CO2-Laser-Micromachined, Polymer Microchannels with a Degassed PDMS slab for the Automatic Production of Monodispersed Water-in-Oil Droplets
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
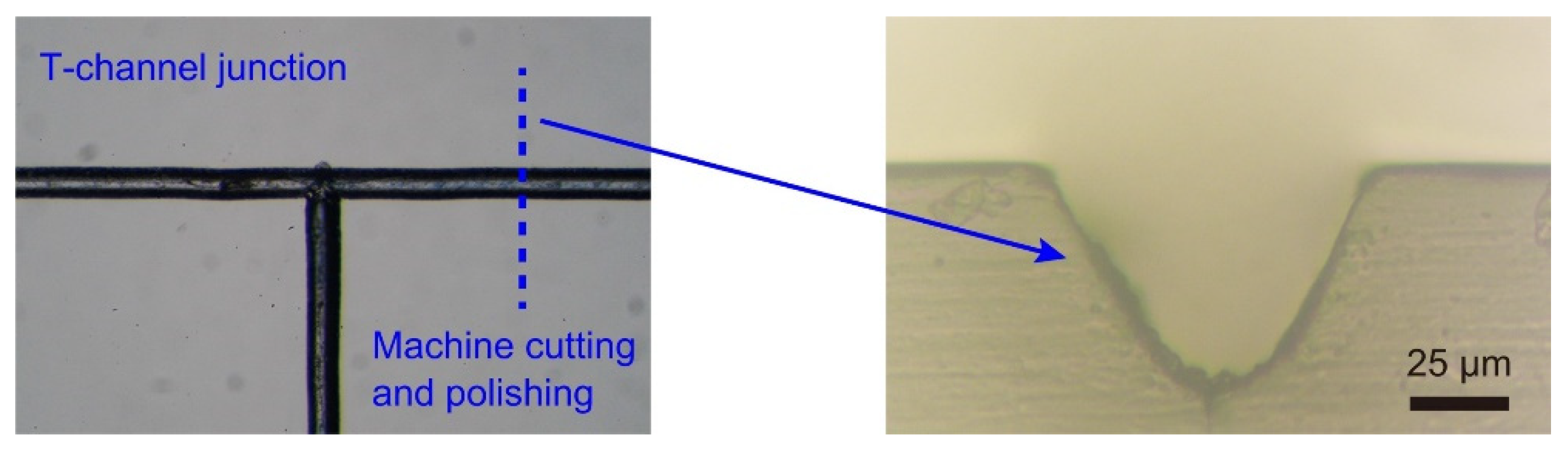
3.1. Laser Micromachining of Microfludic Channels on a PMMA Slab
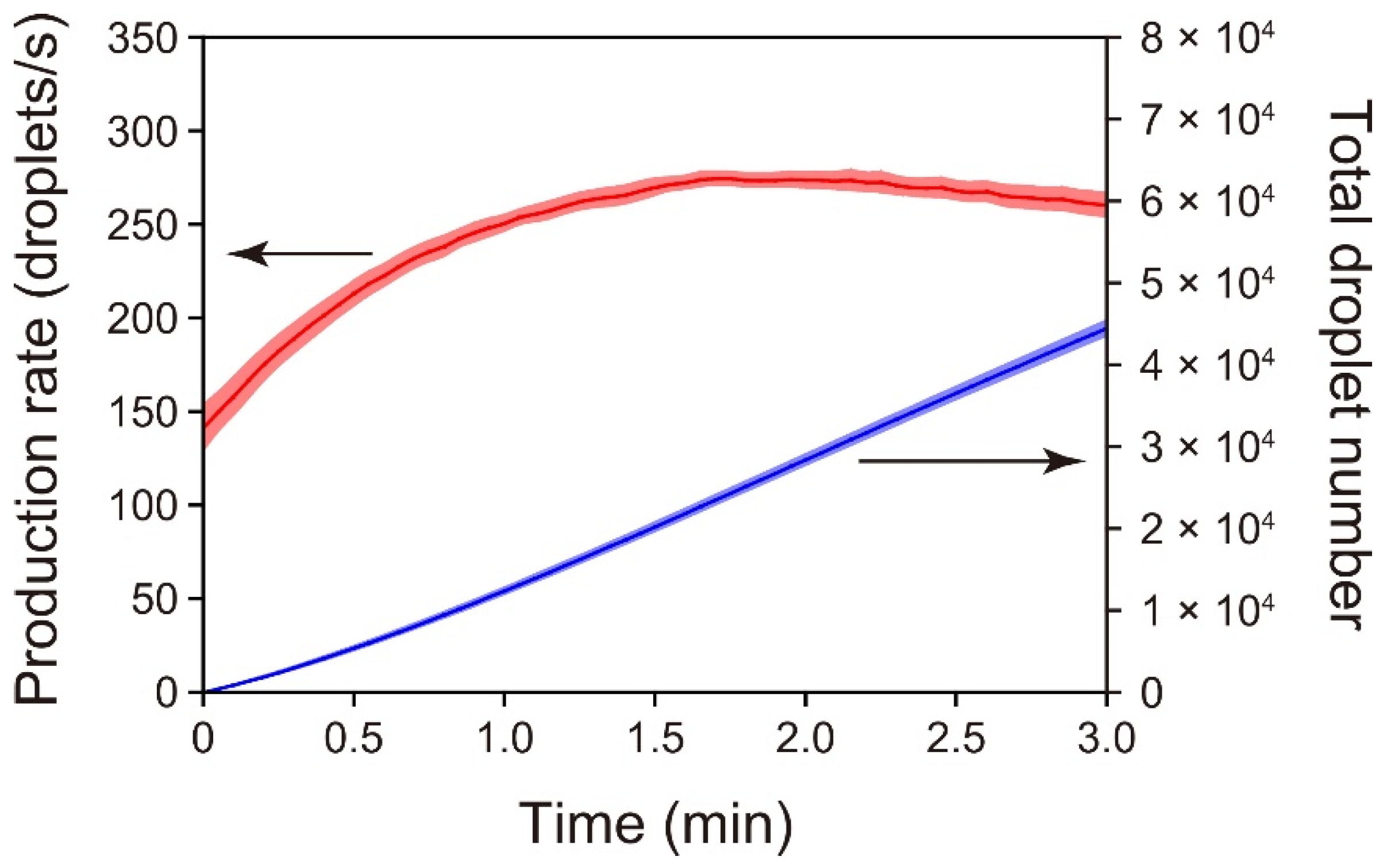
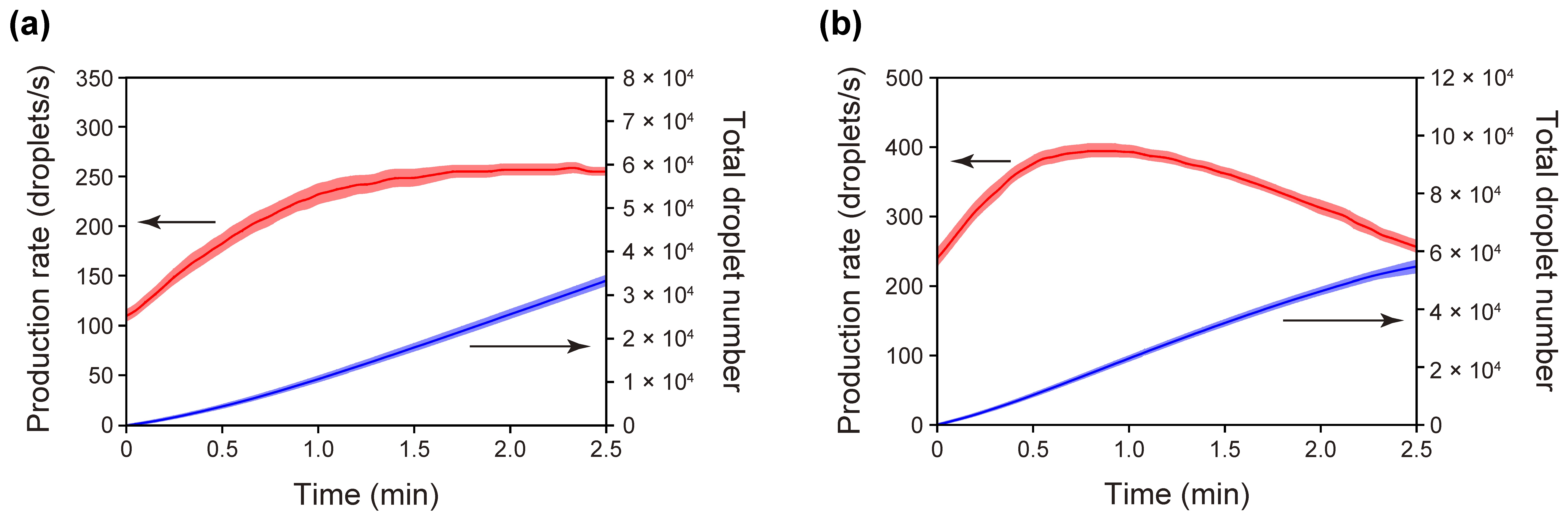
3.2. Time-Course Measurements of Droplet-Production Rates Achieved with the Standard PMMA/PDMS Microchip
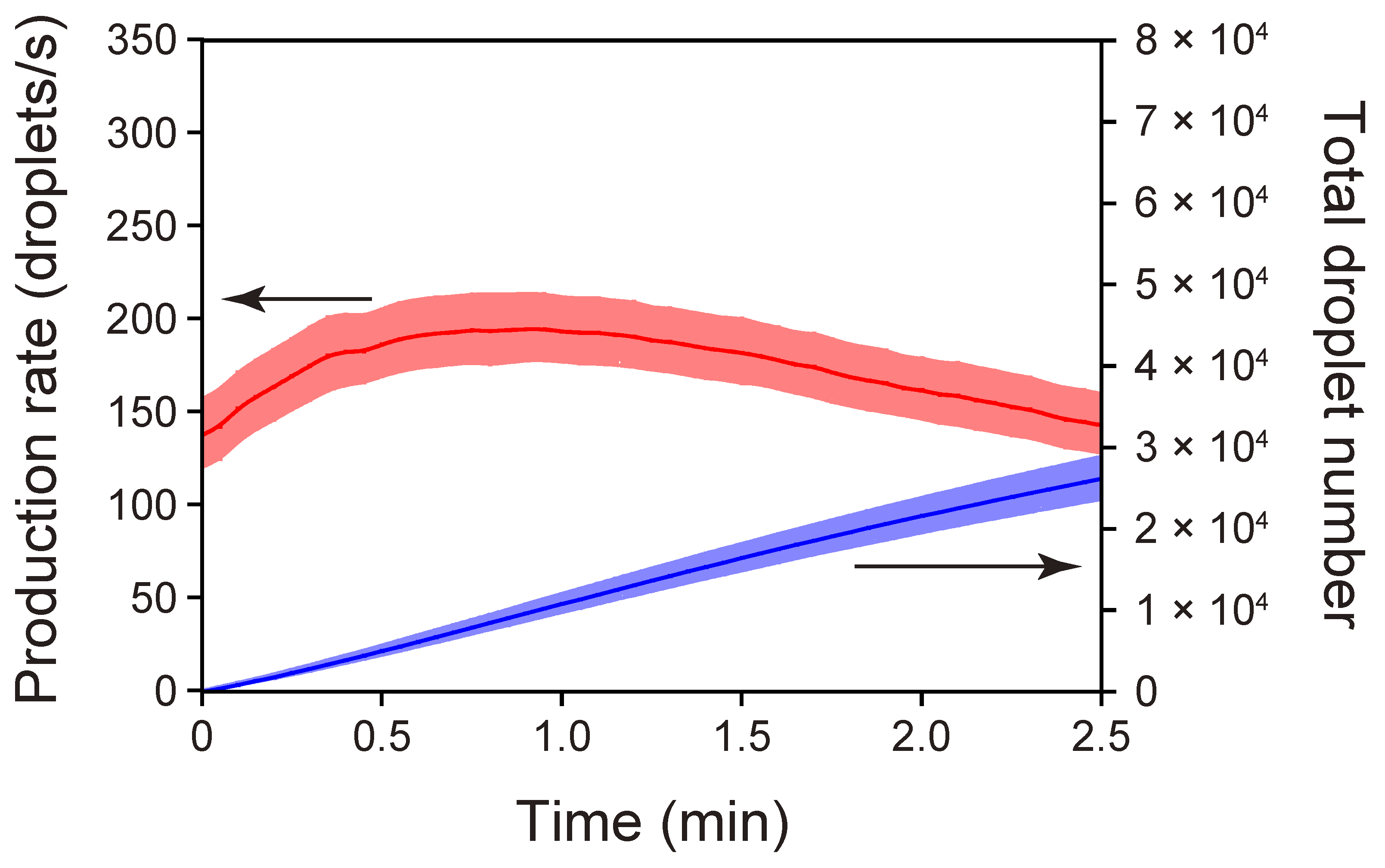
3.3. Droplet Production with a PET/PDMS Microchip
3.4. Droplet-Production Using Different Versions of the PMMA/PDMS Microchip (Variant II and Variant III)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mazaika, E.; Homsy, J. Digital droplet PCR: CNV analysis and other applications. Curr. Protoc. Hum. Genet. 2014, 82, 7.24.1–7.24.13. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.T.; Gu, Z.; Ingersoll, J.; Abdul-Ali, D.; Shi, L.; Pounds, S.; Caliendo, A.M. Comparison of droplet digital PCR to real-time PCR for quantitative detection of cytomegalovirus. J. Clin. Microbiol. 2013, 51, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Demaree, B.; Weisgerber, D.; Lan, F.; Abate, A.R. An ultrahigh-throughput microfluidic platform for single-cell genome sequencing. J. Vis. Exp. 2018, 57598. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.; Light, Y.K.; Meagher, R.J.; Singh, A.K. Digital droplet multiple displacement amplification (ddMDA) for whole genome sequencing of limited DNA samples. PLoS ONE 2016, 11, e0153699. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-K.; Ali, M.M.; Zhang, K.; Huang, S.S.; Peterson, E.; Digman, M.A.; Gratton, E.; Zhao, W. Rapid detection of single bacteria in unprocessed blood using Integrated Comprehensive Droplet Digital Detection. Nat. Commun. 2014, 5, 5427. [Google Scholar] [CrossRef]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef]
- Evans, G.W.H.; Bhuiyan, W.T.; Pang, S.; Warren, B.; Makris, K.; Coleman, S.; Hassan, S.-u.; Niu, X. A portable droplet microfluidic device for cortisol measurements using a competitive heterogeneous assay. Analyst 2021, 146, 4535–4544. [Google Scholar] [CrossRef]
- Shim, J.-u.; Ranasinghe, R.T.; Smith, C.A.; Ibrahim, S.M.; Hollfelder, F.; Huck, W.T.S.; Klenerman, D.; Abell, C. Ultrarapid generation of femtoliter microfluidic droplets for single-molecule-counting immunoassays. ACS Nano 2013, 7, 5955–5964. [Google Scholar] [CrossRef]
- Agresti Jeremy, J.; Antipov, E.; Abate Adam, R.; Ahn, K.; Rowat Amy, C.; Baret, J.-C.; Marquez, M.; Klibanov Alexander, M.; Griffiths Andrew, D.; Weitz David, A. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 4004–4009. [Google Scholar] [CrossRef]
- Fallah-Araghi, A.; Baret, J.-C.; Ryckelynck, M.; Griffiths, A.D. A completely in vitro ultrahigh-throughput droplet-based microfluidic screening system for protein engineering and directed evolution. Lab Chip 2012, 12, 882–891. [Google Scholar] [CrossRef]
- Maeki, M.; Yamaguchi, H.; Yamashita, K.; Nakamura, H.; Miyazaki, M.; Maeda, H. A method for generating single crystals that rely on internal fluid dynamics of microdroplets. Chem. Commun. 2012, 48, 5037–5039. [Google Scholar] [CrossRef] [PubMed]
- Eduati, F.; Utharala, R.; Madhavan, D.; Neumann, U.P.; Longerich, T.; Cramer, T.; Saez-Rodriguez, J.; Merten, C.A. A microfluidics platform for combinatorial drug screening on cancer biopsies. Nat. Commun. 2018, 9, 2434. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.H.-H.; Li, H.; Jia, Y.; Mak, P.-I.; Martins, R.P.d.S.; Liu, Y.; Vong, C.M.; Wong, H.C.; Wong, P.K.; Wang, H.; et al. Drug screening of cancer cell lines and human primary tumors using droplet microfluidics. Sci. Rep. 2017, 7, 9109. [Google Scholar] [CrossRef]
- Bajgiran, K.R.; Cordova, A.S.; Elkhanoufi, R.; Dorman, J.A.; Melvin, A.T. Simultaneous droplet generation with in-series droplet T-junctions induced by gravity-induced flow. Micromachines 2021, 12, 1211. [Google Scholar] [CrossRef]
- Yao, J.; Lin, F.; Kim, H.S.; Park, J. The effect of oil viscosity on droplet generation rate and droplet size in a T-junction microfluidic droplet generator. Micromachines 2019, 10, 808. [Google Scholar] [CrossRef]
- Filatov, N.A.; Evstrapov, A.A.; Bukatin, A.S. Negative pressure provides simple and stable droplet generation in a flow-focusing microfluidic device. Micromachines 2021, 12, 662. [Google Scholar] [CrossRef]
- Kaminski, T.S.; Jakiela, S.; Czekalska, M.A.; Postek, W.; Garstecki, P. Automated generation of libraries of nL droplets. Lab Chip 2012, 12, 3995–4002. [Google Scholar] [CrossRef]
- Zhu, Z.; Jenkins, G.; Zhang, W.; Zhang, M.; Guan, Z.; Yang, C.J. Single-molecule emulsion PCR in microfluidic droplets. Anal. Bioanal. Chem. 2012, 403, 2127–2143. [Google Scholar] [CrossRef]
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; deMello, A.J. Microdroplets: A sea of applications? Lab Chip 2008, 8, 1244–1254. [Google Scholar] [CrossRef]
- Hosokawa, K.; Sato, K.; Ichikawa, N.; Maeda, M. Power-free poly(dimethylsiloxane) microfluidic devices for gold nanoparticle-based DNA analysis. Lab Chip 2004, 4, 181–185. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamamoto, S.; Nakamura, A.; Nakashoji, Y.; Okura, N.; Nakamoto, N.; Tsukagoshi, K.; Hashimoto, M. Hands-off preparation of monodisperse emulsion droplets using a poly(dimethylsiloxane) microfluidic chip for droplet digital PCR. Anal. Chem. 2015, 87, 4134–4143. [Google Scholar] [CrossRef] [PubMed]
- Chau, K.; Millare, B.; Lin, A.; Upadhyayula, S.; Nuñez, V.; Xu, H.; Vullev, V.I. Dependence of the quality of adhesion between poly(dimethylsiloxane) and glass surfaces on the composition of the oxidizing plasma. Microfluid. Nanofluid. 2011, 10, 907–917. [Google Scholar] [CrossRef]
- Kim, J.; Kang, M.; Jensen, E.C.; Mathies, R.A. Lifting gate polydimethylsiloxane microvalves and pumps for microfluidic control. Anal. Chem. 2012, 84, 2067–2071. [Google Scholar] [CrossRef]
- Nakashoji, Y.; Tanaka, H.; Tsukagoshi, K.; Hashimoto, M. A poly(dimethylsiloxane) microfluidic sheet reversibly adhered on a glass plate for creation of emulsion droplets for droplet digital PCR. Electrophoresis 2017, 38, 296–304. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Lassemono, S.; Chollet, F.A. Optical detection for droplet size control in microfluidic droplet-based analysis systems. Sens. Actuators B Chem. 2006, 117, 431–436. [Google Scholar] [CrossRef]
- Bilican, I.; Tahsin Guler, M. Assessment of PMMA and polystyrene based microfluidic chips fabricated using CO2 laser machining. Appl. Surf. Sci. 2020, 534, 147642. [Google Scholar] [CrossRef]
- Nasser, G.A.; Fath El-Bab, A.M.R.; Abdel-Mawgood, A.L.; Mohamed, H.; Saleh, A.M. CO2 laser fabrication of PMMA microfluidic double T-junction device with modified inlet-angle for cost-effective PCR application. Micromachines 2019, 10, 687. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.; Kodzius, R.; Foulds, I.G. Fabrication of polystyrene microfluidic devices using a pulsed CO2 laser system. Microsyst. Technol. 2012, 18, 373–379. [Google Scholar] [CrossRef]
- Liu, S.; Fan, Y.; Gao, K.; Zhang, Y. Fabrication of Cyclo-olefin polymer-based microfluidic devices using CO2 laser ablation. Mater. Res. Express 2018, 5, 095305. [Google Scholar] [CrossRef]
- Konari, P.R.; Clayton, Y.-D.; Vaughan, M.B.; Khandaker, M.; Hossan, M.R. experimental analysis of laser micromachining of microchannels in common microfluidic substrates. Micromachines 2021, 12, 138. [Google Scholar] [CrossRef]
- Benton, M.; Hossan, M.R.; Konari, P.R.; Gamagedara, S. Effect of process parameters and material properties on laser micromachining of microchannels. Micromachines 2019, 10, 123. [Google Scholar] [CrossRef]
- Snakenborg, D.; Klank, H.; Kutter, J.P. Microstructure fabrication with a CO2 laser system. J. Micromechan. Microeng. 2003, 14, 182–189. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Zainal Alam, M.N.H.; Kouzani, A.; Gibson, I. Fabrication of microfluidic devices: Improvement of surface quality of CO2 laser machined poly(methylmethacrylate) polymer. J. Micromechan. Microeng. 2016, 27, 015021. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Z. Study aspect ratio of microchannel on different polymer substrates with CO2 laser and hot bonding for microfluidic chip. AIP Adv. 2018, 8, 015116. [Google Scholar] [CrossRef]
- Hong, T.-F.; Ju, W.-J.; Wu, M.-C.; Tai, C.-H.; Tsai, C.-H.; Fu, L.-M. Rapid prototyping of PMMA microfluidic chips utilizing a CO2 laser. Microfluid. Nanofluid. 2010, 9, 1125–1133. [Google Scholar] [CrossRef]
- Okayama, S.; Nakatani, M.; Hashimoto, M. Rapid fabrication of a pumpless PDMS microfluidic device using CO2 laser micromachining for automated formation of monodisperse water-in-oil droplets. Chem. Lett. 2021, 51, 212–216. [Google Scholar] [CrossRef]
- Du, L.; Chang, H.; Song, M.; Liu, C. The effect of injection molding PMMA microfluidic chips thickness uniformity on the thermal bonding ratio of chips. Microsyst. Technol. 2012, 18, 815–822. [Google Scholar] [CrossRef]
- Kelly, R.T.; Woolley, A.T. Thermal bonding of polymeric capillary electrophoresis microdevices in water. Anal. Chem. 2003, 75, 1941–1945. [Google Scholar] [CrossRef]
- Brown, L.; Koerner, T.; Horton, J.H.; Oleschuk, R.D. Fabrication and characterization of poly(methylmethacrylate) microfluidic devices bonded using surface modifications and solvents. Lab Chip 2006, 6, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.-W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Arata, H.; Komatsu, H.; Han, A.; Hosokawa, K.; Maeda, M. Rapid microRNA detection using power-free microfluidic chip: Coaxial stacking effect enhances the sandwich hybridization. Analyst 2012, 137, 3234–3237. [Google Scholar] [CrossRef] [PubMed]
- Arata, H.; Komatsu, H.; Hosokawa, K.; Maeda, M. Rapid and sensitive microRNA detection with laminar flow-assisted dendritic amplification on power-free microfluidic chip. PLoS ONE 2012, 7, e48329. [Google Scholar] [CrossRef]
- Sato, Y.; Sato, K.; Hosokawa, K.; Maeda, M. Surface plasmon resonance imaging on a microchip for detection of DNA-modified gold nanoparticles deposited onto the surface in a non-cross-linking configuration. Anal. Biochem. 2006, 355, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Y.; Wang, D.; Song, B.; Li, Z.; Song, S.; Wang, L.; Jiang, B.; Zhao, X.; Yan, J.; et al. A power-free microfluidic chip for SNP genotyping using graphene oxide and a DNA intercalating dye. Chem. Commun. 2013, 49, 3125–3127. [Google Scholar] [CrossRef]
- Hosokawa, K.; Omata, M.; Sato, K.; Maeda, M. Power-free sequential injection for microchip immunoassay toward point-of-care testing. Lab Chip 2006, 6, 236–241. [Google Scholar] [CrossRef]
- Hosokawa, K.; Omata, M.; Maeda, M. Immunoassay on a power-free microchip with laminar flow-assisted dendritic amplification. Anal. Chem. 2007, 79, 6000–6004. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, D.; Zhu, C.; Song, S.; Wang, L.; Long, Y.; Fan, C. Design of a gold nanoprobe for rapid and portable mercury detection with the naked eye. Chem. Commun. 2008, 4885–4887. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, B. A PDMS viscometer for assaying endoglucanase activity. Analyst 2011, 136, 1222–1226. [Google Scholar] [CrossRef]
- Han, Z.; Tang, X.; Zheng, B. A PDMS viscometer for microliter Newtonian fluid. J. Micromechan. Microeng. 2007, 17, 1828–1834. [Google Scholar] [CrossRef]
- Nakatani, M.; Tanaka, Y.; Okayama, S.; Hashimoto, M. A simplified PDMS microfluidic device with a built-in suction actuator for rapid production of monodisperse water-in-oil droplets. Electrophoresis 2020, 41, 2114–2121. [Google Scholar] [CrossRef]
- Murata, Y.; Nakashoji, Y.; Kondo, M.; Tanaka, Y.; Hashimoto, M. Rapid automatic creation of monodisperse emulsion droplets by microfluidic device with degassed PDMS slab as a detachable suction actuator. Electrophoresis 2018, 39, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Manoj, P. Droplet digital PCR technology promises new applications and research areas. Mitochondr. DNA Part A 2016, 27, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Hatch, A.C.; Fisher, J.S.; Tovar, A.R.; Hsieh, A.T.; Lin, R.; Pentoney, S.L.; Yang, D.L.; Lee, A.P. 1-Million droplet array with wide-field fluorescence imaging for digital PCR. Lab Chip 2011, 11, 3838–3845. [Google Scholar] [CrossRef] [PubMed]

| Microchip ID | Material for the Bottom slab a | Dimensions of the Bottom slab (W × D × H) | Dimension of the Top PDMS slab (W × D × H) b | Channel Length c |
|---|---|---|---|---|
| Previous model [36] | PDMS | 76 mm × 26 mm × 4 mm | 76 mm × 26 mm × 4 mm | 4 mm each |
| Standard (this work) | PMMA | 76 mm × 26 mm × 2 mm | 76 mm × 26 mm × 4 mm | 4 mm each |
| Variant I (this work) | PET | 76 mm × 26 mm × 2 mm | 76 mm × 26 mm × 4 mm | 4 mm each |
| Variant II (this work) | PMMA | 76 mm × 52 mm × 2 mm | 76 mm × 52 mm × 4 mm | 4 mm each |
| Variant III (this work) | PMMA | 76 mm × 26 mm × 2 mm | 76 mm × 26 mm × 4 mm | 2 mm each |
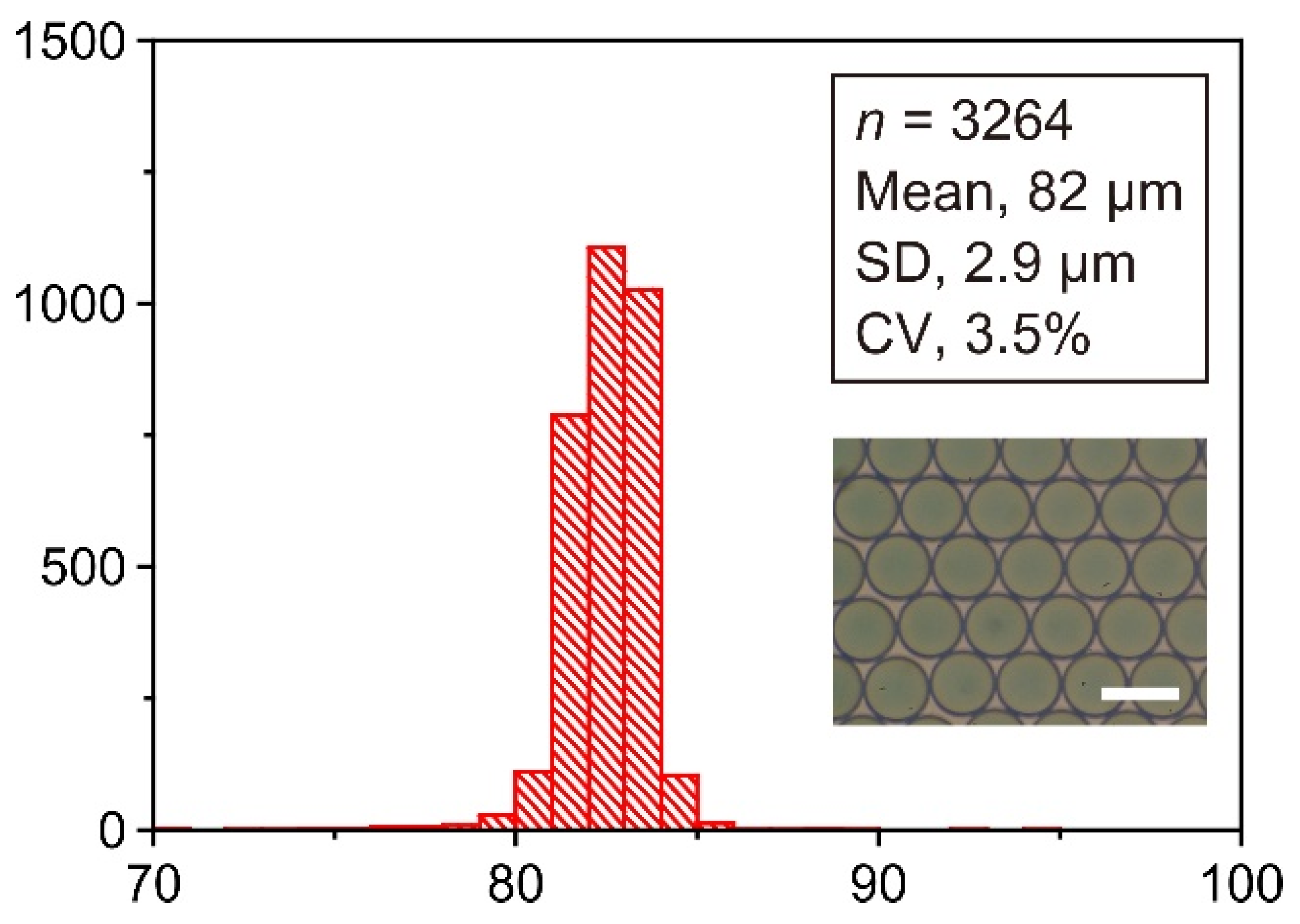
| Run | n a | Mean (µm) | SD (µm) | CV (%) |
|---|---|---|---|---|
| #1 | 3264 | 82 | 2.9 | 3.5 |
| #2 | 3439 | 85 | 3.6 | 4.2 |
| #3 | 2380 | 86 | 1.6 | 1.9 |
| #4 | 4533 | 84 | 1.7 | 2.0 |
| #5 | 3254 | 82 | 3.4 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogo, A.; Okayama, S.; Nakatani, M.; Hashimoto, M. CO2-Laser-Micromachined, Polymer Microchannels with a Degassed PDMS slab for the Automatic Production of Monodispersed Water-in-Oil Droplets. Micromachines 2022, 13, 1389. https://doi.org/10.3390/mi13091389
Ogo A, Okayama S, Nakatani M, Hashimoto M. CO2-Laser-Micromachined, Polymer Microchannels with a Degassed PDMS slab for the Automatic Production of Monodispersed Water-in-Oil Droplets. Micromachines. 2022; 13(9):1389. https://doi.org/10.3390/mi13091389
Chicago/Turabian StyleOgo, Akitsu, Shotaro Okayama, Masaya Nakatani, and Masahiko Hashimoto. 2022. "CO2-Laser-Micromachined, Polymer Microchannels with a Degassed PDMS slab for the Automatic Production of Monodispersed Water-in-Oil Droplets" Micromachines 13, no. 9: 1389. https://doi.org/10.3390/mi13091389
APA StyleOgo, A., Okayama, S., Nakatani, M., & Hashimoto, M. (2022). CO2-Laser-Micromachined, Polymer Microchannels with a Degassed PDMS slab for the Automatic Production of Monodispersed Water-in-Oil Droplets. Micromachines, 13(9), 1389. https://doi.org/10.3390/mi13091389






