A Resistance-Based Microfluidic Chip for Deterministic Single Cell Trapping Followed by Immunofluorescence Staining
Abstract
:1. Introduction
2. Materials and Methods
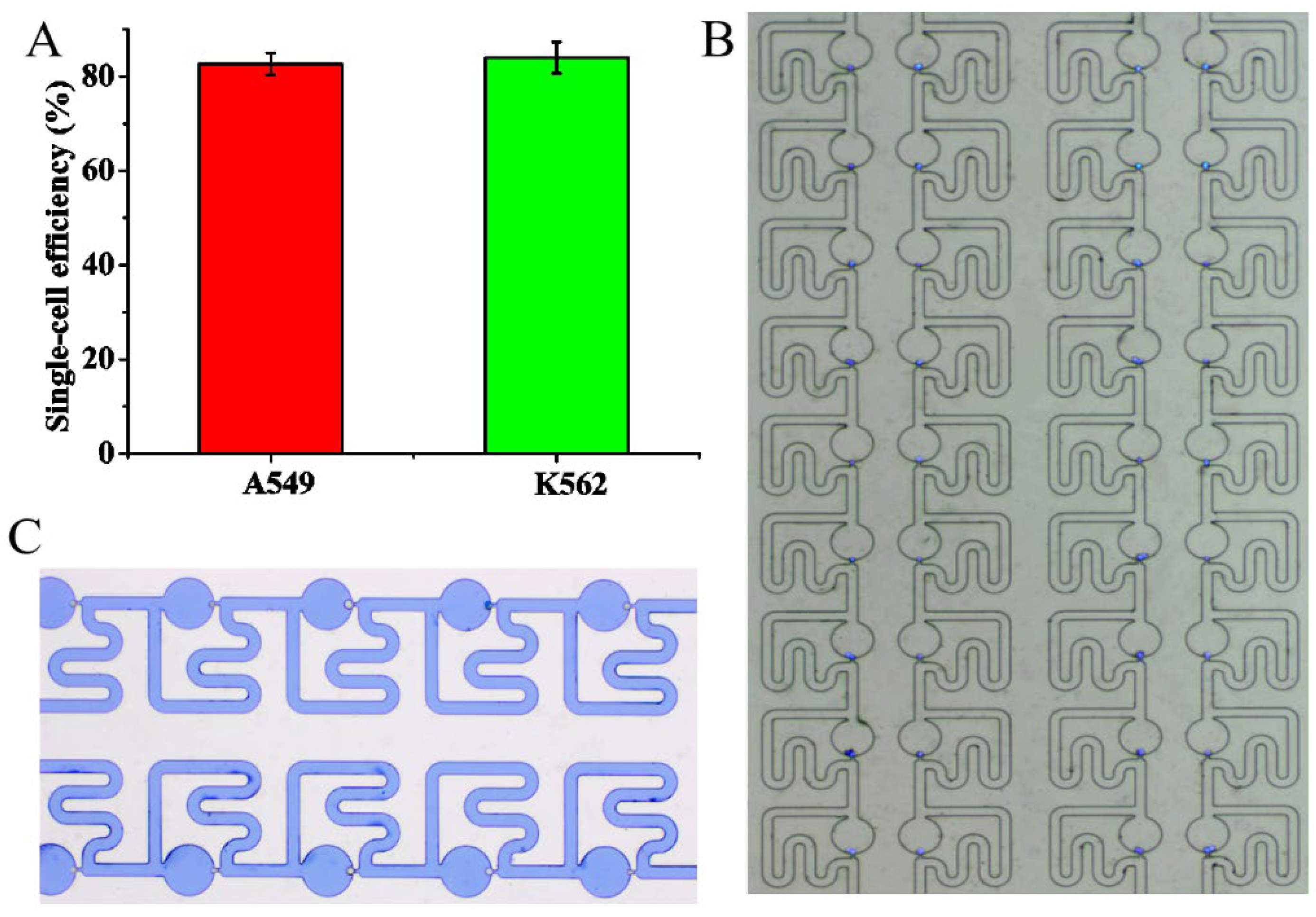
2.1. Design of the Single-Cell Trapping Chip
2.2. Fabrication of the Single-Cell Trapping Microfluidic Chip
2.3. Numerical Simulations
2.4. Cell Culture and Preparation
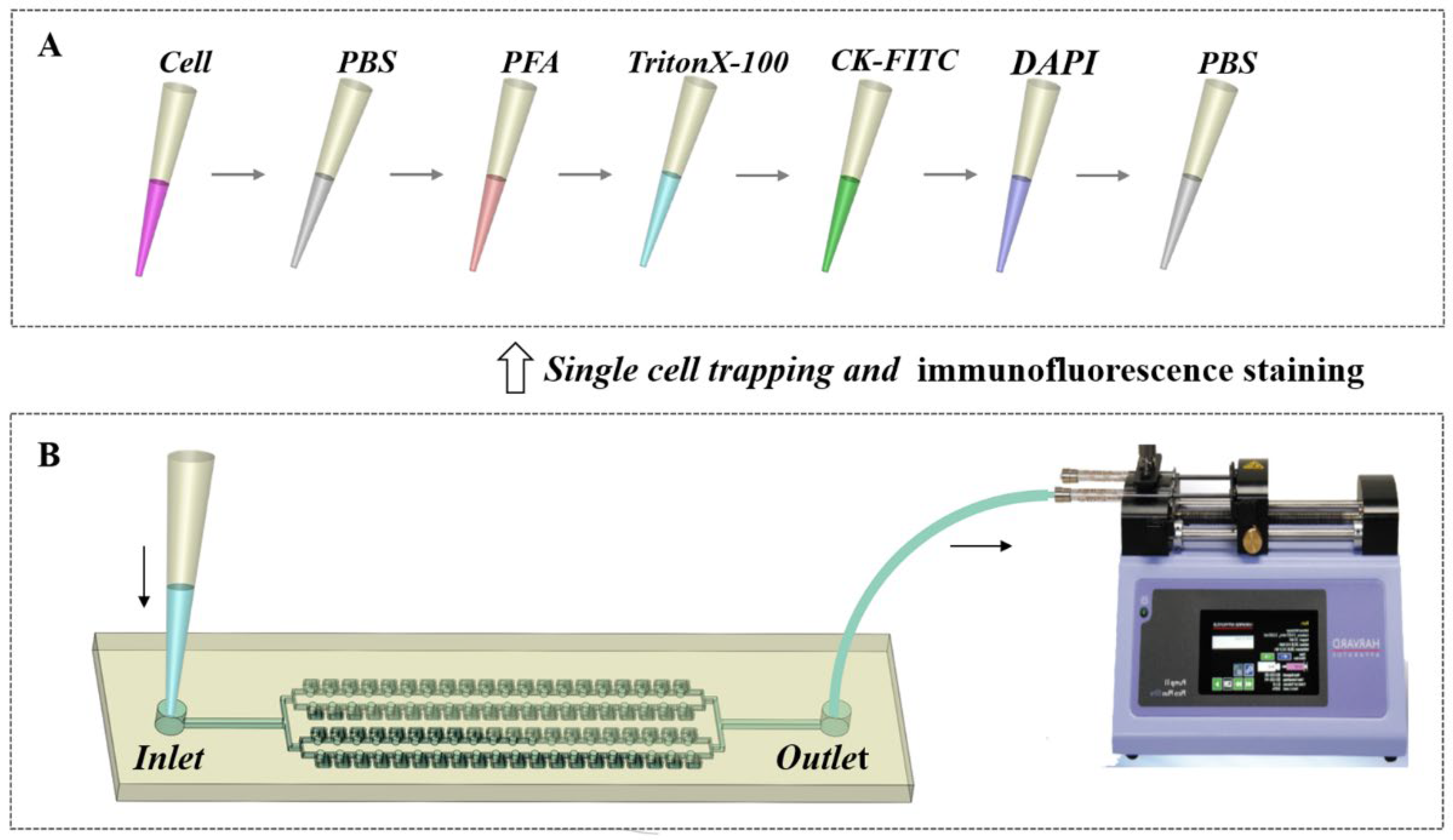
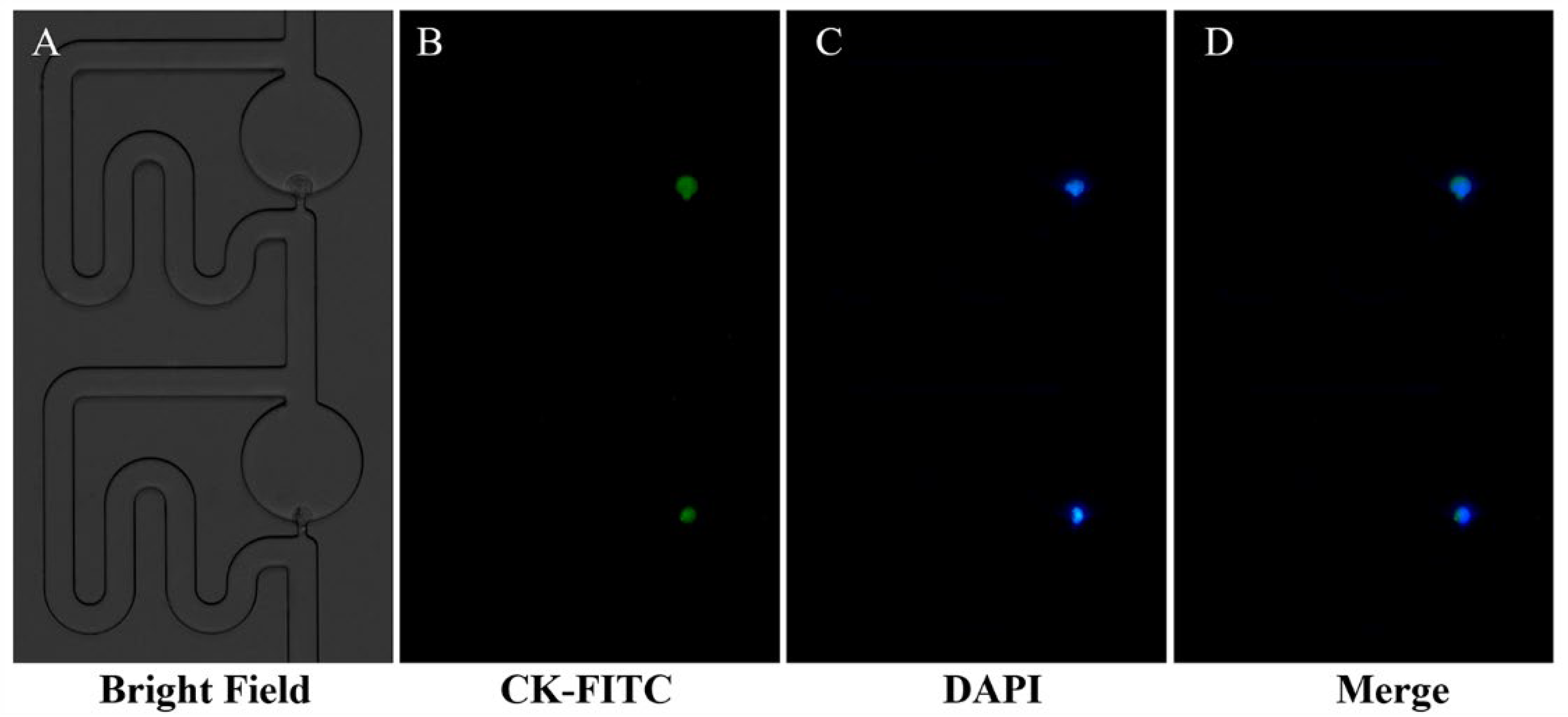
2.5. Single-Cell Trapping and Immunofluorescence Staining Analysis
2.6. Measurement of Cell Viability
3. Results and Discussion
3.1. Principle of Single-Cell Trapping
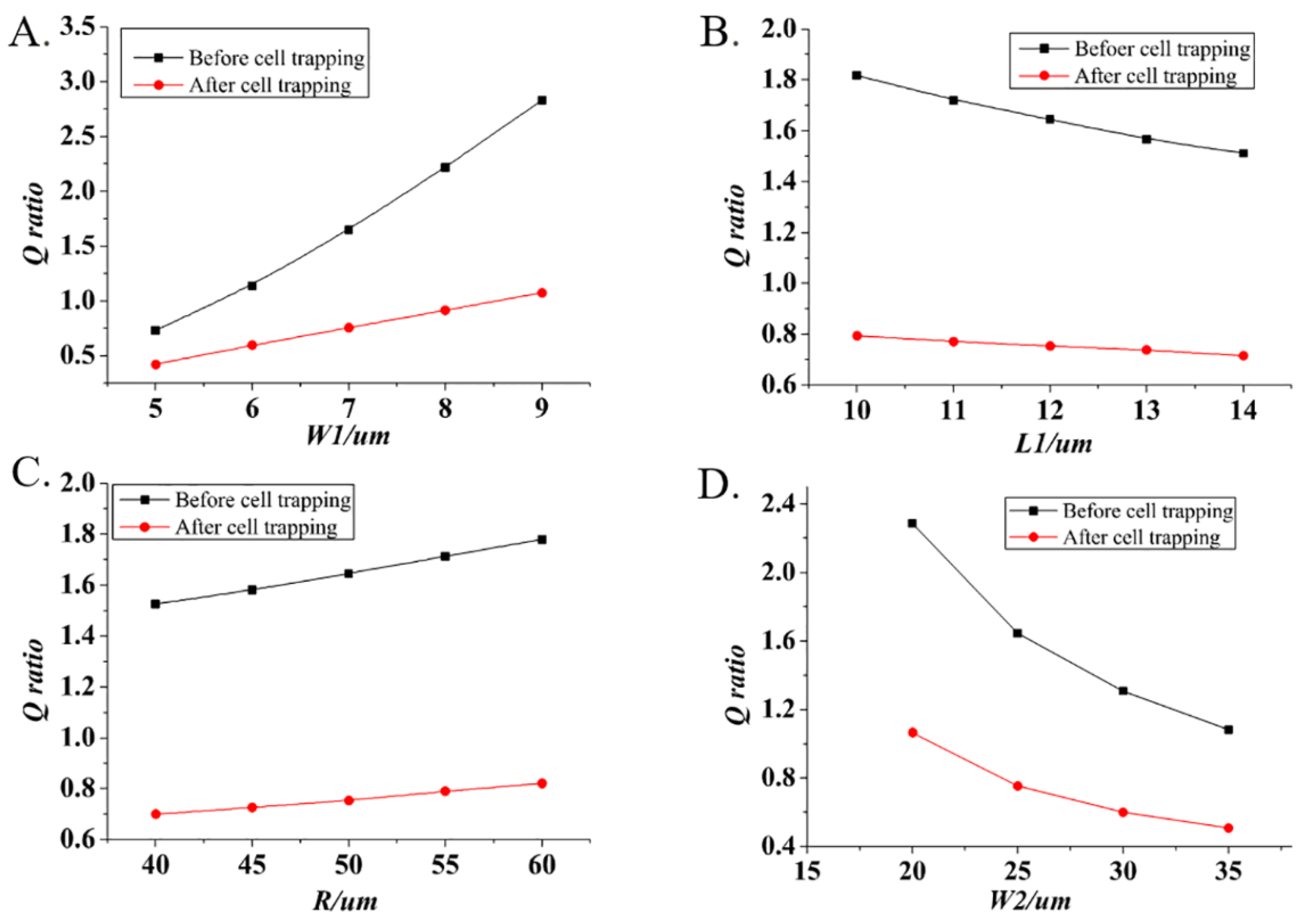
3.2. Theoretical Modeling of Single-Cell Trapping
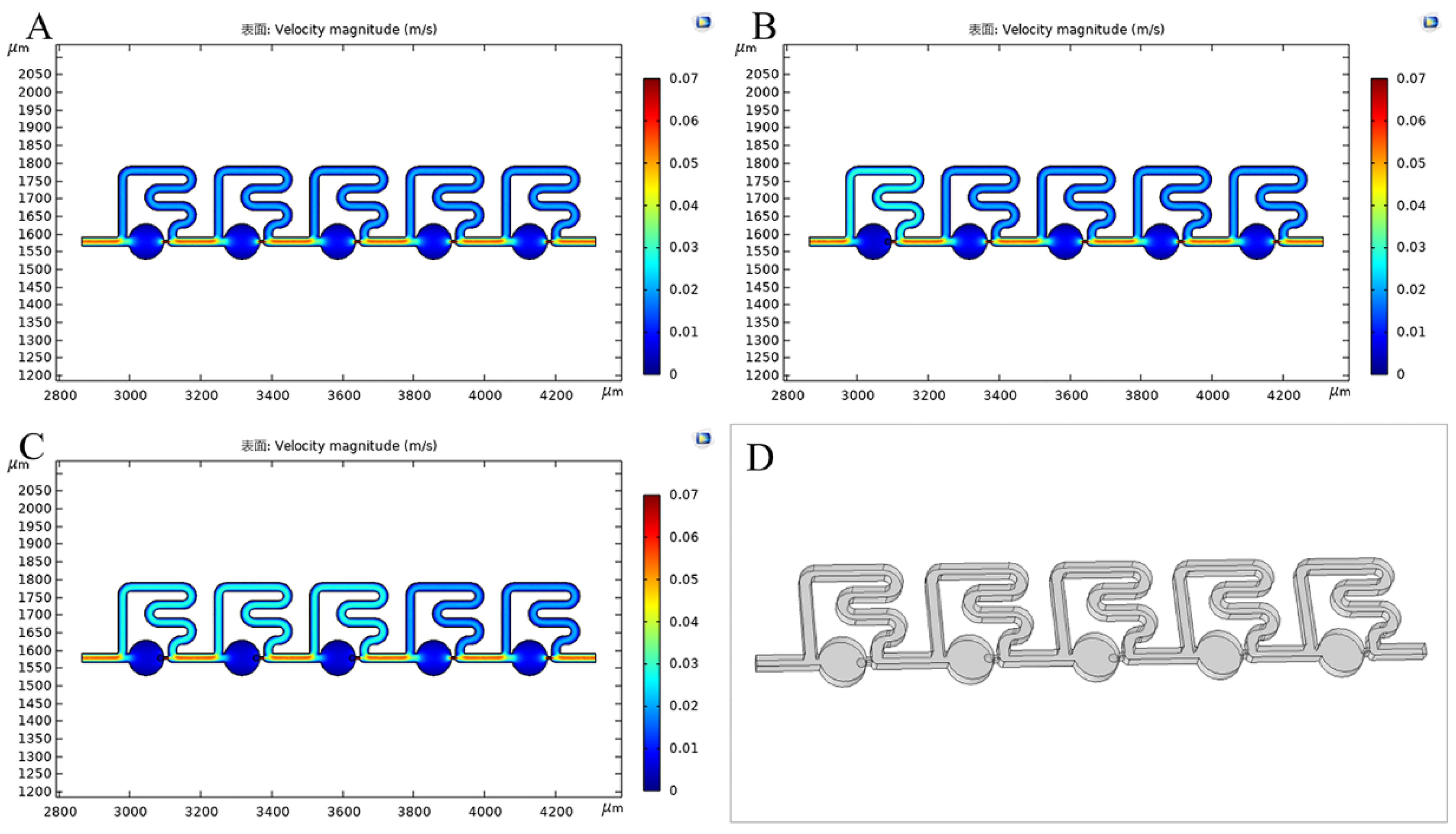
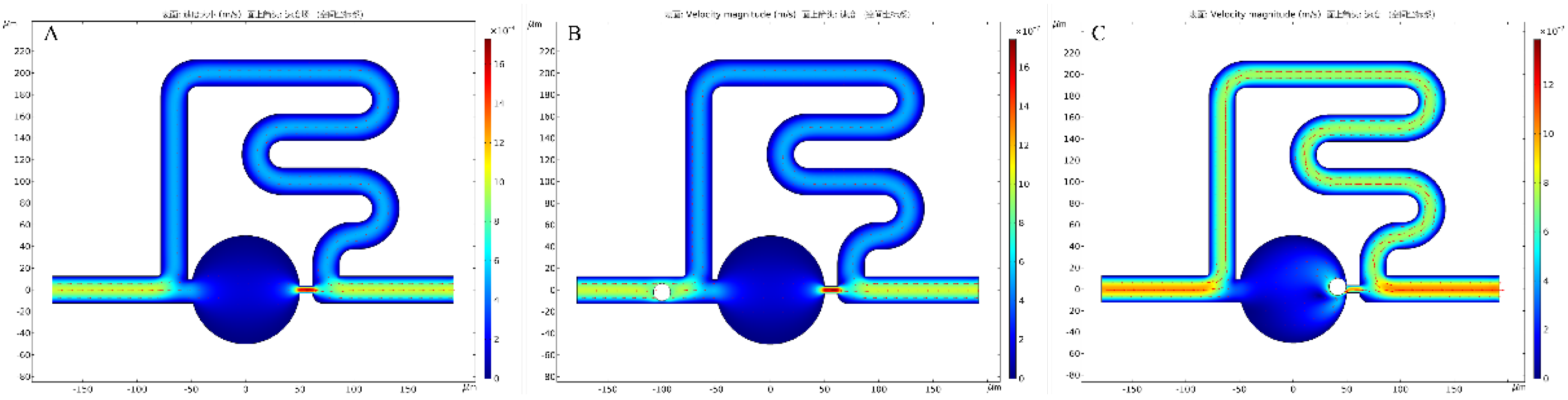
3.3. Numerical Simulations of Single-Cell Trapping
3.4. Single-Cell Trapping and Immunofluorescence Staining Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bedard, P.L.; Hansen, A.R.; Ratain, M.J.; Siu, L.L. Tumour heterogeneity in the clinic. Nature 2013, 501, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellen, B.B.; Zawistowski, J.S.; Czech, E.A.; Sanford, C.I.; SoRelle, E.D.; Luftig, M.A.; Forbes, Z.G.; Wood, K.C.; Hammerbacher, J. Massively parallel quantification of phenotypic heterogeneity in single-cell drug responses. Sci. Adv. 2021, 7, eabf9840. [Google Scholar] [CrossRef] [PubMed]
- Buettner, F.; Natarajan, K.N.; Casale, F.P.; Proserpio, V.; Scialdone, A.; Theis, F.J.; Teichmann, S.A.; Marioni, J.C.; Stegle, O. Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nat. Biotechnol. 2015, 33, 155–160. [Google Scholar] [CrossRef]
- Jung, Y.; Son, M.; Nam, Y.R.; Choi, J.; Heath, J.R.; Yang, S. Microfluidic single-cell proteomics assay chip: Lung cancer cell line case study. Micromachines 2021, 12, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zenobi, R. Single-cell metabolomics: Analytical and biological perspectives. Science 2013, 342, 1243259. [Google Scholar] [CrossRef] [PubMed]
- Gawad, C.; Koh, W.; Quake, S.R. Single-cell genome sequencing: Current state of the science. Nat. Rev. Genet. 2016, 17, 175–188. [Google Scholar] [CrossRef]
- Narayanamurthy, V.; Jeroish, Z.E.; Bhuvaneshwari, K.S.; Bayat, P.; Premkumar, R.; Samsuri, F.; Yusoff, M.M. Advances in passively driven microfluidics and lab-on-chip devices: A comprehensive literature review and patent analysis. Rsc. Adv. 2020, 10, 11652–11680. [Google Scholar] [CrossRef]
- Li, Y.; Motschman, J.D.; Kelly, S.T.; Yellen, B.B. Injection molded microfluidics for establishing high-density single cell arrays in an open hydrogel format. Anal. Chem. 2020, 92, 2794–2801. [Google Scholar] [CrossRef]
- Cheng, D.; Yu, Y.; Han, C.; Cao, M.; Yang, G.; Liu, J.; Chen, X.; Peng, Z. A simple microdevice for single cell capture, array, release, and fast staining using oscillatory method. Biomicrofluidics 2018, 12, 034105. [Google Scholar] [CrossRef]
- Deng, B.; Wang, H.; Tan, Z.; Quan, Y. Microfluidic cell trapping for single-cell analysis. Micromachines 2019, 10, 409. [Google Scholar] [CrossRef] [Green Version]
- Narayanamurthy, V.; Nagarajan, S.; Khan, A.Y.F.; Samsuri, F.; Sridhar, T.M. Microfluidic hydrodynamic trapping for single cell analysis: Mechanisms, methods and applications. Anal. Methods 2017, 9, 3751–3772. [Google Scholar] [CrossRef]
- Lv, D.; Zhang, X.; Xu, M.; Cao, W.; Liu, X.; Deng, J.; Yang, J.; Hu, N. Trapping and releasing of single microparticles and cells in a microfluidic chip. Electrophoresis 2022. [CrossRef]
- Sun, Y.; Cai, B.; Wei, X.; Wang, Z.; Rao, L.; Meng, Q.F.; Liao, Q.; Liu, W.; Guo, S.; Zhao, X. A valve-based microfluidic device for on-chip single cell treatments. Electrophoresis 2019, 40, 961–968. [Google Scholar] [CrossRef]
- Kim, H.S.; Devarenne, T.P.; Han, A. A high-throughput microfluidic single-cell screening platform capable of selective cell extraction. Lab Chip 2015, 15, 2467–2475. [Google Scholar] [CrossRef]
- Keloth, A.; Anderson, O.; Risbridger, D.; Paterson, L. Single cell isolation using optical tweezers. Micromachines 2018, 9, 434. [Google Scholar] [CrossRef]
- Huang, N.T.; Zhang, H.L.; Chung, M.T.; Seo, J.H.; Kurabayashi, K. Recent advancements in optofluidics-based single-cell analysis: Optical on-chip cellular manipulation, treatment, and property detection. Lab Chip 2014, 14, 1230–1245. [Google Scholar] [CrossRef]
- Collins, D.J.; Morahan, B.; Garcia-Bustos, J.; Doerig, C.; Plebanski, M.; Neild, A. Two-dimensional single-cell patterning with one cell per well driven by surface acoustic waves. Nat. Commun. 2015, 6, 8686. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Bachman, H.; Ozcelik, A.; Huang, T.J. Acoustic microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 17–43. [Google Scholar] [CrossRef]
- Khojah, R.; Xiao, Z.; Panduranga, M.K.; Bogumil, M.; Wang, Y.; Goiriena-Goikoetxea, M.; Chopdekar, R.V.; Bokor, J.; Carman, G.P.; Candler, R.N.; et al. Single-domain multiferroic array-addressable terfenol-D (SMArT) micromagnets for programmable single-cell capture and release. Adv. Mater. 2021, 33, 2006651. [Google Scholar] [CrossRef]
- Poudineh, M.; Aldridge, P.M.; Ahmed, S.; Green, B.J.; Kermanshah, L.; Nguyen, V.; Tu, C.; Mohamadi, R.M.; Nam, R.K.; Hansen, A.; et al. Tracking the dynamics of circulating tumour cell phenotypes using nanoparticle-mediated magnetic ranking. Nat. Nanotechnol. 2017, 12, 274–281. [Google Scholar] [CrossRef]
- Thiriet, P.E.; Pezoldt, J.; Gambardella, G.; Keim, K.; Deplancke, B.; Guiducci, C. Selective retrieval of individual cells from microfluidic arrays combining dielectrophoretic force and directed hydrodynamic flow. Micromachines 2020, 11, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Ren, Y.; Tao, Y.; Hou, L.; Jiang, H. High-throughput separation, trapping, and manipulation of single cells and particles by combined dielectrophoresis at a bipolar electrode array. Anal. Chem. 2018, 90, 11461–11469. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Anand, R.K. High-Throughput selective capture of single circulating tumor cells by dielectrophoresis at a wireless electrode array. J. Am. Chem. Soc. 2017, 139, 8950–8959. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Chu, Y.S.; Thiery, J.P.; Lim, C.T.; Rodriguez, I. Microfluidic cell trap array for controlled positioning of single cells on adhesive micropatterns. Lab Chip 2013, 13, 714–721. [Google Scholar] [CrossRef]
- Huang, N.T.; Hwong, Y.J.; Lai, R.L. A microfluidic microwell device for immunomagnetic single-cell trapping. Microfluid. Nanofludics 2018, 22, 16. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Yuan, H.; Wang, Y.; Zhou, H.; Jin, H.; Jia, C.; Jin, Q.; Cong, H.; Zhao, J. EGFR point mutation detection of single circulating tumor cells for lung cancer using a micro-well array. Biosens. Bioelectron. 2019, 139, 111326. [Google Scholar] [CrossRef]
- Park, M.C.; Hur, J.Y.; Cho, H.S.; Park, S.H.; Suh, K.Y. High-throughput single-cell quantification using simple microwell-based cell docking and programmable time-course live-cell imaging. Lab Chip 2011, 11, 79–86. [Google Scholar] [CrossRef]
- Joensson, H.N.; Svahn, H.A. Droplet microfluidics—A tool for single-cell analysis. Angew. Chem. Int. Ed. 2012, 51, 12176–12192. [Google Scholar] [CrossRef]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef]
- Fallahi, H.; Cha, H.; Adelnia, H.; Dai, Y.; Ta, H.T.; Yadav, S.; Zhang, J.; Nguyen, N.T. On-demand deterministic release of particles and cells using stretchable microfluidics. Nanoscale Horiz. 2022, 7, 414–424. [Google Scholar] [CrossRef]
- Chai, H.; Feng, Y.; Liang, F.; Wang, W. A microfluidic device enabling deterministic single cell trapping and release. Lab Chip 2021, 21, 2486–2494. [Google Scholar] [CrossRef]
- Sauzade, M.; Brouzes, E. Deterministic trapping, encapsulation and retrieval of single-cells. Lab Chip 2017, 17, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.H.; Takeuchi, S. A trap-and-release integrated microfluidic system for dynamic microarray applications. Proc. Natl. Acad. Sci. USA 2007, 104, 1146–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frimat, J.P.; Becker, M.; Chiang, Y.Y.; Marggraf, U.; Janasek, D.; Hengstler, J.G.; Franzke, J.; West, J. A microfluidic array with cellular valving for single cell co-culture. Lab Chip 2011, 11, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.; Kim, Y.J.; Yoon, E. Highly-efficient single-cell capture in microfluidic array chips using differential hydrodynamic guiding structures. Appl. Phys. Lett. 2011, 98, 123701. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Deng, B.; Li, J.X.; Cai, W.; Tu, L.; Chen, J.; Wu, Q.; Wang, W.H. A microfluidic device enabling high-efficiency single cell trapping. Biomicrofluidics 2015, 9, 014101. [Google Scholar] [CrossRef] [Green Version]
- Bruus, H. Theoretical Microfluidics; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Judy, J.; Maynes, D.; Webb, B.W. Characterization of frictional pressure drop for liquid flows through microchannels. Int. J. Heat Mass Transfer. 2002, 45, 3477–3489. [Google Scholar] [CrossRef]

| Trap Site | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| No cell trapped | 1.645 | 1.646 | 1.644 | 1.646 | 1.646 |
| One | 0.753 | 1.645 | 1.643 | 1.645 | 1.645 |
| Two | 0.753 | 0.757 | 1.643 | 1.645 | 1.645 |
| Three | 0.753 | 0.757 | 0.754 | 1.645 | 1.645 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Li, B.; Li, W.; Ren, X.; Su, N.; Li, R.; Li, J.; Huang, Q. A Resistance-Based Microfluidic Chip for Deterministic Single Cell Trapping Followed by Immunofluorescence Staining. Micromachines 2022, 13, 1272. https://doi.org/10.3390/mi13081272
Sun X, Li B, Li W, Ren X, Su N, Li R, Li J, Huang Q. A Resistance-Based Microfluidic Chip for Deterministic Single Cell Trapping Followed by Immunofluorescence Staining. Micromachines. 2022; 13(8):1272. https://doi.org/10.3390/mi13081272
Chicago/Turabian StyleSun, Xiange, Bowen Li, Wenman Li, Xiaodong Ren, Ning Su, Ruoxu Li, Jinmi Li, and Qing Huang. 2022. "A Resistance-Based Microfluidic Chip for Deterministic Single Cell Trapping Followed by Immunofluorescence Staining" Micromachines 13, no. 8: 1272. https://doi.org/10.3390/mi13081272






