Mini Review of Reliable Fabrication of Electrode under Stretching for Supercapacitor Application
Abstract
:1. Introduction
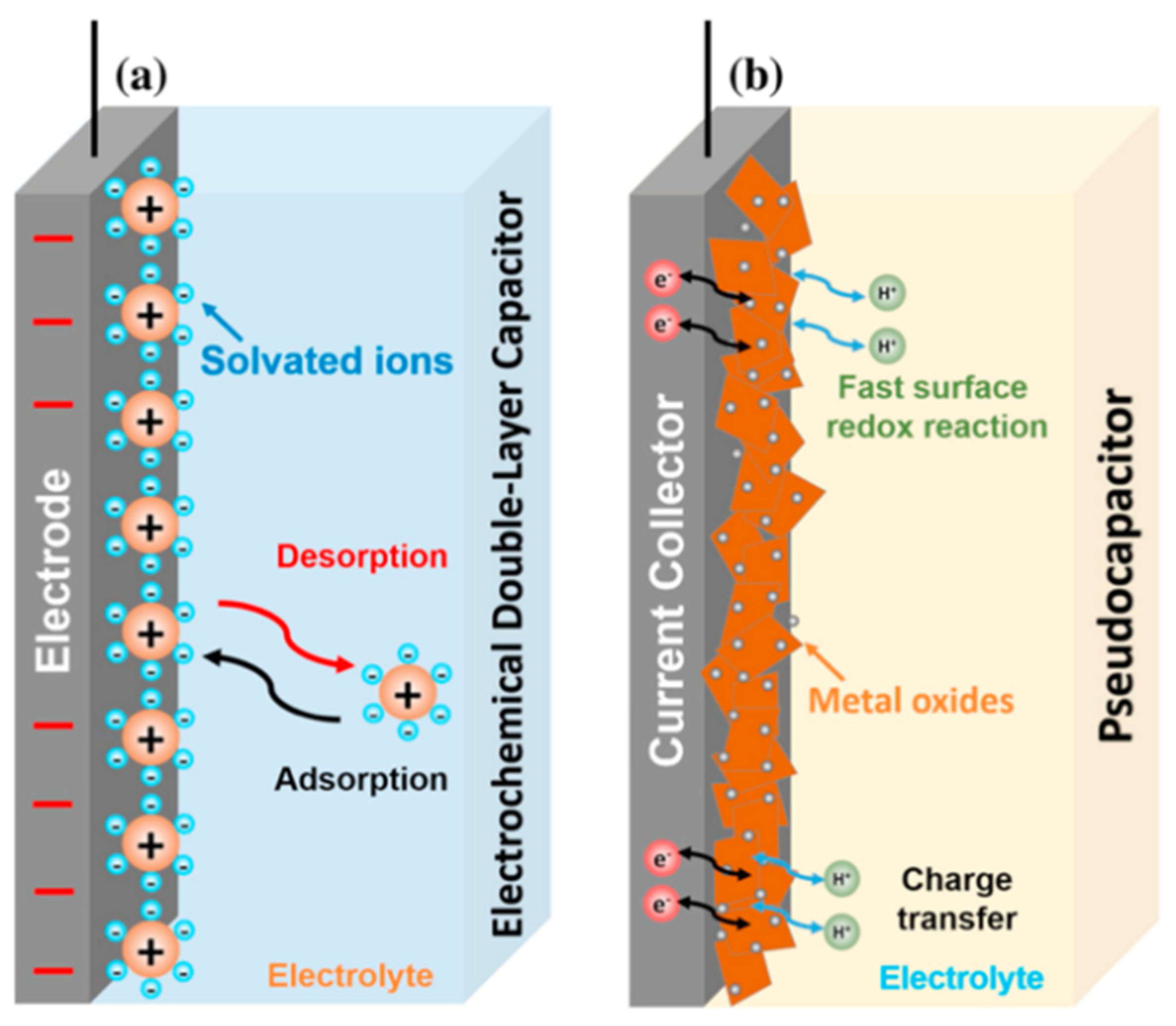
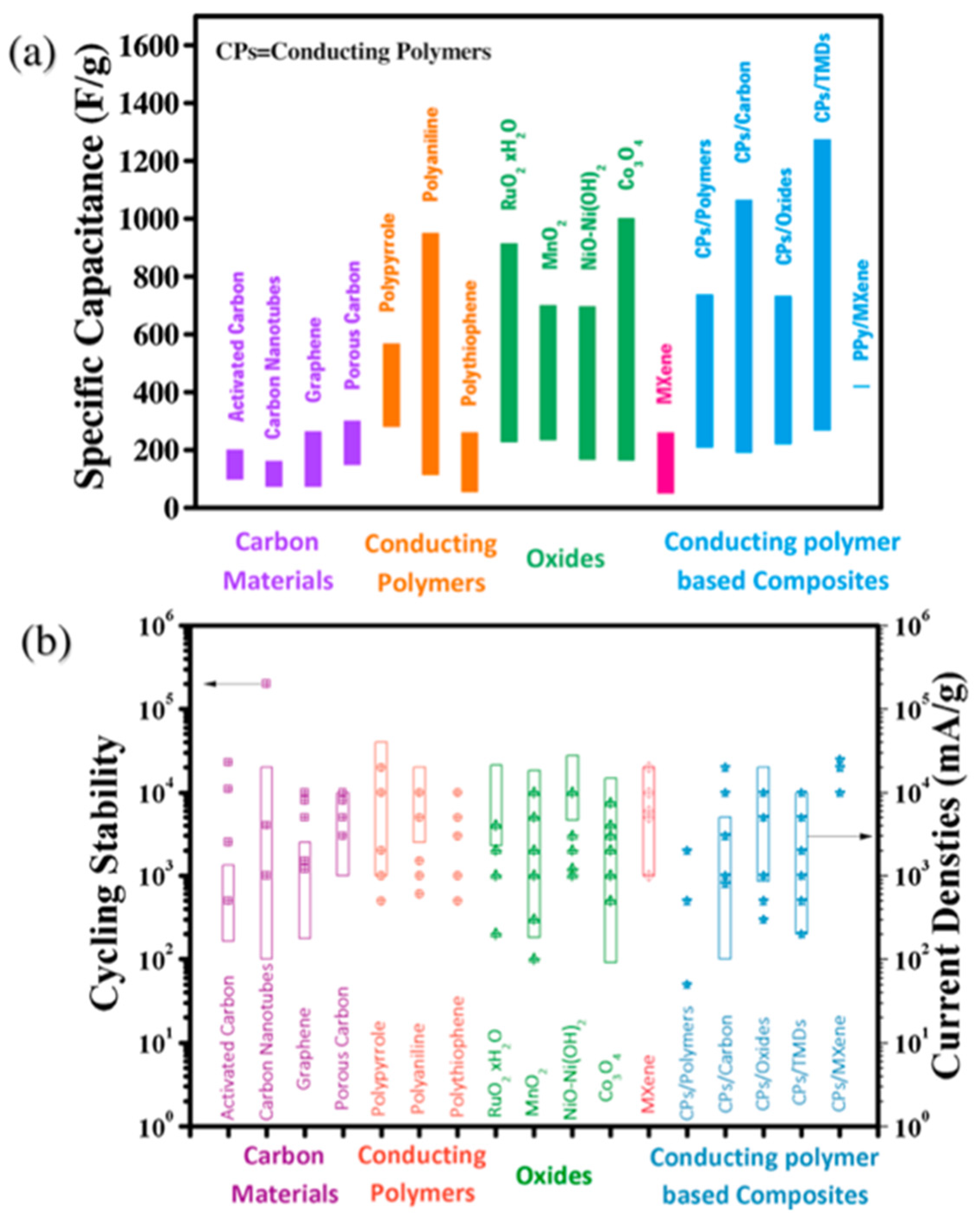
2. Fundamentals of Supercapacitors
3. Stretchable Supercapacitors
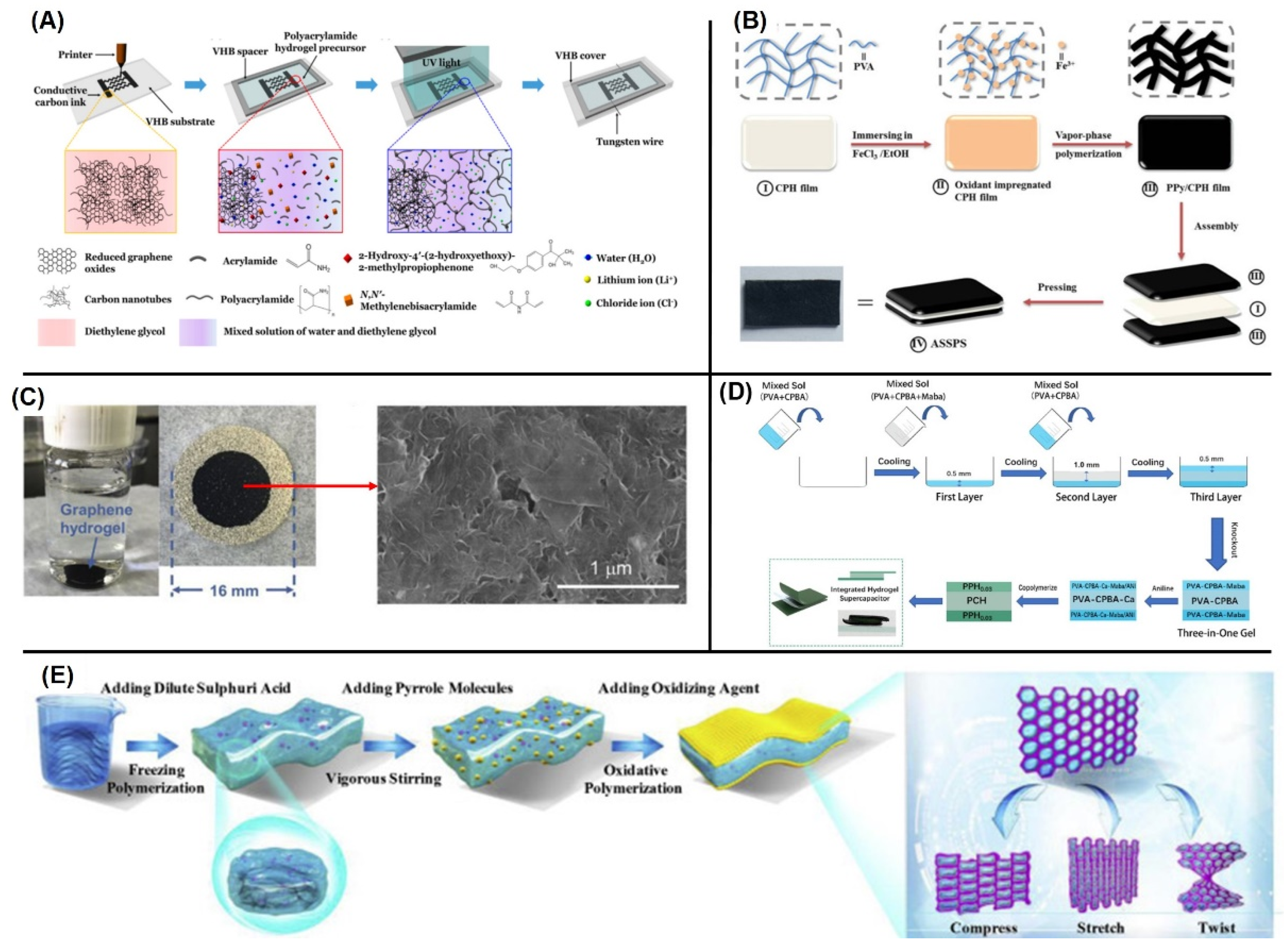
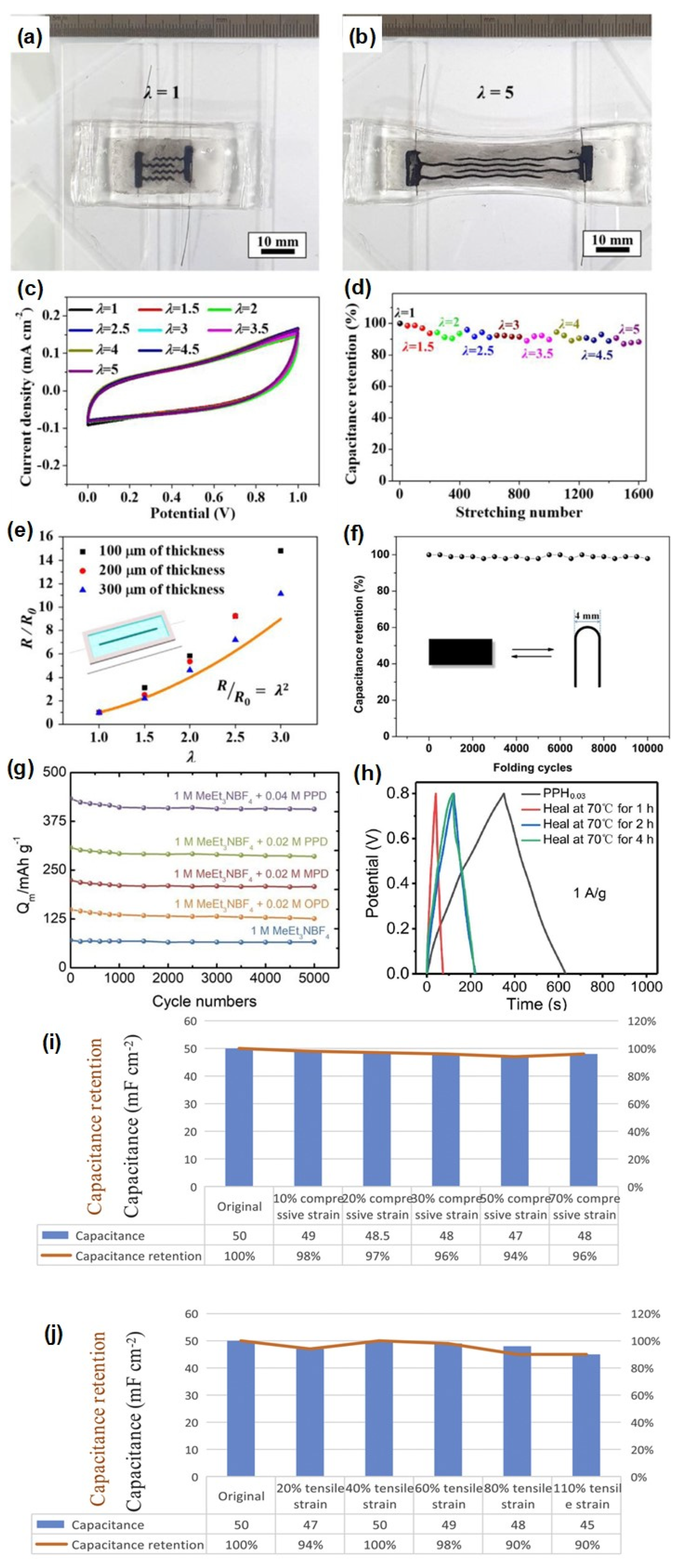
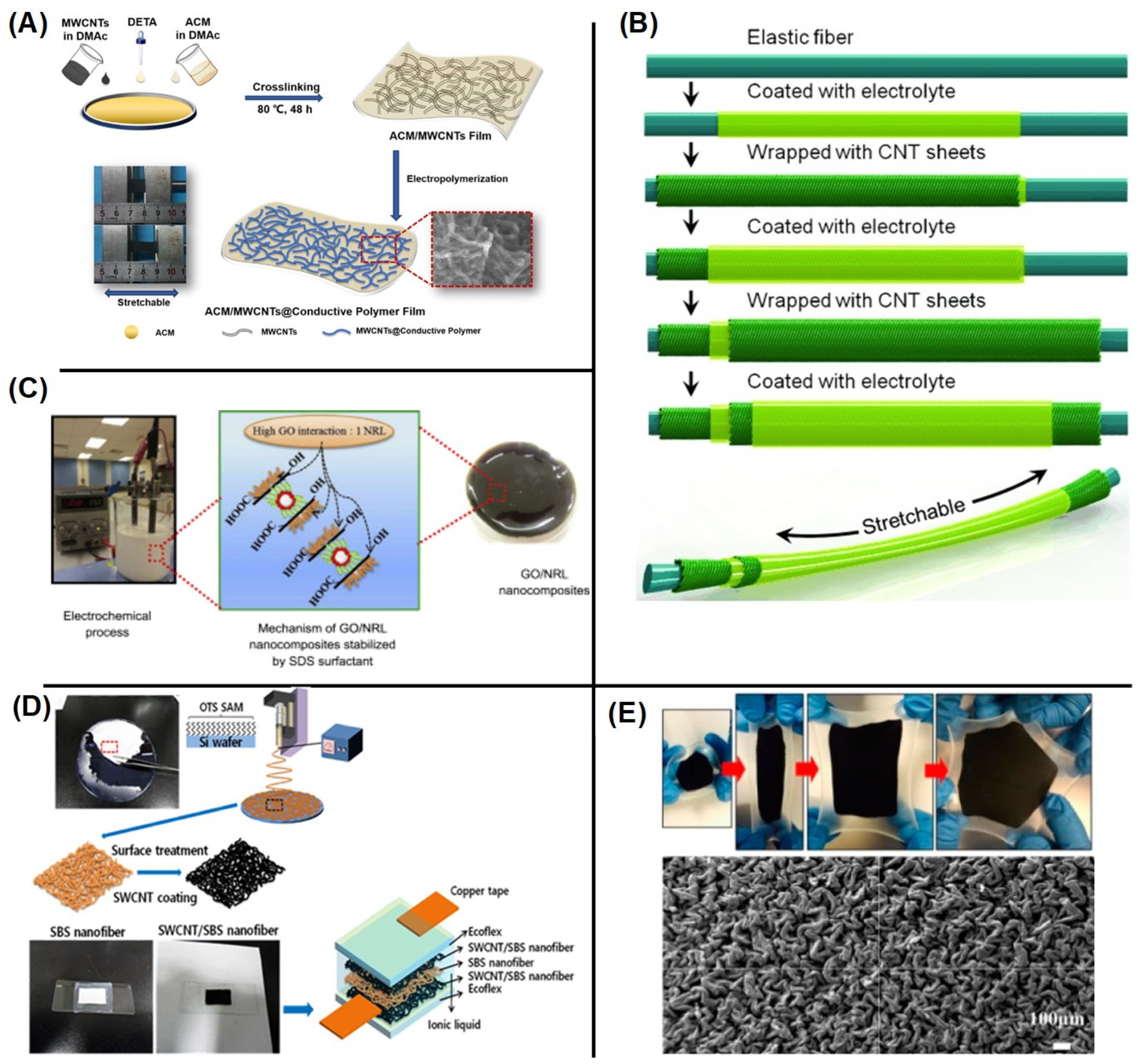
3.1. Hydrogel-Based Supercapacitors
3.2. Rubber Composite-Based Supercapacitors
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACM | Acrylic rubber |
| AN | Acetonitrile |
| ATR-FTIR | Attenuated total reflectance Fourier transform infrared |
| CNT | Carbon nanotube |
| CP | Conducting polymer |
| CV | Cyclic voltammetry |
| DETA | Diethylenetriamine |
| DMAc | Dimethylacetamide |
| EDLC | Electric double-layer capacitor |
| Et4NBF4 | Tetraethylammonium tetrafluoroborate |
| FE-SEM | Field emission scanning electron microscopy |
| GCD | Galvanostatic charge discharge |
| MWCNT | Multiwall carbon nanotube |
| oASSC | Organic semiconducting single crystal |
| PDAA | Poly(1,5-diaminoanthraquinone) |
| PANI | Polyaniline |
| QPE | Quasi-solid polymer electrolyte |
| TGA | Thermogravimetric analysis |
| XPS | X-ray photoelectron spectroscopy |
| UV | Ultraviolet |
References
- Hepel, M. Advances in micro-supercapacitors (MSCs) with high energy density and fast charge-discharge capabilities for flexible bioelectronic devices—A review. Electrochem. Sci. Adv. 2022, e2100222. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S. Characterization of carbon nanofiber (CNF)/polymer composite coated on cotton fabrics prepared with various circuit patterns. Fash. Text. 2018, 5, 7. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S. Characterization of Electrical Heating of Graphene/PLA Honeycomb Structure Composite Manufactured by CFDM 3D Printer. Fash. Text. 2020, 7, 8. [Google Scholar] [CrossRef]
- Lee, H.; Roh, J.-S. Charging device for wearable electromagnetic energy-harvesting textiles. Fash. Text. 2021, 8, 5. [Google Scholar] [CrossRef]
- Lee, Y.-A.; Koo, S.H. Introduction to special collection on 3D printing and wearable technology in fashion. Fash. Text. 2018, 5, 34. [Google Scholar] [CrossRef]
- Mukai, Y.; Li, S.; Suh, M. 3D-printed thermoplastic polyurethane for wearable breast hyperthermia. Fash. Text. 2021, 8, 24. [Google Scholar] [CrossRef]
- Rout, C.S.; Late, D.J. (Eds.) Chapter 1—Introduction. In Fundamentals and Supercapacitor Applications of 2D Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–10. [Google Scholar]
- Al-Osta, A.; Samer, B.S.; Nakate, U.T.; Jadhav, V.V.; Mane, R.S. Electrodeposited spruce leaf-like structured copper bismuth oxide electrode for supercapacitor application. Microelectron. Eng. 2020, 229, 111359. [Google Scholar] [CrossRef]
- Guan, Y.; Xu, F.; Wang, X.; Hui, Y.; Sha, J.; Tian, Y.; Wang, Z.; Zhang, S.; Chen, D.; Yang, L. Implementation of hybrid PDMS-graphite/Ag conductive material for flexible electronic devices and microfluidic applications. Microelectron. Eng. 2021, 235, 111455. [Google Scholar] [CrossRef]
- Kadlečíková, M.; Breza, J.; Dekan, J.; Jesenák, K.; Vančo, Ľ.; Bédiová, K. A study of catalyst particles encapsulated inside multiwalled carbon nanotubes on zeolite and montmorillonite. Microelectron. Eng. 2021, 242–243, 111556. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Lyu, Y.; He, C.; Liu, Z. A novel triboelectric nanogenerator based on carbon fiber reinforced composite lamina and as a self-powered displacement sensor. Microelectron. Eng. 2020, 224, 111231. [Google Scholar] [CrossRef]
- Moschogiannaki, M.; Gagaoudakis, E.; Kiriakidis, G.; Binas, V. Effect of the deposition method and substrate on an ultra-stable CoV2O6 hydrogen gas sensor, operating at room temperature. Microelectron. Eng. 2022, 262, 111819. [Google Scholar] [CrossRef]
- Okutan, M.; Evecan, D.; Yıldırım, S.; Özkan Zayim, E.; Deligöz, H. Investigating the effect of electrolyte types with various ionic liquids on the electrochromic performance of PEDOT:PSS based LbL multilayers. Microelectron. Eng. 2020, 234, 111454. [Google Scholar] [CrossRef]
- Renuka, H.; Enaganti, P.K.; Kundu, S.; Goel, S. Laser-induced graphene electrode based flexible heterojunction photovoltaic cells. Microelectron. Eng. 2022, 251, 111673. [Google Scholar] [CrossRef]
- Siraj, H.; Ahmad, K.S.; Jaffri, S.B.; Sohail, M. Synthesis, characterization and electrochemical investigation of physical vapor deposited barium sulphide doped iron sulphide dithiocarbamate thin films. Microelectron. Eng. 2020, 233, 111400. [Google Scholar] [CrossRef]
- Wang, C.-P.; Hsiao, M.-H.; Lee, G.-H.; Chang, T.-L.; Lee, Y.-W. The investigation of electrothermal response and reliability of flexible graphene micro-heaters. Microelectron. Eng. 2020, 228, 111334. [Google Scholar] [CrossRef]
- Yusof, J.M.; Ismail, I.; Yusop, M.R.; Rashid, S.A.; Nong, M.A.M.; Ali, M.H.M. Effect of zinc oxide nucleation on flexible bio based carbon nanotube cotton via chemical bath deposition method. Microelectron. Eng. 2020, 234, 111439. [Google Scholar] [CrossRef]
- Sarjeant, W.J.; Zirnheld, J.; MacDougall, F.W.; Bowers, J.S.; Clark, N.; Clelland, I.W.; Price, R.A.; Hudis, M.; Kohlberg, I.; McDuff, G.; et al. Chapter 9—Capacitors—Past, Present, and Future. In Handbook of Low and High Dielectric Constant Materials and Their Applications; Singh Nalwa, H., Ed.; Burlington; Academic Press: Cambridge, MA, USA, 1999; pp. 423–491. [Google Scholar]
- Zhao, Q.; Wang, G.; Yan, K.; Yan, J.; Wang, J. Binder-free porous PEDOT electrodes for flexible supercapacitors. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Bird, V.; Ishii, K.; Magazine, G. Electric Double-Layer Capacitor. Available online: https://api.semanticscholar.org/CorpusID:106402787 (accessed on 16 August 2022).
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Wang, R.; Yao, M.; Niu, Z. Smart supercapacitors from materials to devices. InfoMat 2020, 2, 113–125. [Google Scholar] [CrossRef]
- Zaman, N.; Malik, R.A.; Alrobei, H.; Kim, J.; Latif, M.; Hussain, A.; Maqbool, A.; Karim, R.A.; Saleem, M.; Asif Rafiq, M. Structural and electrochemical analysis of decarburized graphene electrodes for supercapacitor applications. Crystals 2020, 10, 1043. [Google Scholar] [CrossRef]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of materials and fabrication methods. J. Energy Eng. 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Yaïci, W.; Entchev, E. Hybrid battery/supercapacitor energy storage system for the electric vehicles. J. Power Sources 2018, 374, 237–248. [Google Scholar] [CrossRef]
- Gidwani, M.; Bhagwani, A.; Rohra, N. Supercapacitors: The near future of batteries. Int. J. Eng. Invent. 2014, 4, 22–27. [Google Scholar]
- Gamby, J.; Taberna, P.; Simon, P.; Fauvarque, J.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube-and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Wang, C.; Zhang, H.; Lu, C.; Wang, J. Hierarchical porous graphene carbon-based supercapacitors. Chem. Mater. 2015, 27, 2107–2113. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Yu, M.; Wang, Z.; Zhao, W.; Liu, P.; Tong, Y.; Lu, X. Nitrogen doped graphene paper as a highly conductive, and light-weight substrate for flexible supercapacitors. RSC Adv. 2014, 4, 51878–51883. [Google Scholar] [CrossRef]
- Rajendran, V.; Mohan, A.V.; Jayaraman, M.; Nakagawa, T. All-printed, interdigitated, freestanding serpentine interconnects based flexible solid state supercapacitor for self powered wearable electronics. Nano Energy 2019, 65, 104055. [Google Scholar] [CrossRef]
- Diao, Y.; Woon, R.; Yang, H.; Chow, A.; Wang, H.; Lu, Y.; D’Arcy, J.M. Kirigami electrodes of conducting polymer nanofibers for wearable humidity dosimeters and stretchable supercapacitors. J. Mater. Chem. A 2021, 9, 9849–9857. [Google Scholar] [CrossRef]
- Xu, J.-L.; Liu, Y.-H.; Gao, X.; Shen, S.; Wang, S.-D. Toward wearable electronics: A lightweight all-solid-state supercapacitor with outstanding transparency, foldability and breathability. Energy Storage Mater. 2019, 22, 402–409. [Google Scholar] [CrossRef]
- Bulusheva, L.; Fedorovskaya, E.; Kurenya, A.; Okotrub, A. Supercapacitor performance of nitrogen-doped carbon nanotube arrays. Phys. Status Solidi B 2013, 250, 2586–2591. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Gao, Q.; Fu, J.-Z.; Chen, Q.-Y.; Zhu, J.-P.; Sun, Y.; He, Y. Multimaterial 3D printing of highly stretchable silicone elastomers. ACS Appl. Mater. Interfaces 2019, 11, 23573–23583. [Google Scholar] [CrossRef]
- Shi, Y.; Ha, H.; Al-Sudani, A.; Ellison, C.J.; Yu, G. Thermoplastic elastomer-enabled smart electrolyte for thermoresponsive self-protection of electrochemical energy storage devices. Adv. Mater. 2016, 28, 7921–7928. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Li, H.; Huang, Y.; Zhi, C. Polymers for supercapacitors: Boosting the development of the flexible and wearable energy storage. Mater. Sci. Eng. R Rep. 2020, 139, 100520. [Google Scholar] [CrossRef]
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Gurunathan, K.; Murugan, A.V.; Marimuthu, R.; Mulik, U.; Amalnerkar, D. Electrochemically synthesised conducting polymeric materials for applications towards technology in electronics, optoelectronics and energy storage devices. Mater. Chem. Phys. 1999, 61, 173–191. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive hydrogels as smart materials for flexible electronic devices. Chem. A Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Tang, L.; Wu, S.; Qu, J.; Gong, L.; Tang, J. A review of conductive hydrogel used in flexible strain sensor. Materials 2020, 13, 3947. [Google Scholar] [CrossRef]
- Song, C.; Chen, B.; Hwang, J.; Lee, S.; Suo, Z.; Ahn, H. A printed highly stretchable supercapacitor by a combination of carbon ink and polymer network. Extrem. Mech. Lett. 2021, 49, 101459. [Google Scholar] [CrossRef]
- Zang, L.; Liu, Q.; Qiu, J.; Yang, C.; Wei, C.; Liu, C.; Lao, L. Design and Fabrication of an All-Solid-State Polymer Supercapacitor with Highly Mechanical Flexibility Based on Polypyrrole Hydrogel. ACS Appl. Mater. Interfaces 2017, 9, 33941–33947. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, X.; Miao, X.; Liu, Y.; Chen, S.; Chen, Y.; Cheng, J.; Wang, W.; Zhang, Y. A phenylenediamine-mediated organic electrolyte for high performance graphene-hydrogel based supercapacitors. Electrochim. Acta 2018, 273, 495–501. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, C.; Sun, Y.; Gan, S.; Dong, L.; Zhao, J.; Rong, J. Flexible, all-hydrogel supercapacitor with self-healing ability. Chem. Eng. J. 2021, 418, 128616. [Google Scholar] [CrossRef]
- Yin, B.-S.; Zhang, S.-W.; Ke, K.; Wang, Z.-B. Advanced deformable all-in-one hydrogel supercapacitor based on conducting polymer: Toward integrated mechanical and capacitive performance. J. Alloys Compd. 2019, 805, 1044–1051. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Jin, J.; Li, X.; Cheng, Q.; Wang, G. High-performance stretchable supercapacitors based on intrinsically stretchable acrylate rubber/MWCNTs@conductive polymer composite electrodes. J. Mater. Chem. A 2018, 6, 4432–4442. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Li, Y.; Wang, Y.; Zhang, J.; Guan, G.; Pan, Z.; Zheng, G.; Peng, H. Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor. Adv. Energy Mater. 2017, 7, 1601814. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, J.; Chen, X.; Ren, J.; Peng, H. A Highly Stretchable, Fiber-Shaped Supercapacitor. Angew. Chem. Int. Ed. 2013, 52, 13453–13457. [Google Scholar] [CrossRef]
- Suriani, A.B.; Nurhafizah, M.D.; Mohamed, A.; Zainol, I.; Masrom, A.K. A facile one-step method for graphene oxide/natural rubber latex nanocomposite production for supercapacitor applications. Mater. Lett. 2015, 161, 665–668. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, J.; Hur, J. Stretchable Supercapacitors Based on Carbon Nanotubes-Deposited Rubber Polymer Nanofibers Electrodes with High Tolerance against Strain. Nanomaterials 2018, 8, 541. [Google Scholar] [CrossRef]
- Yu, J.; Lu, W.; Pei, S.; Gong, K.; Wang, L.; Meng, L.; Huang, Y.; Smith, J.P.; Booksh, K.S.; Li, Q.; et al. Omnidirectionally Stretchable High-Performance Supercapacitor Based on Isotropic Buckled Carbon Nanotube Films. ACS Nano 2016, 10, 5204–5211. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Matteini, P.; Hwang, B. Mini Review of Reliable Fabrication of Electrode under Stretching for Supercapacitor Application. Micromachines 2022, 13, 1470. https://doi.org/10.3390/mi13091470
Kim H, Matteini P, Hwang B. Mini Review of Reliable Fabrication of Electrode under Stretching for Supercapacitor Application. Micromachines. 2022; 13(9):1470. https://doi.org/10.3390/mi13091470
Chicago/Turabian StyleKim, Haeji, Paolo Matteini, and Byungil Hwang. 2022. "Mini Review of Reliable Fabrication of Electrode under Stretching for Supercapacitor Application" Micromachines 13, no. 9: 1470. https://doi.org/10.3390/mi13091470
APA StyleKim, H., Matteini, P., & Hwang, B. (2022). Mini Review of Reliable Fabrication of Electrode under Stretching for Supercapacitor Application. Micromachines, 13(9), 1470. https://doi.org/10.3390/mi13091470




