Ultra-Sensitive Impedimetric Immunosensor Using Copper Oxide Quantum Dots Grafted on the Gold Microelectrode for the Detection of Parathion
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Methods
2.2. Instruments
2.3. Synthesis of Copper Oxide Quantum Dots
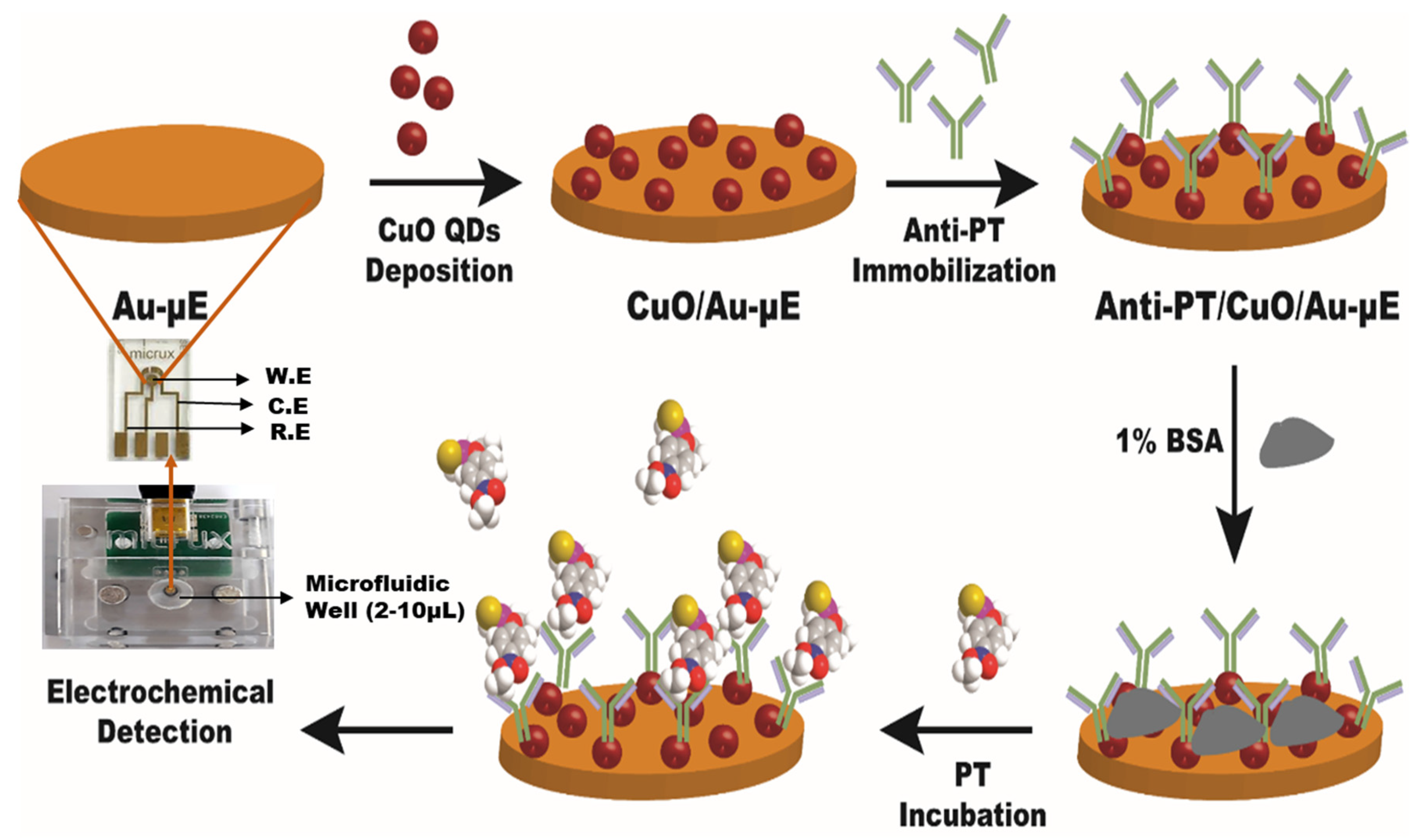
2.4. Development of Anti-PT/CuO/Au-µE
3. Results and Discussion
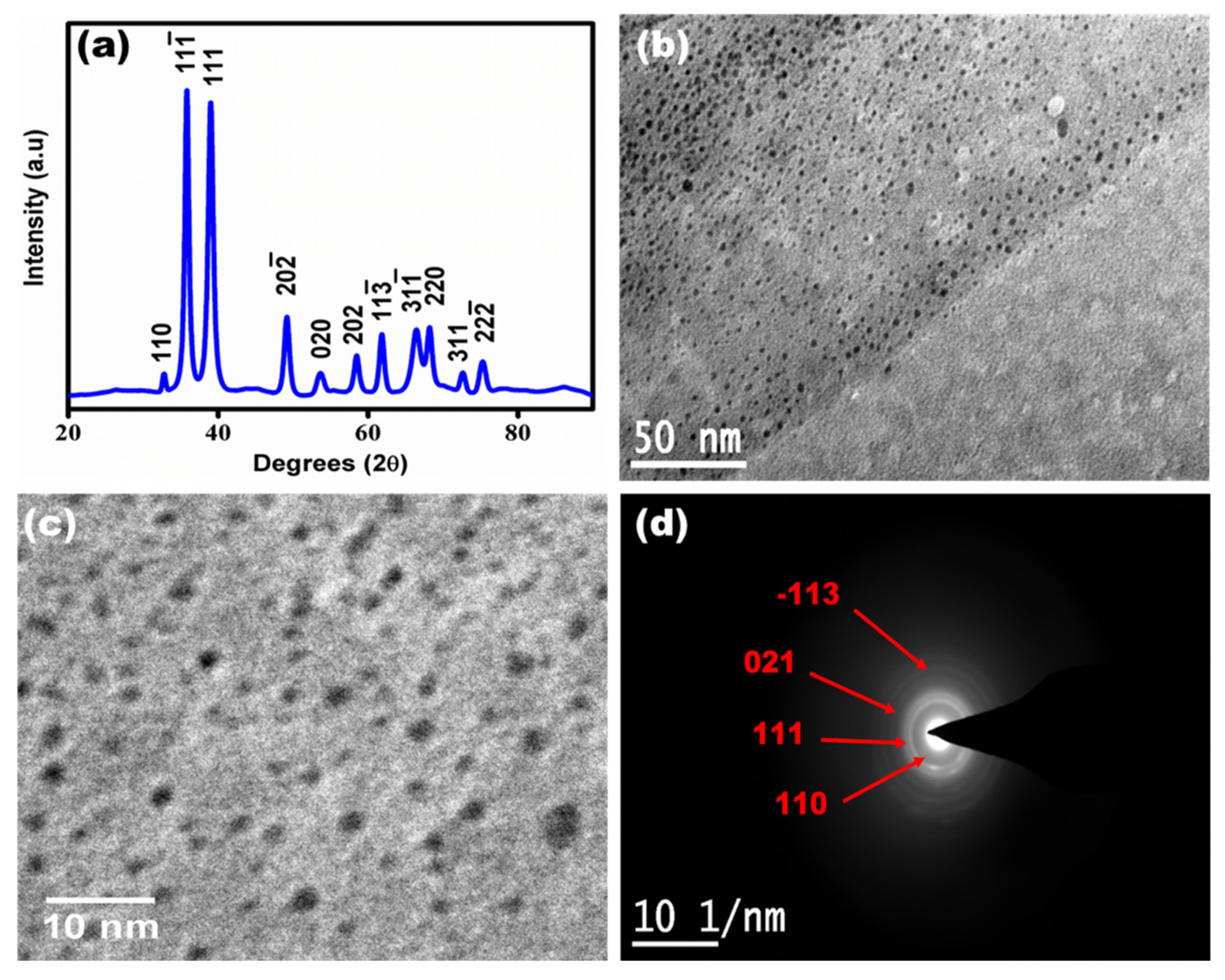
3.1. Characterization of Synthesized Copper Oxide Quantum Dots
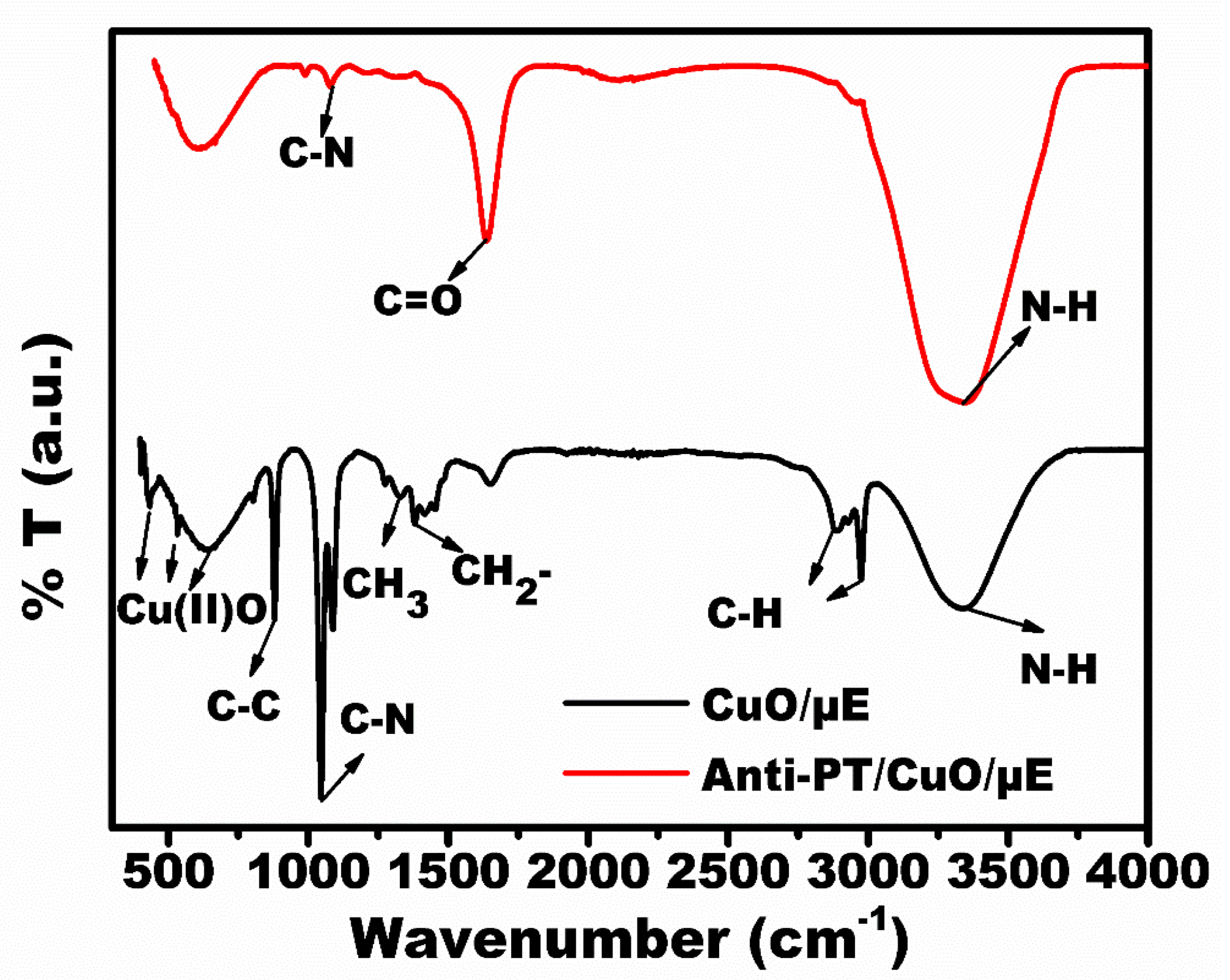
3.2. Characterization of the Developed Immunosensing Platform (Anti-PT/CuO/Au-µE)
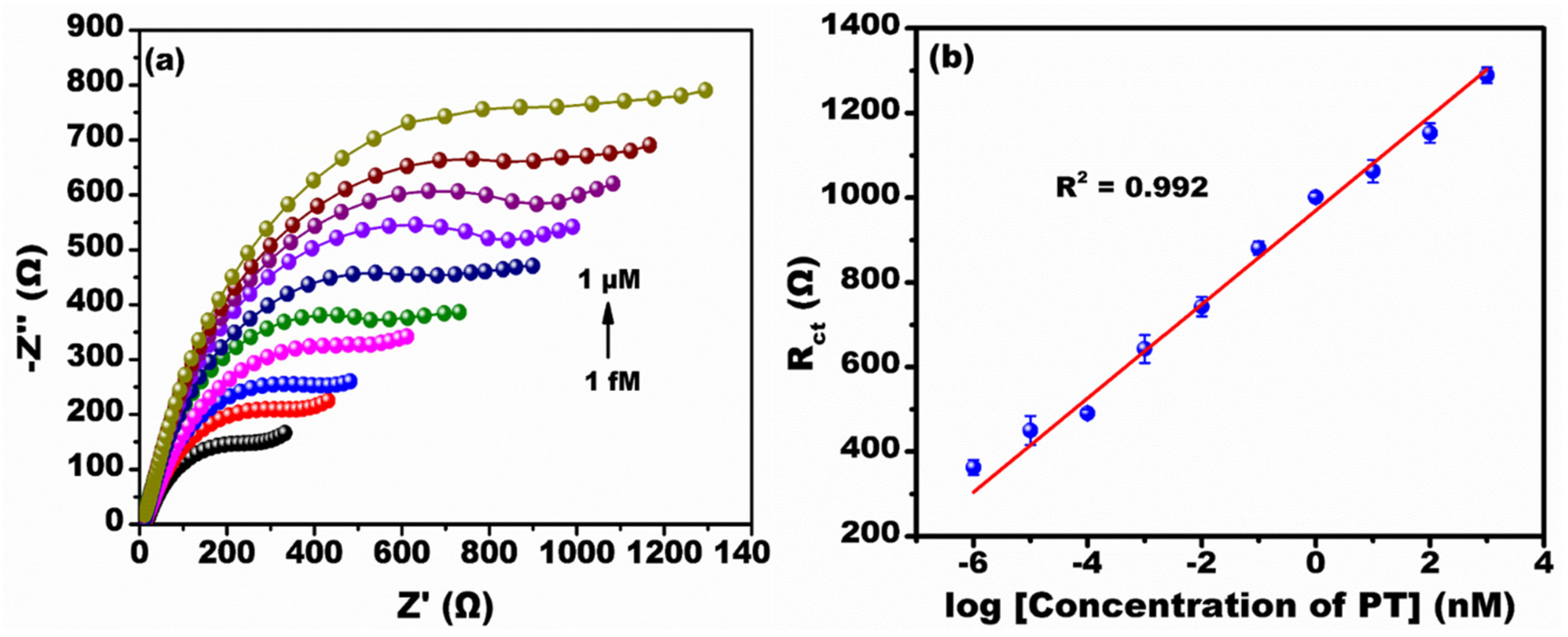
3.3. Impedimetric Detection of Parathion
3.4. Selectivity and Stability Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Hassani, S.; Momtaz, S.; Vakhshiteh, F.; Maghsoudi, A.S.; Ganjali, M.R.; Norouzi, P.; Abdollahi, M. Biosensors and their applications in detection of organophosphorus pesticides in the environment. Arch. Toxicol. 2017, 91, 109–130. [Google Scholar] [CrossRef]
- Sharma, N.; Dutta, S. Scenario of Pesticide Pollution and Toxicity in Rajasthan–A Review Article. IJSRD-Int. J. Sci. Res. Dev. 2017, 5, 1578–1581. [Google Scholar]
- Ragnarsdottir, K.V. Environmental fate and toxicology of organophosphate pesticides. J. Geol. Soc. Lond. 2000, 157, 859–876. [Google Scholar] [CrossRef]
- Dhananjayan, V.; Ravichandran, B. Occupational health risk of farmers exposed to pesticides in agricultural activities. Curr. Opin. Environ. Sci. Health 2018, 4, 31–37. [Google Scholar] [CrossRef]
- Stoytcheva, M.; Zlatev, R. Organophosphorus Pesticides Analysis. Pestic. Mod. World-Trends Pestic. Anal. 2011, Chapter–7, 143–164. [Google Scholar] [CrossRef]
- Ali, J.; Najeeb, J.; Asim Ali, M.; Farhan Aslam, M.; Raza, A. Biosensors: Their Fundamentals, Designs, Types and Most Recent Impactful Applications: A Review. J. Biosens. Bioelectron. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Jiang, X.; Li, D.; Xu, X.; Ying, Y.; Li, Y.; Ye, Z.; Wang, J. Immunosensors for detection of pesticide residues. Biosens. Bioelectron. 2008, 23, 1577–1587. [Google Scholar] [CrossRef]
- Helali, S. Impedimetric Immunosensor for Pesticide Detection. State Art Biosens.-Environ. Med. Appl. 2013, Chapter–6, 53751. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical biosensors for detection of pesticides and heavy metal toxicants in water: Recent trends and progress. ACS EST Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Park, J.; Joo, J.; Soon, G.K.; Jang, Y.; Hyeon, T. Synthesis of monodisperse spherical nanocrystals. Angew. Chem.-Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; Farangi, M. Facile synthesis of graphene quantum dots from corn powder and their application as down conversion effect in quantum dot-dye-sensitized solar cell. J. Mol. Liq. 2018, 251, 267–272. [Google Scholar] [CrossRef]
- Raj, A.S.A.; Biju, V. Nanostructured CuO: Facile synthesis, optical absorption and defect dependent electrical conductivity. Mater. Sci. Semicond. Process. 2017, 68, 38–47. [Google Scholar] [CrossRef]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Talluri, B.; Prasad, E.; Thomas, T. Critical role of surfactants in the formation of digestively-ripened, ultra-small (r <2 nm) copper oxide quantum dots. Superlattices Microstruct. 2018, 116, 122–130. [Google Scholar] [CrossRef]
- Talluri, B.; Prasad, E.; Thomas, T. Ultra-small (r < 2 nm), stable (>1 year) copper oxide quantum dots with wide band gap. Superlattices Microstruct. 2018, 113, 600–607. [Google Scholar] [CrossRef]
- Xu, J.; Ge, J.P.; Li, Y.D. Solvothermal synthesis of monodisperse PbSe nanocrystals. J. Phys. Chem. B 2006, 110, 2497–2501. [Google Scholar] [CrossRef]
- Shimpi, J.R.; Sidhaye, D.S.; Prasad, B.L.V. Digestive Ripening: A Fine Chemical Machining Process on the Nanoscale. Langmuir 2017, 33, 9491–9507. [Google Scholar] [CrossRef]
- Nagabooshanam, S.; Roy, S.; Mathur, A.; Mukherjee, I.; Krishnamurthy, S.; Bhardwaj, L.M. Electrochemical micro analytical device interfaced with portable potentiostat for rapid detection of chlorpyrifos using acetylcholinesterase conjugated metal organic framework using Internet of things. Sci. Rep. 2019, 9, 19862. [Google Scholar] [CrossRef]
- Vaseem, M.; Hong, A.R.; Kim, R.T.; Hahn, Y.B. Copper oxide quantum dot ink for inkjet-driven digitally controlled high mobility field effect transistors. J. Mater. Chem. C 2013, 1, 2112–2120. [Google Scholar] [CrossRef]
- de Regt, J.M.; van Dijk, J.; van der Mullen, J.A.M.; Schram, D.C. Components of continuum radiation in an inductively coupled plasma. J. Phys. D Appl. Phys. 1995, 28, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Rathod, K.N.; Savaliya, C.; Babiya, K.R.; Vasvani, S.H.; Ramani, R.V.; Ramani, B.M.; Joshi, A.D.; Pandya, D.; Shah, N.A.; Markna, J.H. Preparation of CuO Quantum Dots by Cost-Effective Ultrasonication Technique. Int. J. Nanosci. 2017, 16, 1750019. [Google Scholar] [CrossRef]
- Prabhash, P.G.; Nair, S.S. Synthesis of copper quantum dots by chemical reduction method and tailoring of its band gap. AIP Adv. 2016, 6, 055003. [Google Scholar] [CrossRef]
- Wu, J.L.; Zhang, G.M.; Liu, W.M.; Zhang, Q.F.; Wu, Q.D. Quantum yield of photoemission of Ag-BaO thin films for detecting ultrashort infrared laser pulses. Phys. B Condens. Matter 2001, 307, 9–14. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Tang, J.Y.; Wang, G.L.; Zhang, M.; Hu, X.Y. Facile synthesis of submicron Cu2O and CuO crystallites from a solid metallorganic molecular precursor. J. Cryst. Growth 2006, 294, 278–282. [Google Scholar] [CrossRef]
- Borgohain, K.; Singh, J. Quantum size effects in CuO nanoparticles. Phys. Rev. B-Condens. Matter Mater. Phys. 2000, 61, 11093–11096. [Google Scholar] [CrossRef]
- Deep, A.; Bhardwaj, S.K.; Paul, A.K.; Kim, K.H.; Kumar, P. Surface assembly of nano-metal organic framework on amine functionalized indium tin oxide substrate for impedimetric sensing of parathion. Biosens. Bioelectron. 2015, 65, 226–231. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Balakrishnan, S.R.; Hashim, U.; Gopinath, S.C.B.; Poopalan, P.; Ramayya, H.R.; Omar, M.I.; Haarindraprasad, R.; Veeradasan, P. A point-of-care immunosensor for human chorionic gonadotropin in clinical urine samples using a cuneated polysilicon nanogap lab-on-chip. PLoS ONE 2015, 10, e0137891. [Google Scholar] [CrossRef]
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.K.; Kim, K.H.; Deep, A. Graphene modified screen printed immunosensor for highly sensitive detection of parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Tuteja, S.K.; Vinayak, P.; Paul, A.K.; Kim, K.H.; Deep, A. Graphene quantum dot modified screen printed immunosensor for the determination of parathion. Anal. Biochem. 2017, 523, 1–9. [Google Scholar] [CrossRef]



| Senor | Technique | Linear Range | LoD | Reference |
|---|---|---|---|---|
| [Cd(atc)(H2O)2]n/ITO | Impedimetric | 0.1 to 20 ng/mL | 0.1 ng/mL | [27] |
| AntiPT/2ABA/Graphene/SPE | Impedimetric | 0.1 to 1000 ng/L | 52 pg/L (17.8 fM) | [31] |
| GQD/SPE | Impedimetric | 0.01 to 106 ng/L | 46 pg/L (15.8 fM) | [32] |
| Anti-PT/CuO/Au-µE | Impedimetric | 1 fM to 1 µM | 0.69 fM | Present Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagabooshanam, S.; Talluri, B.; Thomas, T.; Krishnamurthy, S.; Mathur, A. Ultra-Sensitive Impedimetric Immunosensor Using Copper Oxide Quantum Dots Grafted on the Gold Microelectrode for the Detection of Parathion. Micromachines 2022, 13, 1385. https://doi.org/10.3390/mi13091385
Nagabooshanam S, Talluri B, Thomas T, Krishnamurthy S, Mathur A. Ultra-Sensitive Impedimetric Immunosensor Using Copper Oxide Quantum Dots Grafted on the Gold Microelectrode for the Detection of Parathion. Micromachines. 2022; 13(9):1385. https://doi.org/10.3390/mi13091385
Chicago/Turabian StyleNagabooshanam, Shalini, Bhusankar Talluri, Tiju Thomas, Satheesh Krishnamurthy, and Ashish Mathur. 2022. "Ultra-Sensitive Impedimetric Immunosensor Using Copper Oxide Quantum Dots Grafted on the Gold Microelectrode for the Detection of Parathion" Micromachines 13, no. 9: 1385. https://doi.org/10.3390/mi13091385






