Protein Albumin Manipulation and Electrical Quantification of Molecular Dielectrophoresis Responses for Biomedical Applications
Abstract
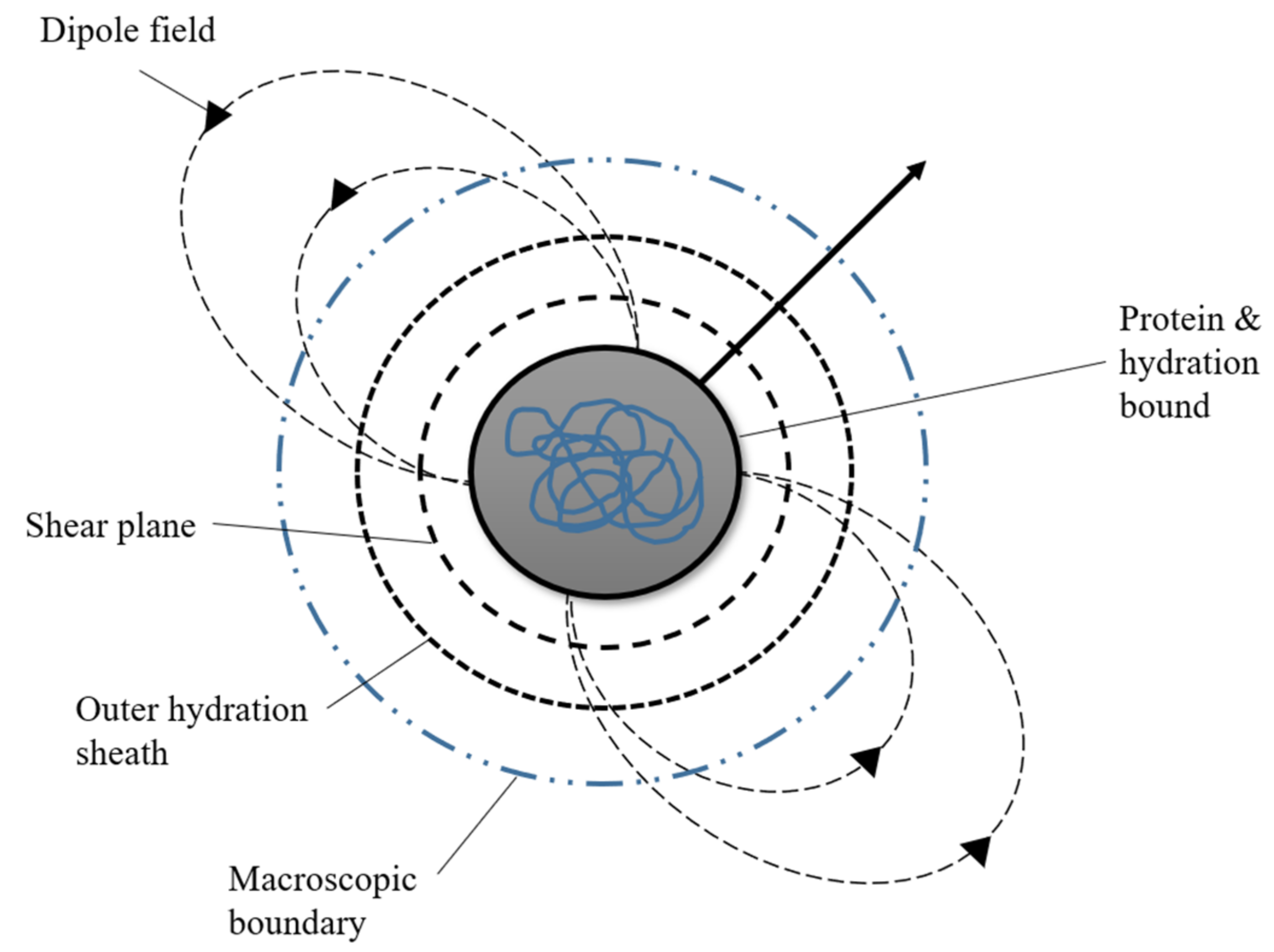
1. Protein as the Basic Component of Life
2. Theory of DEP
3. DEP Studies on Protein Albumin
4. Theories of Electrical Quantification Techniques for DEP Protein
4.1. Electrochemical Impedance Spectroscopy (EIS)
4.2. Cyclic Voltammetry (CV)
4.3. Capacitance Measurement
5. Correlating the Principles of Electrical Quantification Methods to DEP
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakano, A.; Ros, A. Protein dielectrophoresis: Advances, challenges, and applications. Electrophoresis 2013, 34, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Kwak, T.J.; Jung, H.; Allen, B.D.; Demirel, M.C.; Chang, W.-J. Dielectrophoretic separation of randomly shaped protein particles. Sep. Purif. Technol. 2021, 262, 118280. [Google Scholar] [CrossRef]
- Whitford, D. Proteins: Structure and Function; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Pace, C.N.; Scholtz, J.M.; Grimsley, G.R. Forces stabilizing proteins. FEBS Lett. 2014, 588, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Bruno, C.; Parachalil, D.R.; Baker, M.J.; Bonnier, F.; Butler, H.J.; Byrne, H.J. Vibrational spectroscopic analysis and quantification of proteins in human blood plasma and serum. In Vibrational Spectroscopy in Protein Research; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–314. [Google Scholar]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin—More than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Tojo, A.; Kinugasa, S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int. J. Nephrol. 2012, 2012, 481250. [Google Scholar] [CrossRef]
- Mukai, H.; Villafuerte, H.; Qureshi, A.R.; Lindholm, B.; Stenvinkel, P. Serum albumin, inflammation, and nutrition in end-stage renal disease: C-reactive protein is needed for optimal assessment. Semin Dial 2018, 31, 435–439. [Google Scholar] [CrossRef]
- Gounden, V.; Vashisht, R.; Jialal, I. Hypoalbuminemia; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gatta, A.; Verardo, A.; Bolognesi, M. Hypoalbuminemia. Intern. Emerg. Med. 2012, 7, 193–199. [Google Scholar] [CrossRef]
- Throop, J.L.; Kerl, M.E.; Cohn, L.A. Albumin in health and disease: Causes and treatment of hypoalbuminemia. Compendium 2004, 26, 940–948. [Google Scholar]
- Mutlu, E.A.; Keshavarzian, A.; Mutlu, G.M. Hyperalbuminemia and elevated transaminases associated with high-protein diet. Scand. J. Gastroenterol. 2006, 41, 759–760. [Google Scholar] [CrossRef]
- Akirov, A.; Masri-Iraqi, H.; Atamna, A.; Shimon, I. Low albumin levels are associated with mortality risk in hospitalized patients. Am. J. Med. 2017, 130, 1465.e1411–1465.e1419. [Google Scholar] [CrossRef]
- Toto, R.D. Microalbuminuria: Definition, detection, and clinical significance. J. Clin. Hypertens. 2004, 6, 2–7. [Google Scholar] [CrossRef]
- De Jong, P.E.; Curhan, G.C. Screening, monitoring, and treatment of albuminuria: Public health perspectives. J. Am. Soc. Nephrol. 2006, 17, 2120–2126. [Google Scholar] [CrossRef]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef]
- Atmeh, R.F.; Arafa, I.M.; Al-Khateeb, M. Albumin aggregates: Hydrodynamic shape and physico-chemical properties. Jordan J. Chem. JJC 2007, 2, 169–182. [Google Scholar]
- Das, A.; Chitra, R.; Choudhury, R.; Ramanadham, M. Structural changes during the unfolding of Bovine serum albumin in the presence of urea: A small-angle neutron scattering study. Pramana 2004, 63, 363–368. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef]
- Walker, J.; Wilson, K. Protein structure, purification, characterisation and function analysis. In Principles and Techniques of Biochemistry and Molecular Biology; Cambridge University Press: New York, NY, USA, 2005; pp. 349–404. [Google Scholar]
- Yang, Y.; Ma, H. Western blotting and ELISA techniques. Researcher 2009, 1, 67–86. [Google Scholar]
- Domon, B.; Aebersold, R. Mass spectrometry and protein analysis. Science 2006, 312, 212–217. [Google Scholar] [CrossRef]
- Dunn, M.J. Gel Electrophoresis of Proteins; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef]
- Bagherbaigi, S.; Córcoles, E.P.; Wicaksono, D.H. Cotton fabric as an immobilization matrix for low-cost and quick colorimetric enzyme-linked immunosorbent assay (ELISA). Anal. Methods 2014, 6, 7175–7180. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, C.; Li, L.; Song, X.; Xu, K.; Wang, J.; Liu, Y.; Fu, K.; Bao, H.; Song, D. Development of a low-cost paper-based ELISA method for rapid Escherichia coli O157: H7 detection. Anal. Biochem. 2018, 542, 58–62. [Google Scholar] [CrossRef]
- Kumar, H.; Singh, V.; Isha, M.; Mehta, S.; Garg, R.; Shinu, P. Line Immunoassay: A Rapid Test for Screening TORCH Complex in Antenatal Patients with Bad Obstetric History. Mymensingh Med. J. MMJ 2018, 27, 641–644. [Google Scholar]
- Ghosh, R.; Gilda, J.E.; Gomes, A.V. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev. Proteom. 2014, 11, 549–560. [Google Scholar] [CrossRef]
- Mishra, M.; Tiwari, S.; Gomes, A.V. Protein purification and analysis: Next generation Western blotting techniques. Expert Rev. Proteom. 2017, 14, 1037–1053. [Google Scholar] [CrossRef]
- Liu, H. Micro Western Blotting by Dip-Pen Electrophoresis in Capillaries; Chinese University of Hong Kong: Hong Kong, China, 2011. [Google Scholar]
- Lubec, G.; Afjehi-Sadat, L. Limitations and pitfalls in protein identification by mass spectrometry. Chem. Rev. 2007, 107, 3568–3584. [Google Scholar] [CrossRef]
- Frenich, A.G.; Vidal, J.L.M.; Moreno, J.L.F.; Romero-González, R. Compensation for matrix effects in gas chromatography-tandem mass spectrometry using a single point standard addition. J. Chromatogr. A 2009, 1216, 4798–4808. [Google Scholar] [CrossRef]
- Chéry, C.C.; Moens, L.; Cornelis, R.; Vanhaecke, F. Capabilities and limitations of gel electrophoresis for elemental speciation: A laboratory’s experience. Pure Appl. Chem. 2006, 78, 91–103. [Google Scholar] [CrossRef][Green Version]
- Rabilloud, T.; Chevallet, M.; Luche, S.; Lelong, C. Two-dimensional gel electrophoresis in proteomics: Past, present and future. J. Proteom. 2010, 73, 2064–2077. [Google Scholar] [CrossRef]
- Mohd Maidin, N.N.; Buyong, M.R.; Rahim, R.A.; Mohamed, M.A. Dielectrophoresis applications in biomedical field and future perspectives in biomedical technology. Electrophoresis 2021, 42, 2033–2059. [Google Scholar] [CrossRef]
- Pohl, H.A.; Crane, J.S. Dielectrophoresis of cells. Biophys. J. 1971, 11, 711–727. [Google Scholar] [CrossRef]
- Buyong, M.R.; Abd Aziz, N.; Hamzah, A.A.; Majlis, B.Y. Dielectrophoretic characterization of array type microelectrodes. In Proceedings of the 2014 IEEE International Conference on Semiconductor Electronics (ICSE2014), Kuala Lumpur, Malaysia, 27–29 August 2014; pp. 240–243. [Google Scholar]
- Deivasigamani, R.; Abdul Nasir, N.S.; Mohamed, M.A.; Buyong, M.R. In vitro dielectrophoresis of HEK cell migration for stimulating chronic wound epithelialization. Electrophoresis 2021, 43, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.N.; Jiang, A.Y.; Vyas, P.D.; Flanagan, L.A. Separation of neural stem cells by whole cell membrane capacitance using dielectrophoresis. Methods 2018, 133, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.I.; Musso, N.; Romano, A.; Caruso, G.; Petralia, S.; Lanzanò, L.; Broggi, G.; Camarda, M. The role of dielectrophoresis for cancer diagnosis and prognosis. Cancers 2021, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.N.; Abd Aziz, N.; Buyong, M.R.; Majlis, B.Y. Size-based particles separation utilizing dielectrophoresis technique. In Proceedings of the 2017 IEEE Regional Symposium on Micro and Nanoelectronics (RSM), Penang, Malaysia, 23–25 August 2017; pp. 10–13. [Google Scholar]
- Cheah, Y.J.; Buyong, M.R.; Mohd Yunus, M.H. Wound healing with electrical stimulation technologies: A review. Polymers 2021, 13, 3790. [Google Scholar] [CrossRef] [PubMed]
- Buyong, M.R.; Kayani, A.A.; Hamzah, A.A.; Yeop Majlis, B. Dielectrophoresis manipulation: Versatile lateral and vertical mechanisms. Biosensors 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.A. The motion and precipitation of suspensoids in divergent electric fields. J. Appl. Phys. 1951, 22, 869–871. [Google Scholar] [CrossRef]
- Pohl, H.A. Some effects of nonuniform fields on dielectrics. J. Appl. Phys. 1958, 29, 1182–1188. [Google Scholar] [CrossRef]
- Buyong, M.R.; Larki, F.; Takamura, Y.; Abd Aziz, N.; Yunas, J.; Hamzah, A.A.; Majlis, B.Y. Implementing the concept of dielectrophoresis in glomerular filtration of human kidneys. In Proceedings of the 2016 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 17–19 August 2016; pp. 33–37. [Google Scholar]
- Nasir, N.S.A.; Deivasigamani, R.; Rahim, M.K.A.; Nashruddin, S.N.A.M.; Hamzah, A.A.; Wee, M.F.M.R.; Buyong, M.R. Preliminary dielectrophoresis study: Manipulation of protein albumin and electrical quantification by using cyclic voltammetry technique. Microelectron. Int. 2021, 38, 162–171. [Google Scholar] [CrossRef]
- Buyong, M.R.; Larki, F.; Faiz, M.S.; Hamzah, A.A.; Yunas, J.; Majlis, B.Y. A tapered aluminium microelectrode array for improvement of dielectrophoresis-based particle manipulation. Sensors 2015, 15, 10973–10990. [Google Scholar] [CrossRef]
- Kua, C.; Lam, Y.C.; Yang, C.; Youcef-Toumi, K. Review of bio-particle manipulation using dielectrophoresis. Available online: https://dspace.mit.edu/handle/1721.1/7464 (accessed on 2 January 2021).
- Buyong, M.R.; Ismail, A.G.; Yunus, F.W.; Abd Samad, M.I.; Jamaludin, N.M.A.; Rahim, M.K.A.; Hamzah, A.A.; Majlis, B.Y.; Abd Aziz, N. Tapered dielectrophoresis microelectrodes: Device, operation, and application. In Proceedings of the 2019 IEEE Regional Symposium on Micro and Nanoelectronics (RSM), Putrajaya, Malaysia, 21–23 August 2019; pp. 172–175. [Google Scholar]
- Zhou, R.; Wang, P.; Chang, H.C. Bacteria capture, concentration and detection by alternating current dielectrophoresis and self-assembly of dispersed single-wall carbon nanotubes. Electrophoresis 2006, 27, 1376–1385. [Google Scholar] [CrossRef]
- Buyong, M.R.; Larki, F.; Caille, C.E.; Abd Aziz, N.; Ismail, A.G.; Hamzah, A.A.; Majlis, B.Y. Dynamic dielectric properties characterization of tapered dielectrophoresis microelectrodes for selective detection and rapid manipulation of cells. Microelectron. Int. 2020, 37, 189–198. [Google Scholar] [CrossRef]
- Qian, C.; Huang, H.; Chen, L.; Li, X.; Ge, Z.; Chen, T.; Yang, Z.; Sun, L. Dielectrophoresis for bioparticle manipulation. Int. J. Mol. Sci. 2014, 15, 18281–18309. [Google Scholar] [CrossRef]
- Choi, W.; Min, Y.W.; Lee, K.Y.; Jun, S.; Lee, H.G. Dielectrophoresis-based microwire biosensor for rapid detection of Escherichia coli K-12 in ground beef. Lwt 2020, 132, 109230. [Google Scholar] [CrossRef]
- Rashid, N.F.A.; Deivasigamani, R.; Wee, M.M.R.; Hamzah, A.A.; Buyong, M.R. Integration of a Dielectrophoretic Tapered Aluminum Microelectrode Array with a Flow Focusing Technique. Sensors 2021, 21, 4957. [Google Scholar] [CrossRef]
- Rahim, M.K.A.; Jamaludin, N.M.A.; Santhanam, J.; Hamzah, A.A.; Buyong, M.R. Determination of Dielectrophoretic Unique Crossover Frequency by Velocity of Enterobacter Aerogenes Trajectory. Int. J. Nanoelectron. Mater. 2020, 13. [Google Scholar]
- Jamaludin, N.M.A.; Rahim, M.K.A.; Hamzah, A.A.; Abu, N.; Buyong, M.R. Optimization of the Isolation of Extracellular Vesicles via Dielectrophoresis: A preliminary Analysis. Int. J. Nanoelectron. Mater. 2020, 13, 129–142. [Google Scholar]
- Modarres, P.; Tabrizian, M. Alternating current dielectrophoresis of biomacromolecules: The interplay of electrokinetic effects. Sens. Actuators B Chem. 2017, 252, 391–408. [Google Scholar] [CrossRef]
- Cottet, J.; Fabregue, O.; Berger, C.; Buret, F.; Renaud, P.; Frénéa-Robin, M. MyDEP: A new computational tool for dielectric modeling of particles and cells. Biophys. J. 2019, 116, 12–18. [Google Scholar] [CrossRef]
- Honda, C.; Kamizono, H.; Samejima, T.; ENDO, K. Studies on thermal aggregation of bovine serum albumin as a drug carrier. Chem. Pharm. Bull. 2000, 48, 464–466. [Google Scholar] [CrossRef]
- Yang, C.; Lei, U. Quasistatic force and torque on ellipsoidal particles under generalized dielectrophoresis. J. Appl. Phys. 2007, 102, 094702. [Google Scholar] [CrossRef]
- Pethig, R. Limitations of the Clausius-Mossotti function used in dielectrophoresis and electrical impedance studies of biomacromolecules. Electrophoresis 2019, 40, 2575–2583. [Google Scholar] [CrossRef]
- Pethig, R. Protein Dielectrophoresis: A Tale of Two Clausius-Mossottis—Or Something Else? Micromachines 2022, 13, 261. [Google Scholar] [CrossRef]
- Hölzel, R.; Pethig, R. Protein dielectrophoresis: I. Status of experiments and an empirical theory. Micromachines 2020, 11, 533. [Google Scholar] [CrossRef]
- Seyedi, S.S.; Matyushov, D.V. Protein dielectrophoresis in solution. J. Phys. Chem. B 2018, 122, 9119–9127. [Google Scholar] [CrossRef]
- Nakano, A. Protein Dielectrophoresis Using Insulator-Based Microfluidic Platforms; Arizona State University: Tempe, AZ, USA, 2014. [Google Scholar]
- Yan, Y.; Guo, D.; Wen, S. Joule heating effects on two-phase flows in dielectrophoresis microchips. BioChip J. 2017, 11, 196–205. [Google Scholar] [CrossRef]
- Abd Rahman, N.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for biomedical sciences applications: A review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef]
- Wang, Y.; Du, F.; Baune, M.; Thöming, J. Predicting and eliminating Joule heating constraints in large dielectrophoretic IDE separators. Chem. Eng. Sci. 2015, 137, 235–242. [Google Scholar] [CrossRef]
- Zaman, M.A.; Wu, M.; Padhy, P.; Jensen, M.A.; Hesselink, L.; Davis, R.W. Modeling brownian microparticle trajectories in lab-on-a-chip devices with time varying dielectrophoretic or optical forces. Micromachines 2021, 12, 1265. [Google Scholar] [CrossRef]
- Kadaksham, A.T.; Singh, P.; Aubry, N. Dielectrophoresis of nanoparticles. Electrophoresis 2004, 25, 3625–3632. [Google Scholar] [CrossRef]
- Altintas, E.; Böhringer, K.F.; Fujita, H. Micromachined Linear Brownian Motor: Transportation of Nanobeads by Brownian Motion Using Three-Phase Dielectrophoretic Ratchet. Jpn. J. Appl. Phys. 2008, 47, 8673. [Google Scholar] [CrossRef]
- Midelet, C.; Le Pioufle, B.; Werts, M. Brownian Motion and Large Electric Polarizabilities Facilitate Dielectrophoretic Capture of Sub-200 nm Gold Nanoparticles in Water. ChemPhysChem 2019, 20, 3354–3365. [Google Scholar] [CrossRef] [PubMed]
- Benhal, P.; Quashie, D.; Kim, Y.; Ali, J. Insulator Based Dielectrophoresis: Micro, Nano, and Molecular Scale Biological Applications. Sensors 2020, 20, 5095. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Villanueva, R.C.; Sano, M.B.; Lapizco-Encinas, B.H.; Davalos, R.V. Joule heating effects on particle immobilization in insulator-based dielectrophoretic devices. Electrophoresis 2014, 35, 352–361. [Google Scholar] [CrossRef]
- Nakidde, D.; Zellner, P.; Alemi, M.M.; Shake, T.; Hosseini, Y.; Riquelme, M.V.; Pruden, A.; Agah, M. Three dimensional passivated-electrode insulator-based dielectrophoresis. Biomicrofluidics 2015, 9, 014125. [Google Scholar] [CrossRef]
- Jen, C.-P.; Chen, T.-W. Selective trapping of live and dead mammalian cells using insulator-based dielectrophoresis within open-top microstructures. Biomed. Microdevices 2009, 11, 597–607. [Google Scholar] [CrossRef]
- Zellner, P.; Shake, T.; Sahari, A.; Behkam, B.; Agah, M. Off-chip passivated-electrode, insulator-based dielectrophoresis (OπDEP). Anal. Bioanal. Chem. 2013, 405, 6657–6666. [Google Scholar] [CrossRef]
- Pethig, R. Where is dielectrophoresis (DEP) going? J. Electrochem. Soc. 2016, 164, B3049. [Google Scholar] [CrossRef]
- Shayestehpour, H.; Nassiri Nazif, K.; Soufi, A.; Saidi, M. Proposing a high-efficiency dielectrophoretic system for separation of dead and live cells. Sci. Iran. 2018, 25, 186–195. [Google Scholar] [CrossRef]
- Nakano, A.; Chao, T.C.; Camacho-Alanis, F.; Ros, A. Immunoglobulin G and bovine serum albumin streaming dielectrophoresis in a microfluidic device. Electrophoresis 2011, 32, 2314–2322. [Google Scholar] [CrossRef]
- Nakano, A.; Camacho-Alanis, F.; Chao, T.; Ros, A. Systematic investigation of insulator-based protein dielectrophoresis under DC condition. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences 2011, MicroTAS 2011, Seattle, WA, USA, 2–6 October 2011; pp. 644–646. [Google Scholar]
- Lapizco-Encinas, B.H.; Ozuna-Chacón, S.; Rito-Palomares, M. Protein manipulation with insulator-based dielectrophoresis and direct current electric fields. J. Chromatogr. A 2008, 1206, 45–51. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y. DC biased low-frequency insulating constriction dielectrophoresis for protein biomolecules concentration. Biofabrication 2017, 9, 045003. [Google Scholar] [CrossRef]
- Barik, A.; Otto, L.M.; Yoo, D.; Jose, J.; Johnson, T.W.; Oh, S.-H. Dielectrophoresis-enhanced plasmonic sensing with gold nanohole arrays. Nano Lett. 2014, 14, 2006–2012. [Google Scholar] [CrossRef]
- Laux, E.M.; Knigge, X.; Bier, F.F.; Wenger, C.; Hölzel, R. Dielectrophoretic immobilization of proteins: Quantification by atomic force microscopy. Electrophoresis 2015, 36, 2094–2101. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fujii, T. Active immobilization of biomolecules on a hybrid three-dimensional nanoelectrode by dielectrophoresis for single-biomolecule study. Nanotechnology 2007, 18, 495503. [Google Scholar] [CrossRef]
- Zheng, L.; Brody, J.P.; Burke, P.J. Electronic manipulation of DNA, proteins, and nanoparticles for potential circuit assembly. Biosens. Bioelectron. 2004, 20, 606–619. [Google Scholar] [CrossRef]
- Schäfer, C.; Kern, D.P.; Fleischer, M. Capturing molecules with plasmonic nanotips in microfluidic channels by dielectrophoresis. Lab A Chip 2015, 15, 1066–1071. [Google Scholar] [CrossRef]
- Mohamad, A.S.; Hamzah, R.; Hoettges, K.F.; Hughes, M.P. A dielectrophoresis-impedance method for protein detection and analysis. AIP Adv. 2017, 7, 015202. [Google Scholar] [CrossRef]
- Hill, N.; Lapizco-Encinas, B.H. Continuous flow separation of particles with insulator-based dielectrophoresis chromatography. Anal. Bioanal. Chem. 2020, 412, 3891–3902. [Google Scholar] [CrossRef]
- Washizu, M.; Suzuki, S.; Kurosawa, O.; Nishizaka, T.; Shinohara, T. Molecular dielectrophoresis of biopolymers. IEEE Trans. Ind. Appl. 1994, 30, 835–843. [Google Scholar] [CrossRef]
- Ong, J.; Zhao, J.; Levy, G.K.; Macdonald, J.; Justin, A.W.; Markaki, A.E. Functionalisation of a heat-derived and bio-inert albumin hydrogel with extracellular matrix by air plasma treatment. Sci. Rep. 2020, 10, 12429. [Google Scholar] [CrossRef]
- Wischke, C.; Borchert, H.-H. Fluorescein isothiocyanate labelled bovine serum albumin (FITC-BSA) as a model protein drug: Opportunities and drawbacks. Die Pharm.-Int. J. Pharm. Sci. 2006, 61, 770–774. [Google Scholar]
- Luensmann, D.; Jones, L. Impact of fluorescent probes on albumin sorption profiles to ophthalmic biomaterials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 327–336. [Google Scholar] [CrossRef]
- Camacho-Alanis, F.; Ros, A. Protein dielectrophoresis and the link to dielectric properties. Bioanalysis 2015, 7, 353–371. [Google Scholar] [CrossRef]
- Turcan, I.; Caras, I.; Schreiner, T.G.; Tucureanu, C.; Salageanu, A.; Vasile, V.; Avram, M.; Tincu, B.; Olariu, M.A. Dielectrophoretic and Electrical Impedance Differentiation of Cancerous Cells Based on Biophysical Phenotype. Biosensors 2021, 11, 401. [Google Scholar] [CrossRef]
- Valero, A.; Braschler, T.; Renaud, P. A unified approach to dielectric single cell analysis: Impedance and dielectrophoretic force spectroscopy. Lab A Chip 2010, 10, 2216–2225. [Google Scholar] [CrossRef]
- Morgan, H.; Sun, T.; Holmes, D.; Gawad, S.; Green, N.G. Single cell dielectric spectroscopy. J. Phys. D Appl. Phys. 2006, 40, 61. [Google Scholar] [CrossRef]
- Henning, A.; Bier, F.F.; Hölzel, R. Dielectrophoresis of DNA: Quantification by impedance measurements. Biomicrofluidics 2010, 4, 022803. [Google Scholar] [CrossRef]
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of electrochemical impedance spectroscopy in protein biosensing. Anal. Chem. 2009, 81, 3944–3949. [Google Scholar] [CrossRef]
- Madduri, N. Electrochemical Capacitance Measurements to Study Molecular Surface Interactions. Master’s Thesis, Clemson University, Clemson, SC, USA, 2012. [Google Scholar]
- Henning, A.; Henkel, J.; Bier, F.F.; Hölzel, R. Label-free electrical quantification of the dielectrophoretic response of DNA. PMC Biophys. 2008, 1, 4. [Google Scholar] [CrossRef]
- Buyong, M.R.; Larki, F.; Zainal, M.I.H.; Almahi, A.Y.A.; Ismail, A.G.; Hamzah, A.A.; Kayani, A.A.K.; Caille, C.E.; Majlis, B.Y. Implementation of capacitance as simultaneous sensing and actuating tool in tapered microelectrode arrays for dielectrophoresis-on-a-chip application. Microelectron. Int. 2020, 37, 215–224. [Google Scholar] [CrossRef]
- Tran, T.; Nguyen, N.; Nguyen, N.; Bui, T.T.; Duc, T.C. Biological microparticles detection based on differential capacitive sensing and dielectrophoresis manipulation. In Proceedings of the 2016 International Conference on Advanced Technologies for Communications (ATC), Hanoi, Vietnam, 2–14 October 2016; pp. 297–301. [Google Scholar]
- Fischer, O.; Fischerová, E. Basic principles of voltammetry. In Experimental Techniques in Bioelectrochemistry; Springer: Berlin/Heidelberg, Germany, 1995; pp. 41–157. [Google Scholar]
- Ertürk, G.; Mattiasson, B. Capacitive biosensors and molecularly imprinted electrodes. Sensors 2017, 17, 390. [Google Scholar] [CrossRef] [PubMed]
- Buyong, M.R.; Abd Aziz, N.; Majlis, B.Y. Fabrication of thin layer membrane using CMOS process for very low pressure sensor applications. In Proceedings of the 2008 IEEE International Conference on Semiconductor Electronics, Johar, Malaysia, 25–27 November 2008; pp. 363–369. [Google Scholar]
- Páez-Avilés, C.; Juanola-Feliu, E.; Punter-Villagrasa, J.; del Moral Zamora, B.; Homs-Corbera, A.; Colomer-Farrarons, J.; Miribel-Català, P.L.; Samitier, J. Combined dielectrophoresis and impedance systems for bacteria analysis in microfluidic on-chip platforms. Sensors 2016, 16, 1514. [Google Scholar] [CrossRef] [PubMed]
- Mojena-Medina, D.; Hubl, M.; Bäuscher, M.; Jorcano, J.L.; Ngo, H.-D.; Acedo, P. Real-time impedance monitoring of epithelial cultures with inkjet-printed interdigitated-electrode sensors. Sensors 2020, 20, 5711. [Google Scholar] [CrossRef]
- Kadan-Jamal, K.; Sophocleous, M.; Jog, A.; Desagani, D.; Teig-Sussholz, O.; Georgiou, J.; Avni, A.; Shacham-Diamand, Y. Electrical Impedance Spectroscopy of plant cells in aqueous biological buffer solutions and their modelling using a unified electrical equivalent circuit over a wide frequency range: 4Hz to 20 GHz. Biosens. Bioelectron. 2020, 168, 112485. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Varzakas, T.; Erdem, A.; Nikoleli, G.-P. Portable Biosensing of Food Toxicants and Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Yoo, J.; Cha, M.; Lee, J. Bacterial concentration detection with dielectrophoresis and capacitive measurement. In Proceedings of the NSTI Nanotechnology Conference and Trade Show, Boston, MA, USA, 1–5 June 2008; pp. 1–5. [Google Scholar]
- Hirano, A.; Kida, S.; Yamamoto, K.-i.; Sakai, K. Experimental evaluation of flow and dialysis performance of hollow-fiber dialyzers with different packing densities. J. Artif. Organs 2012, 15, 168–175. [Google Scholar] [CrossRef]
- Abd Samad, M.I.; Kayani, A.A.; Zoolfakar, A.S.; Hamzah, A.A.; Majlis, B.Y.; Buyong, M.R. Lab-on-a-chip dielectrophoretic manipulation of beta-2 microglobulin for toxin removal in an artificial kidney. Micro Nanosyst. 2019, 11, 40–46. [Google Scholar] [CrossRef]
- Morin, P. Identification of the bacteriological contamination of a water treatment line used for haemodialysis and its disinfection. J. Hosp. Infect. 2000, 45, 218–224. [Google Scholar] [CrossRef]
- Murray, E.C.; Marek, A.; Thomson, P.C.; Coia, J.E. Gram-negative bacteraemia in haemodialysis. Nephrol. Dial. Transplant. 2015, 30, 1202–1208. [Google Scholar] [CrossRef]
- Demircan, Y.; Özgür, E.; Külah, H. Dielectrophoresis: Applications and future outlook in point of care. Electrophoresis 2013, 34, 1008–1027. [Google Scholar] [CrossRef]
- Yunus, F.W.; Hamzah, A.A.; Norzin, M.S.; Buyong, M.R.; Yunas, J.; Majlis, B.Y. Dielectrophoresis: Iron dificient anemic red blood cells for artificial kidney purposes. In Proceedings of the 2018 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 15–17 August 2018; pp. 5–8. [Google Scholar]
- Yunus, F.W.; Hamzah, A.A.; Buyong, M.R.; Yunas, J.; Majlis, B.Y. Fabrication of 2D Membrane Castellated and Straight Electrode for Dielectrophoresis. Int. J. Nanoelectron. Mater. 2020, 13, 193–202. [Google Scholar]












| Diseases | Definition | References |
|---|---|---|
| Hypoalbuminemia | Decrease of albumin inside the body | [9,10,11] |
| Hyperalbuminemia | Excessive albumin inside the body | [12,13] |
| Albuminuria | Too much albumin found in the urine. | [14,15] |
| Protein Analysis Method | Weakness | References |
|---|---|---|
| Enzyme-linked immunosorbent assay (ELISA) | Tedious procedure and the antibody to be prepared is cost-consuming. | [24,25,26,27] |
| Western blotting | It can only be used if the primary antibody against the specific protein is available. The antibody also can be expensive. | [28,29,30] |
| Mass spectrometry | Relies on calibration. Miscalibration could lead to an inaccurate result. | [31,32] |
| Gel electrophoresis | Susceptible to contamination. | [33,34] |
| Types of DEP | Definition | References |
|---|---|---|
| Insulator-based dielectrophoresis (iDEP) | An insulating layer or structured insulating arrangement is placed between the electrode where nonuniform fields are being created. Mostly applied with DC with high voltage. | [74,75,76,77] |
| Electrode-based dielectrophoresis (eDEP) | Nonuniform fields are created by metal-based, sharp-edged with narrow gaps spaced between the electrodes. | [78,79,80] |
| Frequency/ Type of Current | DEP Principal Application | DEP Response | Geometry of Electrodes | Conductivity of Suspending Medium | Voltage and Frequency Applied | Reference |
|---|---|---|---|---|---|---|
| DC current | Aggregate trapping | pDEP | Elliptical post array | 50 µL phosphate buffer solution; 0.01 S/m | 1900 V/cm | [81] |
| DC current | Enrichment | pDEP | Circular array | Phosphate buffer solution; 0.01 S/m–0.04 S/m | 3000 V/cm | [82] |
| DC current | Enrichment | nDEP | Post array with ‘dove-tail’ geometry on the beginning of the post of both sides. | Deionized water; 25 to 100 S/cm | 700 V/cm | [83] |
| Both AC and DC current | Enrichment | nDEP | Parallel micro ridges | Phosphate buffered saline (PBS); 0.08–0.1 S/m | 5 V to 15 V; 10 Hz to 100 kHz | [84] |
| Frequency/ Type of Current | DEP Principal Application | DEP Response | Geometry of Electrodes | Conductivity of Suspending Medium | Voltage and Frequency Applied | Reference |
|---|---|---|---|---|---|---|
| AC current | Trapping | pDEP | Nanohole array | Water medium; 0.28 mS/m | 6 Vpp; 1 kHz | [85] |
| AC current | Enrichment | pDEP | Subarray | Ultrapure water; less than 1 S/cm | 0.7–14.1 Vrms; 10 kHz | [86] |
| AC current | Trapping | pDEP | Column-shaped nanopillar with a flat top | De-ionized water; 2 µS/cm | From 3 MV/m to 5 MV/m | [87] |
| AC current | Trapping | pDEP | Quadrupole | De-ionized water; 1 mS/m | Up to 8Vpp; 50 kHz and 5 MHz | [88] |
| AC current | Accumulation | pDEP | Nanocones | Water; S/m | 10 V, 2.5 MHz | [89] |
| Electrical Quantification Techniques | Explanation | References |
|---|---|---|
| EIS | Defined as the reaction relationship between a biological body and electrical impetus (e.g., extrinsic electrical field) with taking the dielectric properties and frequency applied into account of the reaction | [97] |
| CV | Described as an electrolytic cell in which three electrodes are dipped into an electrolytic cell, and the measurement of the electric current flow through the cell system. | [106] |
| Capacitance Measurement | The measurement of changes in the dielectric properties (includes the conductivity and permittivity of the biosample and suspending medium) and the dielectric layer of the sample and suspending medium with the electrode interaction. | [107] |
| DEP Responses | Definition | Correlation between DEP and CV |
|---|---|---|
| pDEP | Load can be seen attracted on the edge of the electrodes | The adsorption of the sample onto the CV electrodes increases. The peak current of the voltammogram also increases. |
| nDEP | Load can be seen accumulated in the middle of the electrodes | The adsorption of the sample onto the CV electrodes decreases. The peak current of the voltammogram also decreases. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasir, N.S.A.; Deivasigamani, R.; Wee, M.F.M.R.; Hamzah, A.A.; Zaid, M.H.M.; Rahim, M.K.A.; Kayani, A.A.; Abdulhameed, A.; Buyong, M.R. Protein Albumin Manipulation and Electrical Quantification of Molecular Dielectrophoresis Responses for Biomedical Applications. Micromachines 2022, 13, 1308. https://doi.org/10.3390/mi13081308
Nasir NSA, Deivasigamani R, Wee MFMR, Hamzah AA, Zaid MHM, Rahim MKA, Kayani AA, Abdulhameed A, Buyong MR. Protein Albumin Manipulation and Electrical Quantification of Molecular Dielectrophoresis Responses for Biomedical Applications. Micromachines. 2022; 13(8):1308. https://doi.org/10.3390/mi13081308
Chicago/Turabian StyleNasir, Nur Shahira Abdul, Revathy Deivasigamani, M. F. Mohd Razip Wee, Azrul Azlan Hamzah, Mohd Hazani Mat Zaid, Muhammad Khairulanwar Abdul Rahim, Aminuddin Ahmad Kayani, Abdullah Abdulhameed, and Muhamad Ramdzan Buyong. 2022. "Protein Albumin Manipulation and Electrical Quantification of Molecular Dielectrophoresis Responses for Biomedical Applications" Micromachines 13, no. 8: 1308. https://doi.org/10.3390/mi13081308
APA StyleNasir, N. S. A., Deivasigamani, R., Wee, M. F. M. R., Hamzah, A. A., Zaid, M. H. M., Rahim, M. K. A., Kayani, A. A., Abdulhameed, A., & Buyong, M. R. (2022). Protein Albumin Manipulation and Electrical Quantification of Molecular Dielectrophoresis Responses for Biomedical Applications. Micromachines, 13(8), 1308. https://doi.org/10.3390/mi13081308








