Versatile and Low-Cost Fabrication of Modular Lock-and-Key Microfluidics for Integrated Connector Mixer Using a Stereolithography 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design of Primary Modular Fluidic Platform
2.3. Evaluation of Printing Parameters
2.4. Approach for Submillimeter-Scale Chamber Module
3. Results and Discussion
3.1. Basic Optimization Using the Test Model Setting
3.2. Dimension Limit Investigation from Simple Structures
3.3. Post-Processing and Printed Module Evaluation
3.4. Chamber Modules and Strategy to Approach a Submillimeter Scale Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puiu, M.; Bala, C. Microfluidics-integrated biosensing platforms as emergency tools for on-site field detection of foodborne pathogens. TrAC-Trends Anal. Chem. 2020, 125, 115831. [Google Scholar] [CrossRef]
- Camarillo-Escobedo, R.M.; Flores-Nuñez, J.L.; Garcia-Torales, G.; Hernandez-Campos, E.; Camarillo-Escobedo, J.M. 3D printed opto-microfluidic autonomous analyzer for photometric applications. Sens. Actuators A Phys. 2022, 337. [Google Scholar] [CrossRef]
- Xiao, C.; Eriksson, J.; Suska, A.; Filippini, D.; Mak, W.C. Print-and-stick unibody microfluidics coupled surface plasmon resonance (SPR) chip for smartphone imaging SPR (Smart-iSRP). Anal. Chim. Acta 2022, 1201, 339606. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, A.; Alhassan, S.; Senel, M. Lab-In-A-Syringe: A Novel Electrochemical Biosensor for On-Site and Real-Time Monitoring of Dopamine in Freely Behaving Mice. ACS Sens. 2022, 7, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Stobiecka, M. High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: A concise review. Carbohydr. Polym. 2020, 229. [Google Scholar] [CrossRef]
- Cowell, T.W.; Valera, E.; Jankelow, A.; Park, J.; Schrader, A.W.; Ding, R.; Berger, J.; Bashir, R.; Han, H.S. Rapid, multiplexed detection of biomolecules using electrically distinct hydrogel beads. Lab Chip 2020, 20, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.R.; Musgrove, H.B.; Throckmorton, A.L.; Pompano, R.R. Microscale impeller pump for recirculating flow in organs-on-chip and microreactors. Lab Chip 2022, 22, 605–620. [Google Scholar] [CrossRef]
- Hermann, C.A.; Mayer, M.; Griesche, C.; Beck, F.; Baeumner, A.J. Microfluidic-enabled magnetic labelling of nanovesicles for bioanalytical applications. Analyst 2021, 146, 997–1003. [Google Scholar] [CrossRef]
- Mosavati, B.; Oleinikov, A.V.; Du, E. Development of an organ-on-a-chip-device for study of placental pathologies. Int. J. Mol. Sci. 2020, 21, 8755. [Google Scholar] [CrossRef]
- Sunkara, V.; Kumar, S.; Sabaté Del Río, J.; Kim, I.; Cho, Y.K. Lab-on-a-Disc for Point-of-Care Infection Diagnostics. Acc. Chem. Res. 2021, 54, 3643–3655. [Google Scholar] [CrossRef]
- Zuo, Z.-Q.; Pan, J.-Z.; Fang, Q. An integrated microfluidic system for multi-target biochemical analysis of a single drop of blood. Talanta 2022, 249, 123585. [Google Scholar] [CrossRef]
- Svensson, K.; Södergren, S.; Hjort, K. Thermally controlled microfluidic back pressure regulator. Sci. Rep. 2022, 12, 569. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Ipatov, A.; Domingues, T.; Borme, J.; Martins, M.; Alpuim, P.; Prado, M. Programmable graphene-based microfluidic sensor for DNA detection. Sens. Actuators B Chem. 2022, 367, 132044. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Fernandes, E.; Freitas, P. A pump-free microfluidic device for fast magnetic labeling of ischemic stroke biomarkers. Anal. Bioanal. Chem. 2022, 414, 2571–2583. [Google Scholar] [CrossRef]
- Wlodarczyk, K.L.; Hand, D.P.; Maroto-Valer, M.M. Maskless, rapid manufacturing of glass microfluidic devices using a picosecond pulsed laser. Sci. Rep. 2019, 9, 20215. [Google Scholar] [CrossRef]
- Szymborski, T.; Jankowski, P.; Ogończyk, D.; Garstecki, P. An FEP microfluidic reactor for photochemical reactions. Micromachines 2018, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Cong, H.; Zhang, N. Perspectives in translating microfluidic devices from laboratory prototyping into scale-up production. Biomicrofluidics 2022, 16, 021301. [Google Scholar] [CrossRef]
- Kalkal, A.; Kumar, S.; Kumar, P.; Pradhan, R.; Willander, M.; Packirisamy, G.; Kumar, S.; Malhotra, B.D. Recent advances in 3D printing technologies for wearable (bio)sensors. Addit. Manuf. 2021, 46, 102088. [Google Scholar] [CrossRef]
- Fritschen, A.; Bell, A.K.; Königstein, I.; Stühn, L.; Stark, R.W.; Blaeser, A. Investigation and comparison of resin materials in transparent DLP-printing for application in cell culture and organs-on-a-chip. Biomater. Sci. 2022, 10, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.Y.; Wang, X.; Sweet, E.; Liu, N.; Gong, X.; Lin, L. 3D printed microfluidic devices for circulating tumor cells (CTCs) isolation. Biosens. Bioelectron. 2020, 150, 111900. [Google Scholar] [CrossRef] [PubMed]
- Brenda, B.M.; Griveau, S.; Bedioui, F.; d’Orlye, F.; da Silva, J.A.F.; Varenne, A. Stereolithography based 3D-printed microfluidic device with integrated electrochemical detection. Electrochim. Acta 2022, 407, 139888. [Google Scholar] [CrossRef]
- Gonzalez, G.; Roppolo, I.; Pirri, C.F.; Chiappone, A. Current and emerging trends in polymeric 3D printed microfluidic devices. Addit. Manuf. 2022, 55, 102867. [Google Scholar] [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; De Groot, T.E.; Wan, A.M.D.; Beebe, D.J.; Young, E.W.K. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Gao, Q.; Qiu, J.; Sun, M.; Liu, A.; Shao, L.; Fu, J.; Zhao, P.; He, Y. 3D printed Lego®-like modular microfluidic devices based on capillary driving. Biofabrication 2018, 10, 35001. [Google Scholar] [CrossRef]
- Convery, N.; Samardzhieva, I.; Stormonth-Darling, J.M.; Harrison, S.; Sullivan, G.J.; Gadegaard, N. 3D Printed Tooling for Injection Molded Microfluidics. Macromol. Mater. Eng. 2021, 306, 2100464. [Google Scholar] [CrossRef]
- Tech, R.P.G.; Ferdinand, J.-P.; Dopfer, M. Open source hardware startups and their communities. In The Decentralized and Networked Future of Value Creation; Springer: Cham, Switzerland, 2016; pp. 129–145. [Google Scholar]
- Chmayssem, A.; Verplanck, N.; Tanase, C.E.; Costa, G.; Monsalve-Grijalba, K.; Amigues, S.; Alias, M.; Gougis, M.; Mourier, V.; Vignoud, S.; et al. Development of a multiparametric (bio)sensing platform for continuous monitoring of stress metabolites. Talanta 2021, 229, 122275. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [Green Version]
- Ymbern, O.; Berenguel-Alonso, M.; Calvo-López, A.; Gómez-De Pedro, S.; Izquierdo, D.; Alonso-Chamarro, J. Versatile lock and key assembly for optical measurements with microfluidic platforms and cartridges. Anal. Chem. 2015, 87, 1503–1508. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, F.; Mao, H.; Li, S.; Zhu, Y.; Gong, J.; Wang, L.; Malmstadt, N.; Chen, Y. In-situ transfer vat photopolymerization for transparent microfluidic device fabrication. Nat. Commun. 2022, 13, 918. [Google Scholar] [CrossRef] [PubMed]
- Brimmo, A.; Goyette, P.-A.; Alnemari, R.; Gervais, T.; Qasaimeh, M.A. 3D printed microfluidic probes. Sci. Rep. 2018, 8, 10995. [Google Scholar] [CrossRef] [Green Version]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic surface modification of pdms for droplet microfluidics using a simple, quick, and robust method via pva deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef]
- Amoyav, B.; Goldstein, Y.; Steinberg, E.; Benny, O. 3D Printed Microfluidic Devices for Drug Release Assays. Pharmaceutics 2021, 13, 13. [Google Scholar] [CrossRef]
- Lee, J.M.; Zhang, M.; Yeong, W.Y. Characterization and evaluation of 3D printed microfluidic chip for cell processing. Microfluid. Nanofluidics 2016, 20, 5. [Google Scholar] [CrossRef]
- Rogers, C.I.; Qaderi, K.; Woolley, A.T.; Nordin, G.P. 3D printed microfluidic devices with integrated valves. Biomicrofluidics 2015, 9, 16501. [Google Scholar] [CrossRef]
- Shallan, A.I.; Smejkal, P.; Corban, M.; Guijt, R.M.; Breadmore, M.C. Cost-effective three-dimensional printing of visibly transparent microchips within minutes. Anal. Chem. 2014, 86, 3124–3130. [Google Scholar] [CrossRef]
- Au, A.K.; Lee, W.; Folch, A. Mail-order microfluidics: Evaluation of stereolithography for the production of microfluidic devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.; Bhagat, A.A.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134–148. [Google Scholar] [CrossRef]
- Kristensen, M.F.; Leonhardt, D.; Neland, M.L.B.; Schlafer, S. A 3D printed microfluidic flow-cell for microscopy analysis of in situ-grown biofilms. J. Microbiol. Methods 2020, 171, 105876. [Google Scholar] [CrossRef]
- Ymbern, O.; Sández, N.; Calvo-López, A.; Puyol, M.; Alonso-Chamarro, J. Gas diffusion as a new fluidic unit operation for centrifugal microfluidic platforms. Lab Chip 2014, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Felton, H.; Hughes, R.; Diaz-Gaxiola, A. Negligible-cost microfluidic device fabrication using 3D-printed interconnecting channel scaffolds. PLoS ONE 2021, 16, e0245206. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.-S.; Vincent, P.A.; Dai, G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35, 8092–8102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Cui, D.; Li, H. A hard–soft microfluidic-based biosensor flow cell for SPR imaging application. Biosens. Bioelectron. 2010, 26, 255–261. [Google Scholar] [CrossRef]

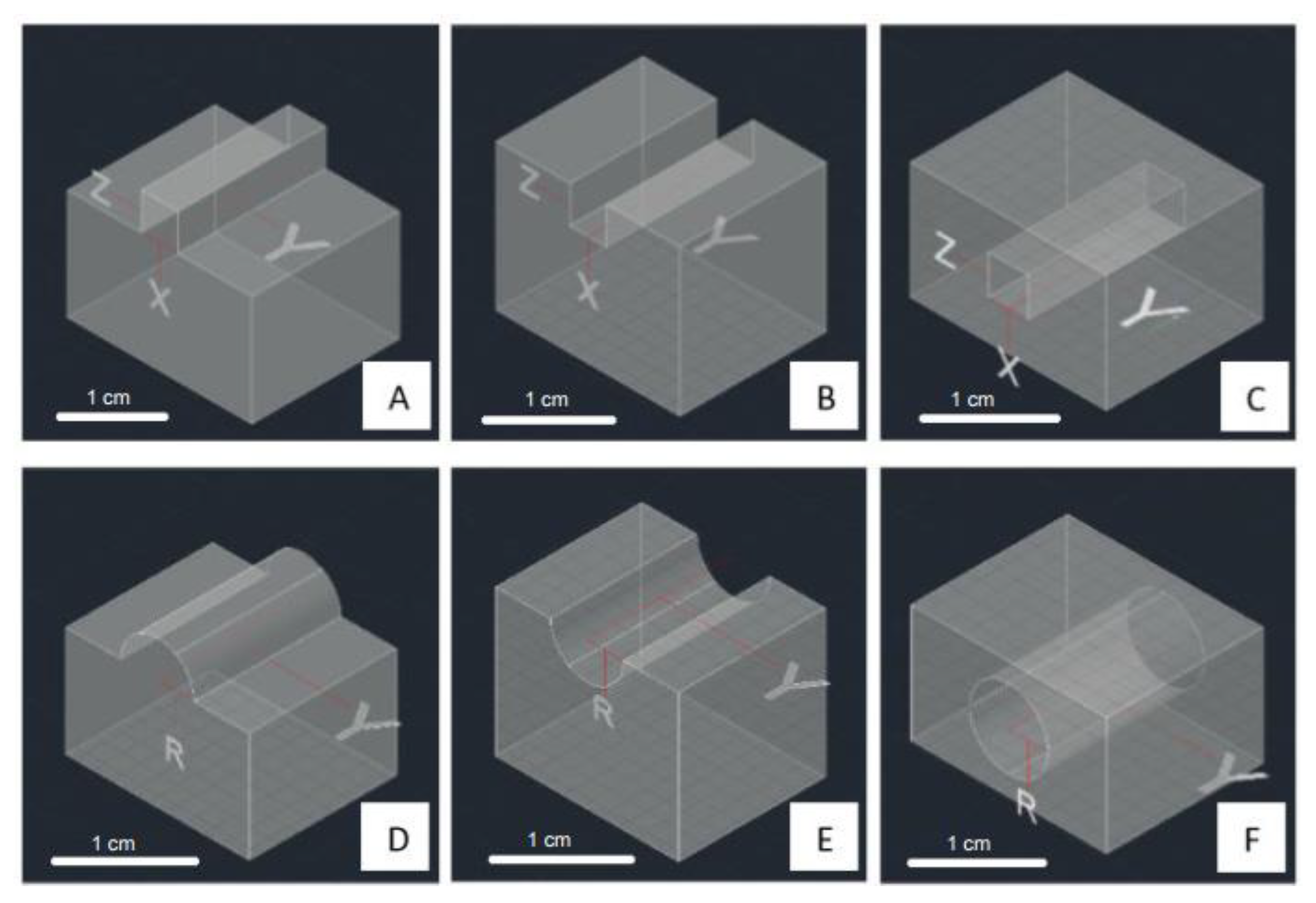


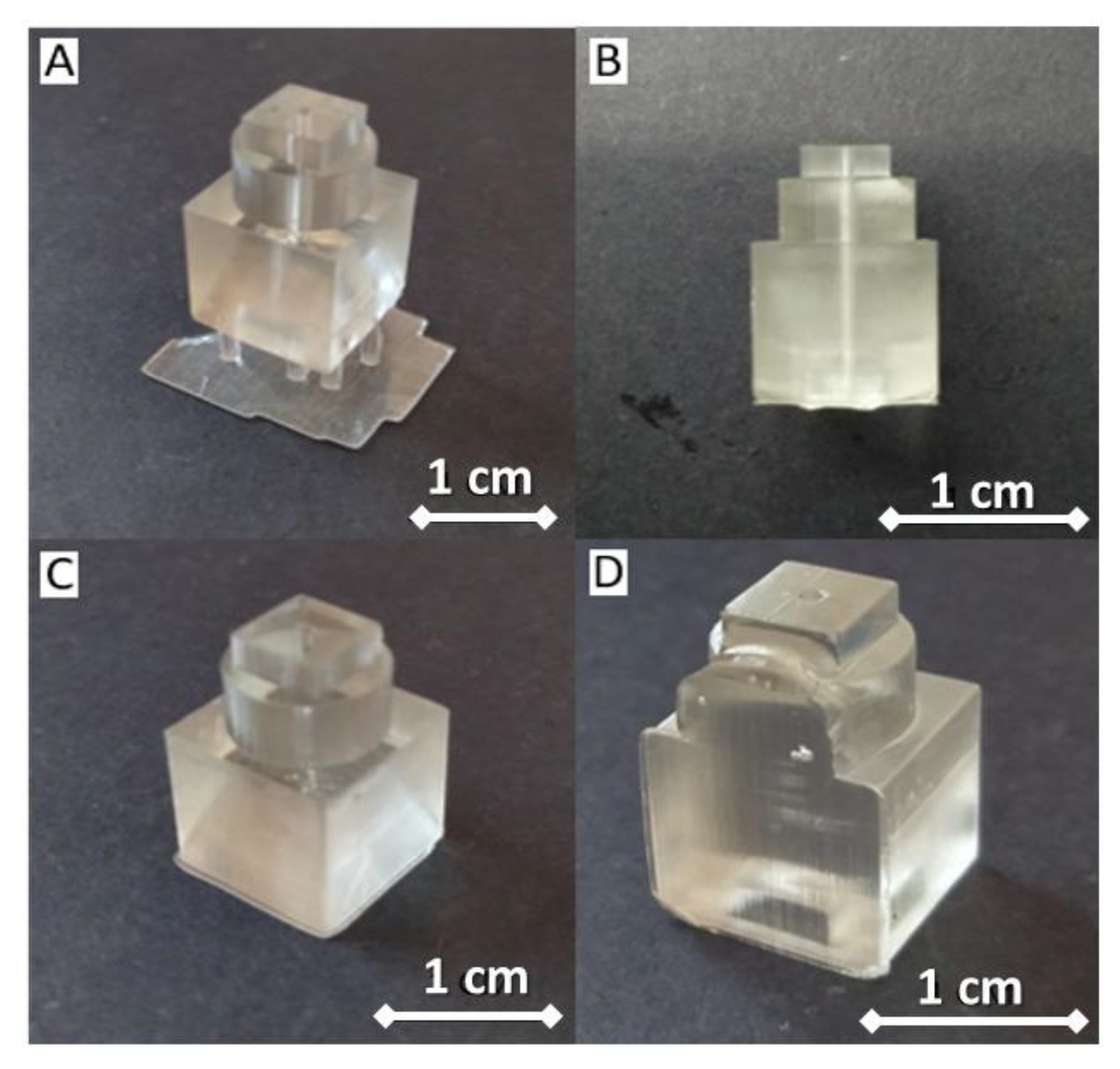

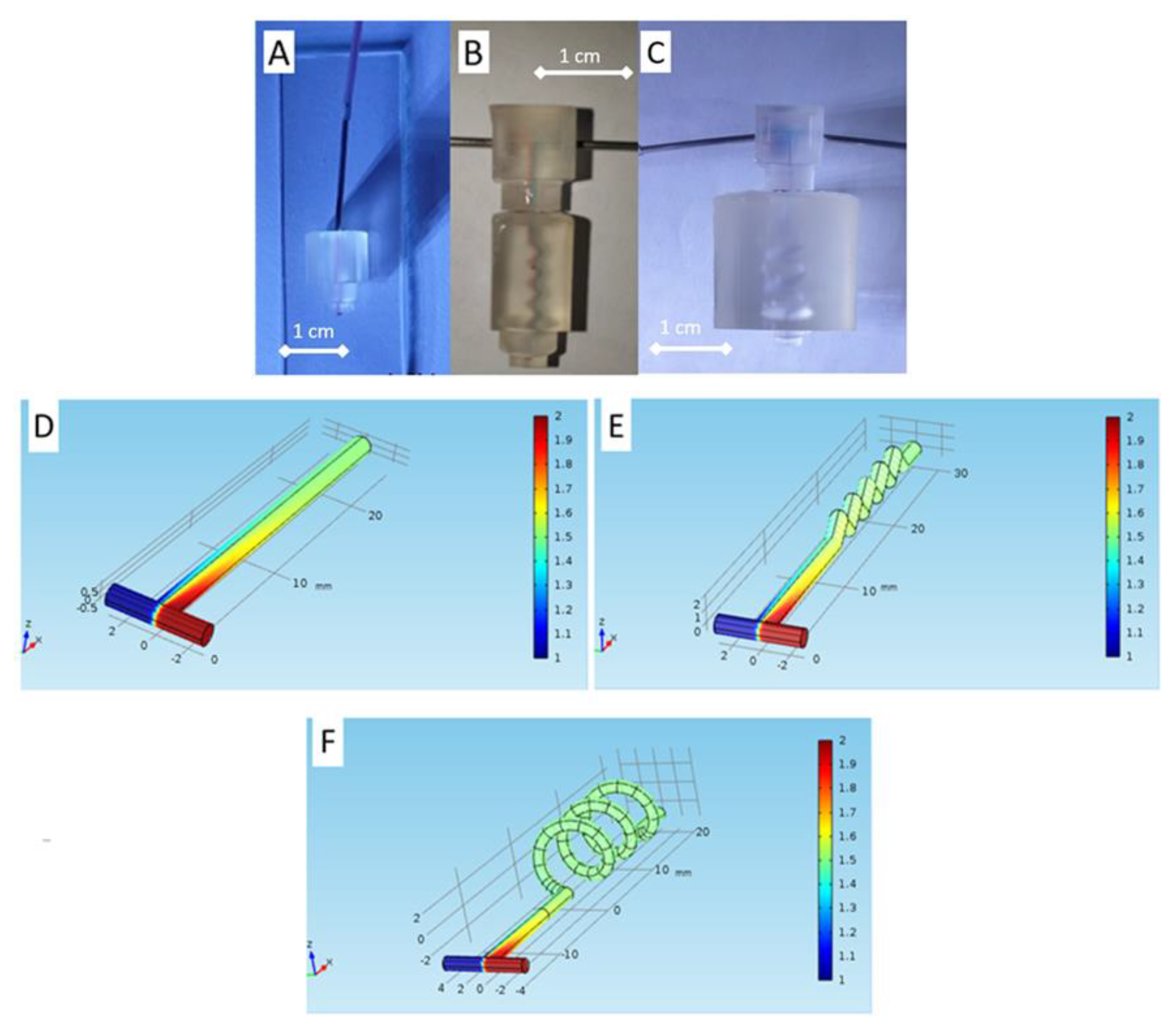


| Desired Structure | Optimum Setting |
|---|---|
| Square embossed | Vertical orientation; 6, 9, or 10 s exposure time |
| Circle embossed | Horizontal orientation; 6–10 s exposure time |
| Square debossed | Vertical orientation; 5–10 s exposure time |
| Circle debossed | Horizontal orientation; 5 s exposure time |
| Square hollow | Vertical orientation; 5 s exposure time |
| Circle hollow | Vertical orientation; 5–6 s exposure time |
| No. | System | Printing Type | Pixel Resolution | Smallest Printed Dimension | Unit Price (Current Estimation) | Resin Price | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Miicraft | DLP-SLA | 30–78 µm | 250 µm | USD 8500–USD 12,500 | USD 510/ 1 kg | [39] |
| 2 | Asiga Pico Plus 27 | DLP-SLA | 27 µm | 150 µm | >USD 10,000 | USD 175/1000 mL | [34] |
| 3 | Asiga Max-X27 UV | DLP-SLA | 27 µm | 120 µm | >USD 10,000 | USD 175/1000 mL | [36] |
| 4 | 3D Systems Viper SL system | DLP-SLA | 75 µm | 500 µm | >USD 10,000 | - | [40] |
| 5 | Stratasys Objet Eden 350V | Polyjet | 16 µm | 100 µm | >USD 10,000 | - | [37] |
| 6 | B9 Creator 3D printer | DLP-SLA | 15 µm | 250 µm | >USD 10,000 | USD 299/1 kg | [38] |
| 7 | Anycubic Photon S | DLP-SLA | 47 µm | 550 µm | <USD 400 | USD 25/500 mL | Our work |
| No. | Microfluidics System | Fabrication Method | Technical Remarks | Smallest Dimension | Potential Application | Ref. |
|---|---|---|---|---|---|---|
| 1 | USC-shaped fluid router and microfiller | 3D printing | Vat photopolymerization | 10 µm gap (microfiller) | Particles sorter | [32] |
| 2 | Centifugal microfluidic | CNC micromilling | Integrated to colorimetric | 80 μm | Gas diffusion in analyte | [43] |
| 3 | Biosensing flowcell | Cyclic olefin copolymer (COC) | Integrated electrochemical | 300 μm | Cell culture | [30] |
| 4 | Spiral microfluidics | Lithography | Simple particle size separation | 75 μm | Circulating tumor cells (CTC) sorter | [41] |
| 5 | Interconnecting channel scaffolds | Material Extrusion 3D printers (mold) and PDMS casting | Single-extrusion scaffolds | 100 μm | Mixer iquid sample and droplet generator | [44] |
| 6 | Microscale impeller pump for recirculating fluid flow | 3D printing | magnetically-driven impeller pump system | 500 μm | Organ-on-chip and mircoreactor | [7] |
| 7 | Modular key–lock and mixer connector | 3D printing | Fully portable integrated 3D spiral mixer | 550 µm | Biofluid mixer, such as urine and blood | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anshori, I.; Lukito, V.; Adhawiyah, R.; Putri, D.; Harimurti, S.; Rajab, T.L.E.; Pradana, A.; Akbar, M.; Syamsunarno, M.R.A.A.; Handayani, M.; et al. Versatile and Low-Cost Fabrication of Modular Lock-and-Key Microfluidics for Integrated Connector Mixer Using a Stereolithography 3D Printing. Micromachines 2022, 13, 1197. https://doi.org/10.3390/mi13081197
Anshori I, Lukito V, Adhawiyah R, Putri D, Harimurti S, Rajab TLE, Pradana A, Akbar M, Syamsunarno MRAA, Handayani M, et al. Versatile and Low-Cost Fabrication of Modular Lock-and-Key Microfluidics for Integrated Connector Mixer Using a Stereolithography 3D Printing. Micromachines. 2022; 13(8):1197. https://doi.org/10.3390/mi13081197
Chicago/Turabian StyleAnshori, Isa, Vincent Lukito, Rafita Adhawiyah, Delpita Putri, Suksmandhira Harimurti, Tati Latifah Erawati Rajab, Arfat Pradana, Mohammad Akbar, Mas Rizky Anggun Adipurna Syamsunarno, Murni Handayani, and et al. 2022. "Versatile and Low-Cost Fabrication of Modular Lock-and-Key Microfluidics for Integrated Connector Mixer Using a Stereolithography 3D Printing" Micromachines 13, no. 8: 1197. https://doi.org/10.3390/mi13081197
APA StyleAnshori, I., Lukito, V., Adhawiyah, R., Putri, D., Harimurti, S., Rajab, T. L. E., Pradana, A., Akbar, M., Syamsunarno, M. R. A. A., Handayani, M., Purwidyantri, A., & Prabowo, B. A. (2022). Versatile and Low-Cost Fabrication of Modular Lock-and-Key Microfluidics for Integrated Connector Mixer Using a Stereolithography 3D Printing. Micromachines, 13(8), 1197. https://doi.org/10.3390/mi13081197








