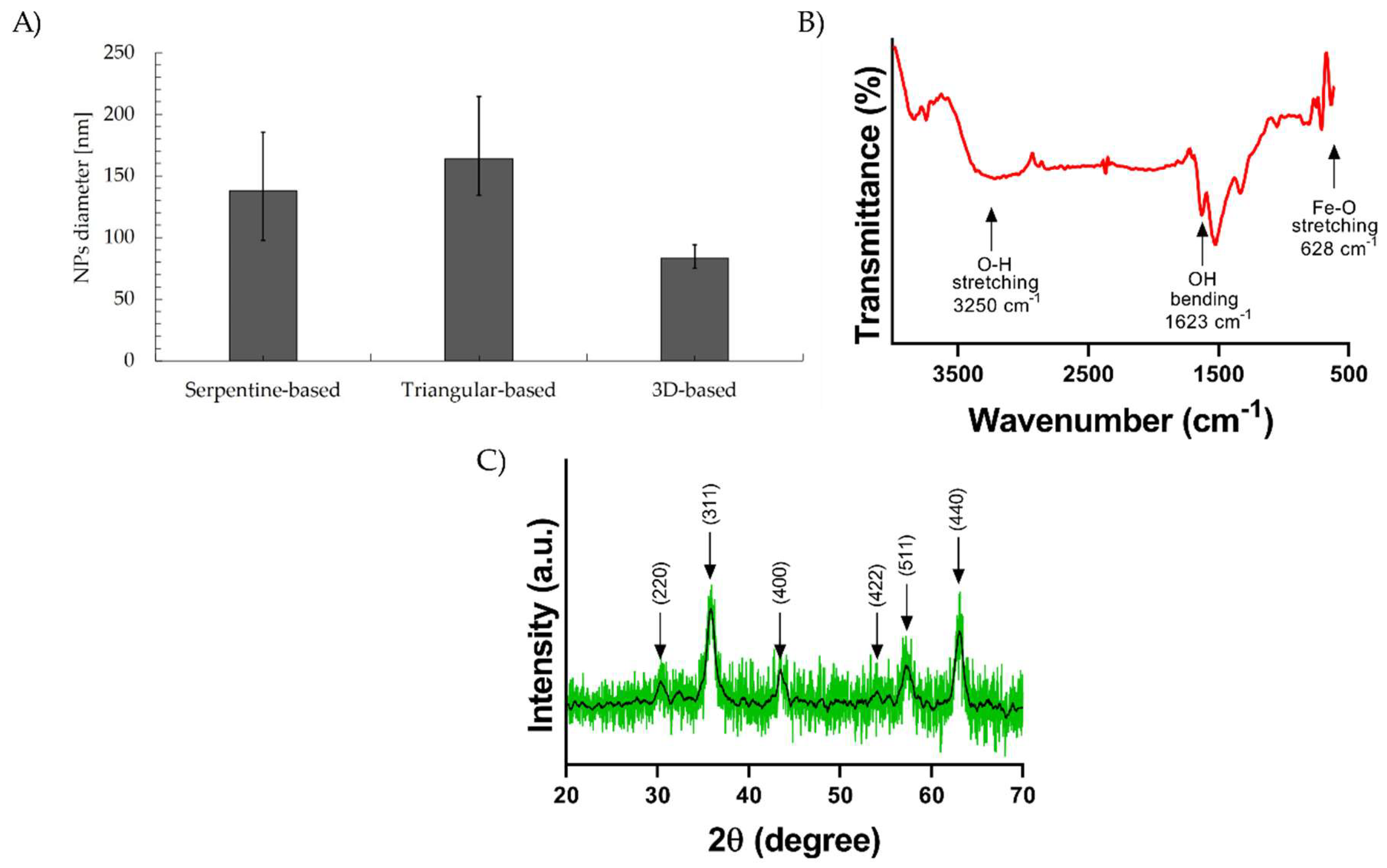
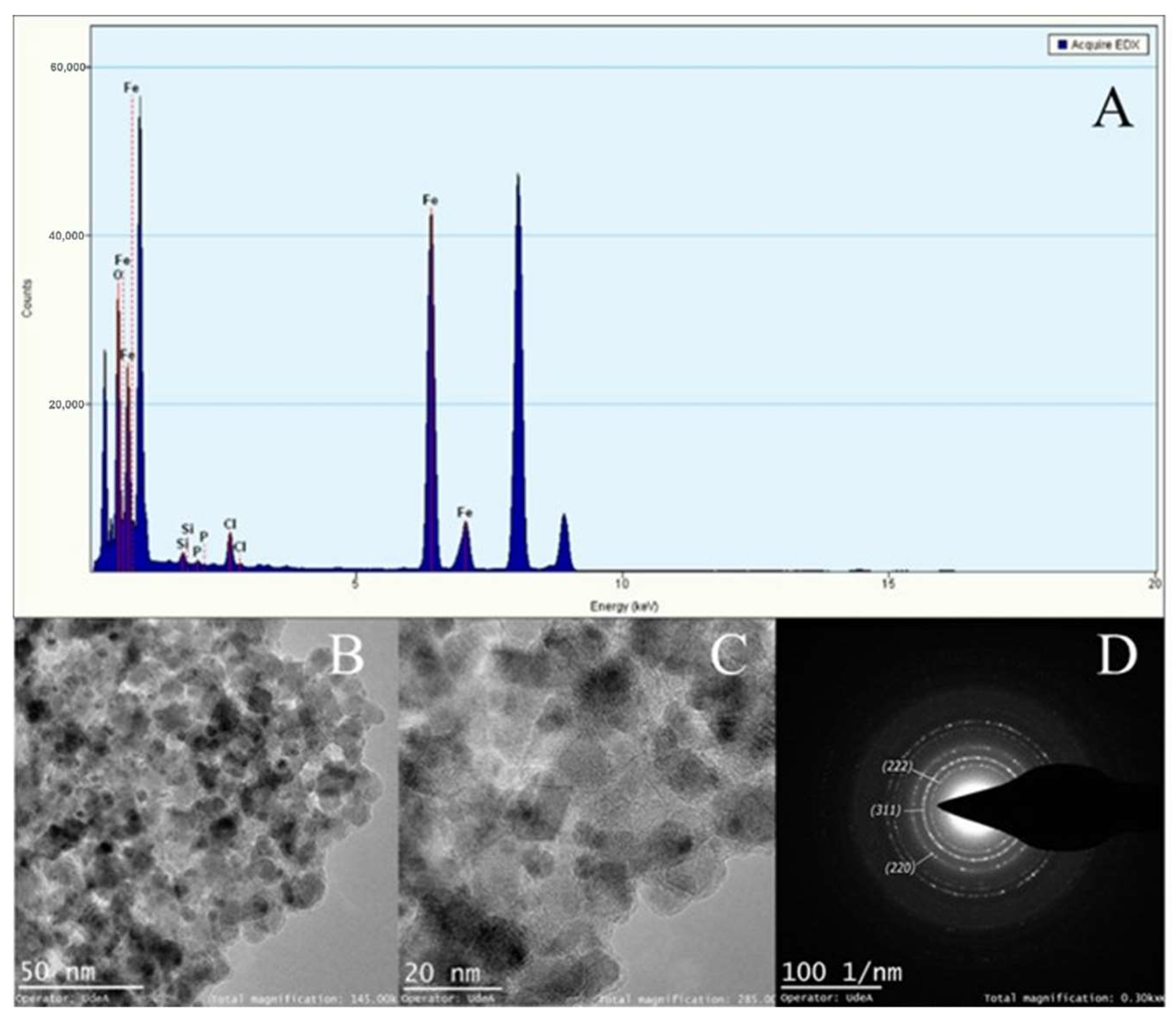
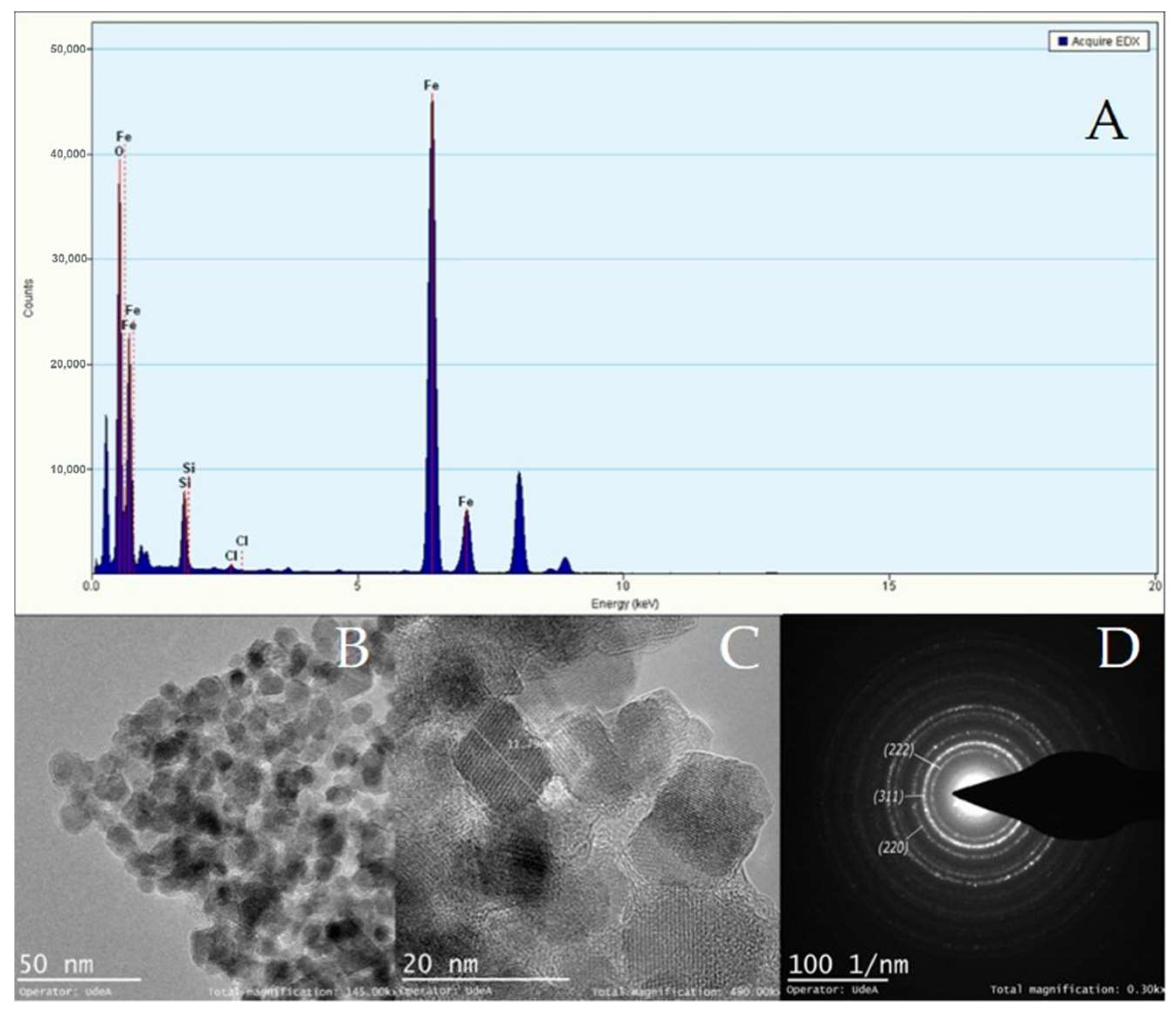
3.3. Synthesis of Magnetite Particles and Characterization of Magnetic Particles
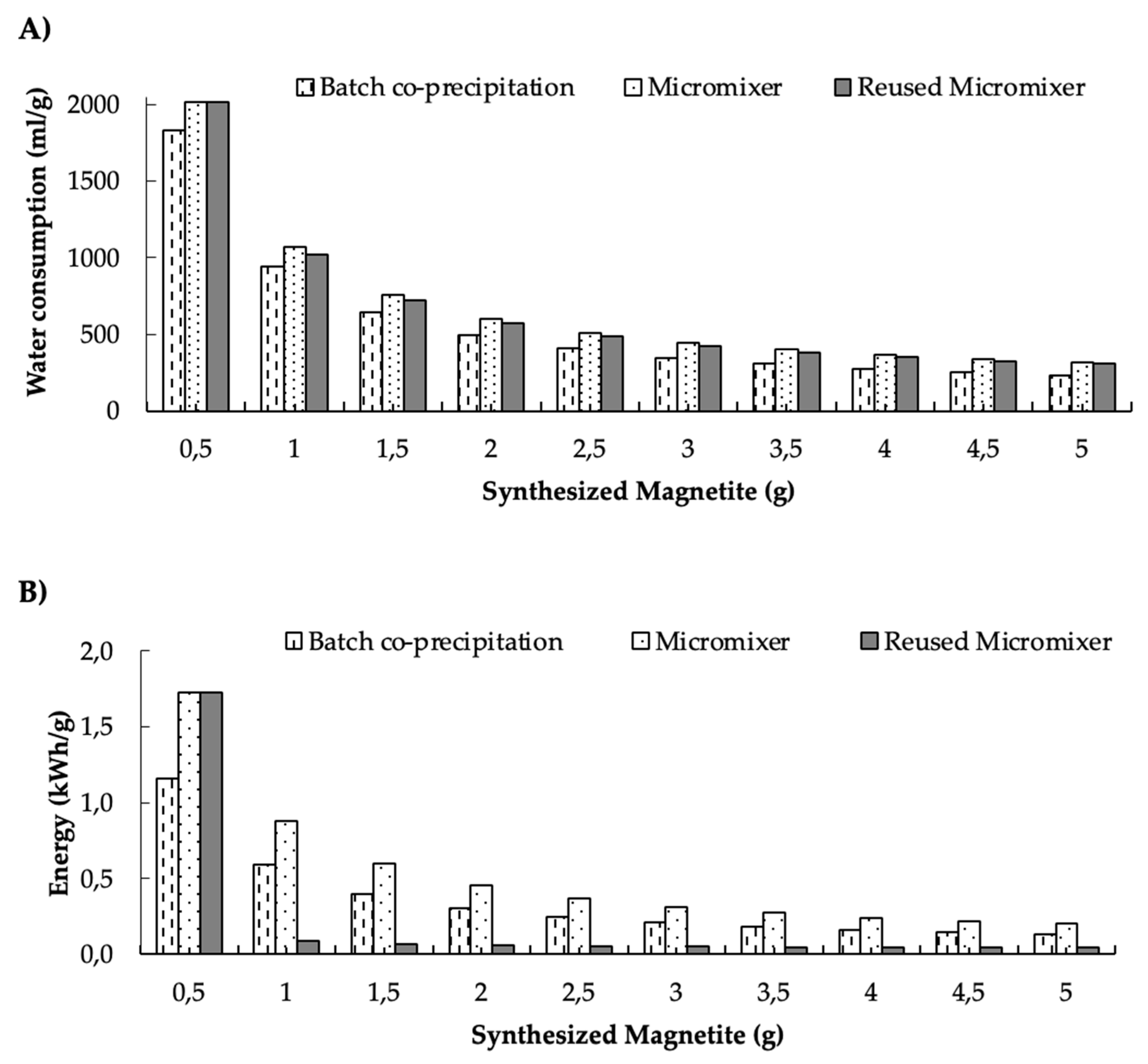
3.4. Water and Energy Consumption Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thu, V.T.; Mai, A.N.; Tam, L.T.; Van Trung, H.; Thu, P.T.; Tien, B.Q.; Thuat, N.T.; Lam, T.D. Fabrication of PDMS-Based Microfluidic Devices: Application for Synthesis of Magnetic Nanoparticles. J. Electron. Mater. 2016, 45, 2576–2581. [Google Scholar] [CrossRef]
- Bemetz, J.; Wegemann, A.; Saatchi, K.; Haase, A.; Häfeli, U.O.; Niessner, R.; Gleich, B.; Seidel, M. Microfluidic-Based Synthesis of Magnetic Nanoparticles Coupled with Miniaturized NMR for Online Relaxation Studies. Anal. Chem. 2018, 90, 9975–9982. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.H.; Harford, J.B.; Eaton, M.A.; Boisseau, P.M.; Dube, A.; Hayeshi, R.; Swai, H.; Lee, D.S. Nanomedicine: Past, present and future—A global perspective. Biochem. Biophys. Res. Commun. 2015, 468, 511–517. [Google Scholar] [CrossRef]
- Herranz, F.; Salinas, B.; Groult, H.; Pellico, J.; Lechuga-Vieco, A.V.; Bhavesh, R.; Ruiz-Cabello, J. Superparamagnetic Nanoparticles for Atherosclerosis Imaging. Nanomaterials 2014, 4, 408–438. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, U.M.; Buhl, E.M.; Draack, S.; Viereck, T.; Ludwig, F.; Schmitz-Rode, T.; Slabu, I. Magnetic Relaxation of Agglomerated and Immobilized Iron Oxide Nanoparticles for Hyperthermia and Imaging Applications. IEEE Magn. Lett. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Meloni, M.M.; Barton, S.; Chivu, A.; Kaski, J.C.; Song, W.; He, T. Facile, productive, and cost-effective synthesis of a novel tetrazine-based iron oxide nanoparticle for targeted image contrast agents and nanomedicines. J. Nanoparticle Res. 2018, 20, 265. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Agnihotri, S.; Roy, I. Preparation and characterization of superparamagnetic iron oxide nanoparticles for magnetically guided drug delivery. Int. J. Nanomed. 2018, 13, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Bedford, E.E.; Boujday, S.; Pradier, C.-M.; Gu, F.X. Spiky gold shells on magnetic particles for DNA biosensors. Talanta 2018, 182, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-Y.; Wu, L.-P.; Chou, T.-T.; Hsieh, Y.-Z. Functional magnetic nanoparticles–assisted electrochemical biosensor for eosinophil cationic protein in cell culture. Sensors Actuators B Chem. 2018, 257, 672–677. [Google Scholar] [CrossRef]
- Tay, A.; Pfeiffer, D.; Rowe, K.; Tannenbaum, A.; Popp, F.; Strangeway, R.; Schüler, D.; Di Carlo, D. High-Throughput Microfluidic Sorting of Live Magnetotactic Bacteria. Appl. Environ. Microbiol. 2018, 84, 1308–1326. [Google Scholar] [CrossRef] [Green Version]
- Rajput, S.; Pittman, C.U., Jr.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; Barandiarán, J.M.; Barquín, L.F.; García-Arribas, A. Magnetic Nanoparticles, Synthesis, Properties, and Applications. In Magnetic Nanostructured Materials: From Lab. to Fab; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–40. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Barahuie, F.; Dorniani, D.; Saifullah, B.; Gothai, S.; Hussein, M.Z.; Pandurangan, A.K.; Arulselvan, P. Sustained release of anticancer agent phytic acid from its chitosan-coated magnetic nanoparticles for drug-delivery system. Int. J. Nanomed. 2017, 12, 2361–2372. [Google Scholar] [CrossRef] [Green Version]
- Roca, A.G.; Gutiérrez, L.; Gavilán, H.; Fortes Brollo, M.E.; Veintemillas-Verdaguer, S.; del Puerto Morales, M. Design strategies for shape-controlled magnetic iron oxide nanoparticles, Adv. Drug Deliv. Rev. 2019, 138, 68–104. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.-P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2018, 138, 56–67. [Google Scholar] [CrossRef]
- Villegas, V.A.R.; Ramírez, J.I.D.L.; Guevara, E.H.; Sicairos, S.P.; Ayala, L.A.H.; Sanchez, B.L. Synthesis and characterization of magnetite nanoparticles for photocatalysis of nitrobenzene. J. Saudi Chem. Soc. 2019, 24, 223–235. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, X.; Zhang, Y.; Natalia, A.; Sun, X.; Wang, Z.; Shao, H. Design and synthesis of magnetic nanoparticles for biomedical diagnostics. Quant. Imaging Med. Surg. 2018, 8, 957–970. [Google Scholar] [CrossRef]
- Yang, C.-H.; Wang, C.-Y.; Huang, K.-S.; Kung, C.-P.; Chang, Y.-C.; Shaw, J.-F. Microfluidic one-step synthesis of Fe3O4-chitosan composite particles and their applications. Int. J. Pharm. 2014, 463, 155–160. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab Chip 2016, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Gonidec, M.; Puigmartí-Luis, J. Continuous-versus Segmented-Flow Microfluidic Synthesis in Materials Science. Crystals 2018, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.M.; Abouezzi, J.; Ristroph, L. Early turbulence and pulsatile flows enhance diodicity of Tesla’s macrofluidic valve. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Habhab, M.-B.; Ismail, T.; Lo, J. A Laminar Flow-Based Microfluidic Tesla Pump via Lithography Enabled 3D Printing. Sensors 2016, 16, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, C.-C.; Choi, J.-W.; Ahn, C.H. A novel in-plane passive microfluidic mixer with modified Tesla structures. Lab a Chip 2004, 4, 109–113. [Google Scholar] [CrossRef]
- Hossain, S.; Ansari, M.A.; Husain, A.; Kim, K.-Y. Analysis and optimization of a micromixer with a modified Tesla structure. Chem. Eng. J. 2010, 158, 305–314. [Google Scholar] [CrossRef]
- Li, J.; Xia, G.; Li, Y. Numerical and experimental analyses of planar asymmetric split-and-recombine micromixer with dislocation sub-channels. J. Chem. Technol. Biotechnol. 2013, 88, 1757–1765. [Google Scholar] [CrossRef]
- Ansari, M.A.; Kim, K.-Y. Mixing performance of unbalanced split and recombine micomixers with circular and rhombic sub-channels. Chem. Eng. J. 2010, 162, 760–767. [Google Scholar] [CrossRef]
- Xia, G.; Li, J.; Tian, X.; Zhou, M. Analysis of Flow and Mixing Characteristics of Planar Asymmetric Split-and-Recombine (P-SAR) Micromixers with Fan-Shaped Cavities. Ind. Eng. Chem. Res. 2012, 51, 7816–7827. [Google Scholar] [CrossRef]
- Hossain, S.; Ansari, M.; Kim, K.-Y. Evaluation of the mixing performance of three passive micromixers. Chem. Eng. J. 2009, 150, 492–501. [Google Scholar] [CrossRef]
- Alam, A.; Kim, K.-Y. Analysis of mixing in a curved microchannel with rectangular grooves. Chem. Eng. J. 2012, 181–182, 708–716. [Google Scholar] [CrossRef]
- Ansari, M.A.; Kim, K.-Y. Parametric study on mixing of two fluids in a three-dimensional serpentine microchannel. Chem. Eng. J. 2009, 146, 439–448. [Google Scholar] [CrossRef]
- Hossain, S.; Kim, K.-Y. Mixing analysis in a three-dimensional serpentine split-and-recombine micromixer. Chem. Eng. Res. Des. 2015, 100, 95–103. [Google Scholar] [CrossRef]
- Liu, R.H.; Stremler, M.A.; Sharp, K.V.; Olsen, M.G.; Santiago, J.G.; Adrian, R.J.; Aref, H.; Beebe, D.J. Passive mixing in a three-dimensional serpentine microchannel. J. Microelectromechanical Syst. 2000, 9, 190–197. [Google Scholar] [CrossRef]
- Lin, Y. Numerical characterization of simple three-dimensional chaotic micromixers. Chem. Eng. J. 2015, 277, 303–311. [Google Scholar] [CrossRef]
- Liga, A.; Morton, J.A.S.; Kersaudy-Kerhoas, M. Safe and cost-effective rapid-prototyping of multilayer PMMA microfluidic devices. Microfluid. Nanofluidics 2016, 20, 164. [Google Scholar] [CrossRef] [Green Version]
- Trinh, K.T.L.; Thai, D.A.; Chae, W.R.; Lee, N.Y. Rapid Fabrication of Poly(methyl methacrylate) Devices for Lab-on-a-Chip Applications Using Acetic Acid and UV Treatment. ACS Omega 2020, 5, 17396–17404. [Google Scholar] [CrossRef]
- Bruus, H. Theoretical Microfluidics; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Choi, S.; Lee, M.G.; Park, J.-K. Microfluidic parallel circuit for measurement of hydraulic resistance. Biomicrofluidics 2010, 4, 034110. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, N.; Chen, J.; Rodgers, V.G.J.; Brisk, P.; Grover, W.H. Finding the optimal design of a passive microfluidic mixer. Lab Chip 2019, 19, 3618–3627. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Wang, W.-T.; Liu, C.-C.; Fu, L.-M. Passive mixers in microfluidic systems: A review. Chem. Eng. J. 2016, 288, 146–160. [Google Scholar] [CrossRef]
- Suh, S.; Traore, M.A.; Behkam, B. Bacterial chemotaxis-enabled autonomous sorting of nanoparticles of comparable sizes. Lab a Chip 2016, 16, 1254–1260. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Chen, X.; Wu, Z.; Zheng, Y. Three-dimensional numerical simulation of droplet formation in a microfluidic flow-focusing device. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 265. [Google Scholar] [CrossRef]
- Plevniak, K.; Campbell, M.; He, M. 3D Printed Microfluidic Mixer for Point-of-Care Diagnosis of Anemia. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS 2016, 2016, 267–270. [Google Scholar]
- Yin, P.; Zhao, L.; Chen, Z.; Jiao, Z.; Shi, H.; Hu, B.; Yuan, S.; Tian, J. Simulation and practice of particle inertial focusing in 3D-printed serpentine microfluidic chips via commercial 3D-printers. Soft Matter 2020, 16, 3096–3105. [Google Scholar] [CrossRef] [PubMed]
- Peiró, J.; Sherwin, S. Finite Difference, Finite Element and Finite Volume Methods for Partial Differential Equations. In Handbook of Materials Modeling; Springer: Cham, Switzerland, 2005; pp. 2415–2446. [Google Scholar] [CrossRef]
- Shamloo, A.; Boodaghi, M. Design and simulation of a microfluidic device for acoustic cell separation. Ultrasonics 2018, 84, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Plevniak, K.; Campbell, M.; Myers, T.; Hodges, A.; He, M. 3D printed auto-mixing chip enables rapid smartphone diagnosis of anemia. Biomicrofluidics 2016, 10, 054113. [Google Scholar] [CrossRef] [Green Version]
- Shamloo, A.; Parast, F.Y. Simulation of Blood Particle Separation in a Trapezoidal Microfluidic Device by Acoustic Force. IEEE Trans. Electron Devices 2019, 66, 1495–1503. [Google Scholar] [CrossRef]
- Campaña, A.L.; Florez, S.L.; Noguera, M.J.; Fuentes, O.P.; Puentes, P.R.; Cruz, J.C.; Osma, J.F. Enzyme-Based Electrochemical Biosensors for Microfluidic Platforms to Detect Pharmaceutical Residues in Wastewater. Biosensors 2019, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, X. Dielectrophoretic microfluidic device for separation of red blood cells and platelets: A model-based study. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 89. [Google Scholar] [CrossRef]
- Levasseur, A.; Cavalett, O.; Fuglestvedt, J.S.; Gasser, T.; Johansson, D.J.; Jørgensen, S.V.; Raugei, M.; Reisinger, A.; Schivley, G.; Strømman, A.; et al. Enhancing life cycle impact assessment from climate science: Review of recent findings and recommendations for application to LCA. Ecol. Indic. 2016, 71, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Marimón-Bolívar, W.; Gonzalez, E.E. Green synthesis with enhanced magnetization and life cycle assessment of Fe3O4 nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018, 9, 58–66. [Google Scholar] [CrossRef]
- Grimaldi, F.; Pucciarelli, M.; Gavriilidis, A.; Dobson, P.; Lettieri, P. Anticipatory life cycle assessment of gold nanoparticles production: Comparison of milli-continuous flow and batch synthesis. J. Clean. Prod. 2020, 269, 122335. [Google Scholar] [CrossRef]
- Chen, S.; Fu, X.; Chu, M.; Liu, Z.; Tang, J. Life cycle assessment of the comprehensive utilisation of vanadium titano-magnetite. J. Clean. Prod. 2015, 101, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Corominas, L.; Byrne, D.M.; Guest, J.S.; Hospido, A.; Roux, P.; Shaw, A.; Short, M.D. The application of life cycle assessment (LCA) to wastewater treatment: A best practice guide and critical review. Water Res. 2020, 184, 116058. [Google Scholar] [CrossRef] [PubMed]
- Salieri, B.; Turner, D.A.; Nowack, B.; Hischier, R. Life cycle assessment of manufactured nanomaterials: Where are we? NanoImpact 2018, 10, 108–120. [Google Scholar] [CrossRef]
- Shahid, M.K.; Choi, Y. Characterization and application of magnetite Particles, synthesized by reverse coprecipitation method in open air from mill scale. J. Magn. Magn. Mater. 2019, 495, 165823. [Google Scholar] [CrossRef]
- Schenk, O.; Gärtner, K.; Fichtner, W.; Stricker, A. PARDISO: A high-performance serial and parallel sparse linear solver in semiconductor device simulation. Futur. Gener. Comput. Syst. 2001, 18, 69–78. [Google Scholar] [CrossRef]
- Hischier, R.; Walser, T. Life cycle assessment of engineered nanomaterials: State of the art and strategies to overcome existing gaps. Sci. Total Environ. 2012, 425, 271–282. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Mitra, D. Multiphysical phenomenon of air bubble growth in polydimethylsiloxane channel corners under microfluidic negative pressure-driven flow. Int. J. Heat Mass Transf. 2015, 91, 611–618. [Google Scholar] [CrossRef]
- Pereiro, I.; Khartchenko, A.F.; Petrini, L.; Kaigala, G.V. Nip the bubble in the bud: A guide to avoid gas nucleation in microfluidics. Lab a Chip 2019, 19, 2296–2314. [Google Scholar] [CrossRef]
- McGinty, J.; Yazdanpanah, N.; Price, C.; ter Horst, J.H.; Sefcik, J. CHAPTER 1. Nucleation and Crystal Growth in Continuous Crystallization. In The Handbook of Continuous Crystallization; Royal Society of Chemistry: London, UK, 2020; pp. 1–50. [Google Scholar] [CrossRef]
- Wilson, J.F.; Kroupa, M.; Kosek, J.; Soos, M. Numerical Study of Soft Colloidal Nanoparticles Interaction in Shear Flow. Langmuir 2018, 34, 15600–15611. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lin, C.-H.; Fu, L.-M. Passive Micromixers. In Encyclopedia of Microfluidics and Nanofluidics; Springer: Boston, MA, USA, 2008; pp. 1602–1610. [Google Scholar] [CrossRef]
- Clark, J.; Kaufman, M.; Fodor, P.S. Mixing Enhancement in Serpentine Micromixers with a Non-Rectangular Cross-Section. Micromachines 2018, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Morel, A.-L.; Nikitenko, S.I.; Gionnet, K.; Wattiaux, A.; Lai-Kee-Him, J.; Labrugere, C.; Chevalier, B.; Deleris, G.; Petibois, C.; Brisson, A.; et al. Sonochemical Approach to the Synthesis of Fe3O4@SiO2 Core−Shell Nanoparticles with Tunable Properties. ACS Nano 2008, 2, 847–856. [Google Scholar] [CrossRef]
- Franco, F.; Pérez-Maqueda, L.; Pérez-Rodríguez, J. The effect of ultrasound on the particle size and structural disorder of a well-ordered kaolinite. J. Colloid Interface Sci. 2004, 274, 107–117. [Google Scholar] [CrossRef]
- Nalbandian, L.; Patrikiadou, E.; Zaspalis, V.; Patrikidou, A.; Hatzidaki, E.; Papandreou, C.N. Magnetic Nanoparticles in Medical Diagnostic Applications: Synthesis, Characterization and Proteins Conjugation. Curr. Nanosci. 2016, 12, 455–468. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Mohammadi, H.; Nekobahr, E.; Akhtari, J.; Saeedi, M.; Akbari, J.; Fathi, F. Synthesis and characterization of magnetite nanoparticles by co-precipitation method coated with biocompatible compounds and evaluation of in-vitro cytotoxicity. Toxicol. Rep. 2021, 8, 331–336. [Google Scholar] [CrossRef]
- Ramírez-Acosta, C.M.; Cifuentes, J.; Castellanos, M.C.; Moreno, R.J.; Muñoz-Camargo, C.; Cruz, J.C.; Reyes, L.H. PH-Responsive, Cell-Penetrating, Core/Shell Magnetite/Silver Nanoparticles for the Delivery of Plasmids: Preparation, Characterization, and Preliminary In Vitro Evaluation. Pharmaceutics 2020, 12, 561. [Google Scholar] [CrossRef]
- Fuentes, O.P.; Noguera, M.J.; Peñaranda, P.A.; Flores, S.L.; Cruz, J.C.; Osma, J.F. Micromixers for Wastewater Treatment and Their Life Cycle Assessment (LCA). In Advances in Microfluidics and Nanofluids; InTechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Badilescu, S.; Packirisamy, M. Microfluidics-Nano-Integration for Synthesis and Sensing. Polymers 2012, 4, 1278–1310. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Kim, K.-J.; Chang, C.-H. Segmented Microfluidic Flow Reactors for Nanomaterial Synthesis. Nanomaterials 2020, 10, 1421. [Google Scholar] [CrossRef]
- Hakke, V.; Sonawane, S.; Anandan, S.; Sonawane, S.; AshokKumar, M. Process Intensification Approach Using Microreactors for Synthesizing Nanomaterials—A Critical Review. Nanomaterials 2021, 11, 98. [Google Scholar] [CrossRef]
- Dai, J.; Yang, X.; Hamon, M.; Kong, L. Particle size controlled synthesis of CdS nanoparticles on a microfluidic chip. Chem. Eng. J. 2015, 280, 385–390. [Google Scholar] [CrossRef]
- Baek, S.; Song, S.; Lee, J.; Kim, J.-M. Nanoscale diameter control of sensory polydiacetylene nanoparticles on microfluidic chip for enhanced fluorescence signal. Sensors Actuators B Chem. 2016, 230, 623–629. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Bazzi, R.; Cabuil, V. Multistep Continuous-Flow Microsynthesis of Magnetic and Fluorescent γ-Fe2O3@SiO2Core/Shell Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 7180–7183. [Google Scholar] [CrossRef]
- Li, L.; Sanchez, J.R.; Kohler, F.; Røyne, A.; Dysthe, D.K. Microfluidic Control of Nucleation and Growth of CaCO3. Cryst. Growth Des. 2018, 18, 4528–4535. [Google Scholar] [CrossRef] [Green Version]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Barbosa, N.; Garcia, J.G.; Cifuentes, J.; Castro, L.M.; Vargas, F.; Ostos, C.; Cardona-Gomez, G.P.; Hernandez, A.M.; Cruz, J.C. Multifunctional magnetite nanoparticles to enable delivery of siRNA for the potential treatment of Alzheimer’s. Drug Deliv. 2020, 27, 864–875. [Google Scholar] [CrossRef]
- Rangel-Muñoz, N.; González-Barrios, A.; Pradilla, D.; Osma, J.; Cruz, J. Novel Bionanocompounds: Outer Membrane Protein A and Lacasse Co-Immobilized on Magnetite Nanoparticles for Produced Water Treatment. Nanomaterials 2020, 10, 2278. [Google Scholar] [CrossRef]
- Yazdani, F.; Seddigh, M. Magnetite nanoparticles synthesized by co-precipitation method: The effects of various iron anions on specifications. Mater. Chem. Phys. 2016, 184, 318–323. [Google Scholar] [CrossRef]
- Roth, H.-C.; Schwaminger, S.P.; Schindler, M.; Wagner, F.E.; Berensmeier, S. Influencing factors in the CO-precipitation process of superparamagnetic iron oxide nano particles: A model based study. J. Magn. Magn. Mater. 2015, 377, 81–89. [Google Scholar] [CrossRef]
- Suppiah, D.D.; Hamid, S.B.A. One step facile synthesis of ferromagnetic magnetite nanoparticles. J. Magn. Magn. Mater. 2016, 414, 204–208. [Google Scholar] [CrossRef]
- Scott, S.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Meramo-Hurtado, S.; Moreno-Sader, K.; González-Delgado, D. Computer-aided simulation and exergy analysis of TiO2 nanoparticles production via green chemistry. PeerJ 2019, 7, e8113. [Google Scholar] [CrossRef] [Green Version]
- Bartolozzi, I.; Daddi, T.; Punta, C.; Fiorati, A.; Iraldo, F. Life cycle assessment of emerging environmental technologies in the early stage of development: A case study on nanostructured materials. J. Ind. Ecol. 2019, 24, 101–115. [Google Scholar] [CrossRef]



| Parameters | Value | Units |
|---|---|---|
| Density of the fluid | 1000 | kg/m3 |
| Viscosity of the fluid | 1 | mPa.s |
| Rate constant | 1 × 10−24 | m30/(s·mol10) |
| FeCl2 molar mass | 0.199 | kg/mol |
| FeCl3 molar mass | 0.270 | kg/mol |
| NaOH molar mass | 0.040 | kg/mol |
| Fe3O4 molar mass | 0.232 | kg/mol |
| NaCl molar mass | 0.058 | kg/mol |
| H2O molar mass | 0.018 | kg/mol |
| FeCl2 inflow concentration | 100 | mM |
| FeCl3 inflow concentration | 200 | mM |
| NaOH inflow concentration | 800 | mM |
| Central inlet normal inflow rate | 1 | ml/min |
| Lateral inlet normal inflow rate | 0.5 | ml/min |
| Method | Inventory | Amount | Unit |
|---|---|---|---|
| Co-precipitation | Inputs | ||
| Reagents preparation | |||
| Iron (II) chloride tetrahydrate ) | 0.43 | g | |
| Iron (III) chloride hexahydrate ) | 1.17 | g | |
| Sodium hydroxide (NaOH) | 0.69 | g | |
| Tetramethylammonium hydroxide (TMAH) | 0.0012 | L | |
| Energy (Precision scale) | 0.0033 | kWh | |
| Water consumption | 0.026 | L | |
| Energy (Stirring plate) | 0.0343 | kWh | |
| Synthesis | |||
| Energy (Syringe pump) | 0.01067 | kWh | |
| Energy (Stirring plate) | 0.5150 | kWh | |
| Washing and re-dispersion | |||
| Water consumption | 0.89 | L | |
| Energy (Vortex) | 0.0176 | kWh | |
| Outputs | |||
| Wastewater | 0.9 | L | |
| Micromixer | Inputs | ||
| Micromixers | |||
| Acrylic (PMMA 3 mm) | 0.000039375 | m3 | |
| Energy (Laser cut) | 0.7 | kWh | |
| Ethanol 70% | 10 | mL | |
| Ethanol 96% | 5 | mL | |
| Energy (hot plate) | 0.09 | kWh | |
| Acrylic glue | 1.5 | g | |
| Water consumption | 0.053 | L | |
| Reagents preparation | |||
| Iron (II) chloride tetrahydrate ) | 0.43 | g | |
| Iron (III) chloride hexahydrate ) | 1.17 | g | |
| Sodium hydroxide (NaOH) | 0.69 | g | |
| Tetramethylammonium hydroxide (TMAH) | 1 | mL | |
| Energy (Precision scale) | 0.005 | kWh | |
| Water consumption | 0.06579 | L | |
| Energy (Stirring plate) | 0.0343 | kWh | |
| Synthesis | |||
| Energy (Syringe pump) | 0.0072 | kWh | |
| Washing and re-dispersion | |||
| Water consumption | 0.89 | L | |
| Energy (Vortex) | 0.0176 | kWh | |
| Outputs | |||
| Water consumption | 0.0525 | L | |
| Wastewater | 0.9 | L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florez, S.L.; Campaña, A.L.; Noguera, M.J.; Quezada, V.; Fuentes, O.P.; Cruz, J.C.; Osma, J.F. CFD Analysis and Life Cycle Assessment of Continuous Synthesis of Magnetite Nanoparticles Using 2D and 3D Micromixers. Micromachines 2022, 13, 970. https://doi.org/10.3390/mi13060970
Florez SL, Campaña AL, Noguera MJ, Quezada V, Fuentes OP, Cruz JC, Osma JF. CFD Analysis and Life Cycle Assessment of Continuous Synthesis of Magnetite Nanoparticles Using 2D and 3D Micromixers. Micromachines. 2022; 13(6):970. https://doi.org/10.3390/mi13060970
Chicago/Turabian StyleFlorez, Sergio Leonardo, Ana Lucia Campaña, M. Juliana Noguera, Valentina Quezada, Olga P. Fuentes, Juan C. Cruz, and Johann F. Osma. 2022. "CFD Analysis and Life Cycle Assessment of Continuous Synthesis of Magnetite Nanoparticles Using 2D and 3D Micromixers" Micromachines 13, no. 6: 970. https://doi.org/10.3390/mi13060970
APA StyleFlorez, S. L., Campaña, A. L., Noguera, M. J., Quezada, V., Fuentes, O. P., Cruz, J. C., & Osma, J. F. (2022). CFD Analysis and Life Cycle Assessment of Continuous Synthesis of Magnetite Nanoparticles Using 2D and 3D Micromixers. Micromachines, 13(6), 970. https://doi.org/10.3390/mi13060970








