Electrochemical Detection of Ascorbic Acid in Finger-Actuated Microfluidic Chip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Equipment
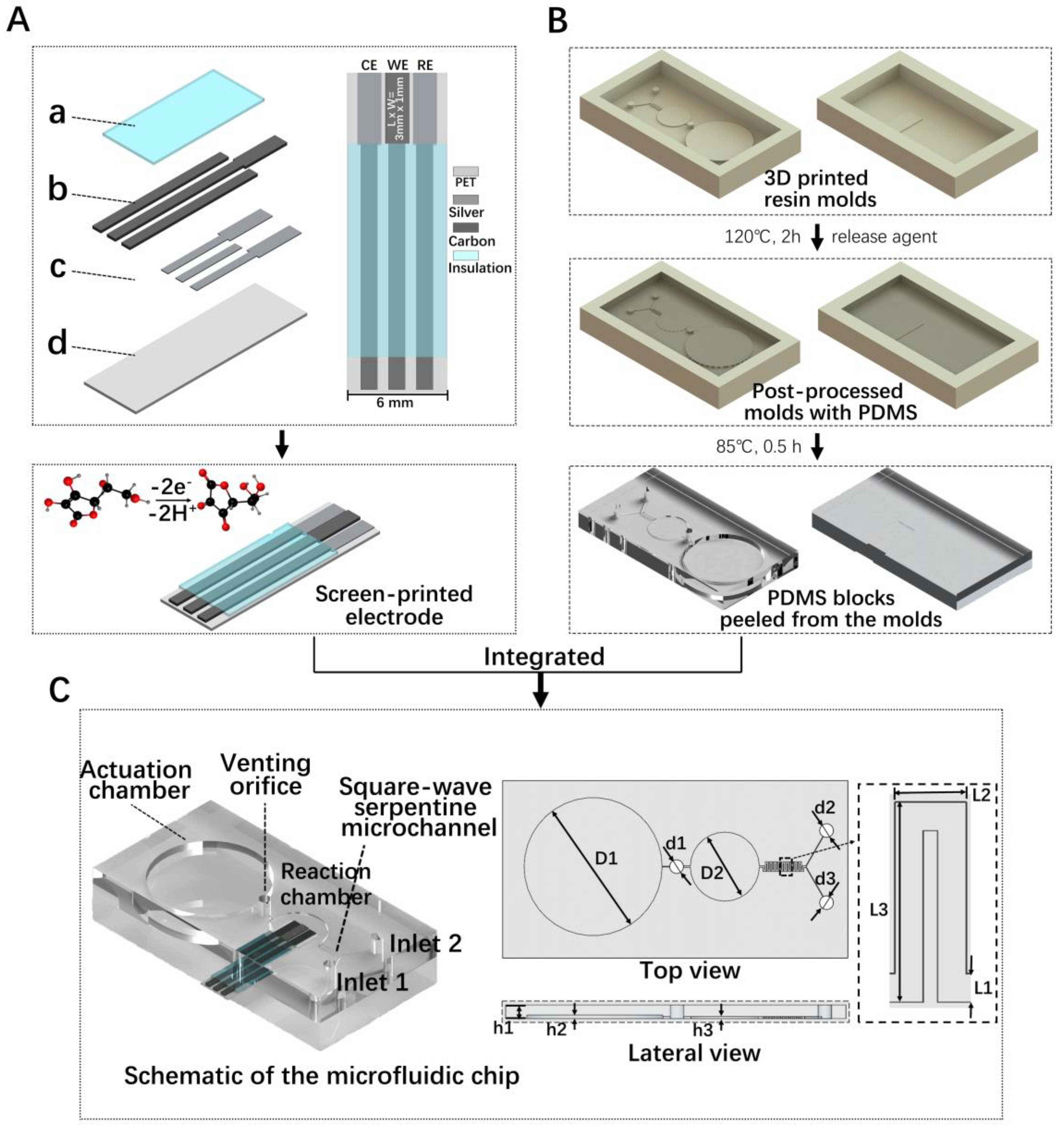
2.2. Preparation of Electrochemical Sensor Chip
2.3. Sample Preparation and Electrochemical Methods
3. Results and Discussion
3.1. Characterization and Electrochemical Behavior of SPEs
3.2. The Evaluation of Finger-Actuated Micropump and On-Chip Detection
3.3. Real Sample Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balunkeswar, N.; Rui Hai, L.; Juming, T. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains--a review. Crit. Rev. Food Sci. Nutr. 2014, 55, 887–918. [Google Scholar]
- Li, Y.R.; Hong, Z. Vitamin c for sepsis intervention: From redox biochemistry to clinical medicine. Mol. Cell. Biochem. 2021, 476, 4449–4460. [Google Scholar] [CrossRef] [PubMed]
- Jens, L. On the effect of vitamin c intake on human health: How to (mis)interprete the clinical evidence. Redox Biol. 2020, 34, 101532. [Google Scholar]
- Sok Kuan, W.; Kok-Yong, C.; Soelaiman, I.-N. Vitamin c: A review on its role in the management of metabolic syndrome. Int. J. Med. Sci. 2020, 17, 1625–1638. [Google Scholar]
- Seth, R.B.; Aanchal, K.; Mary, R.; Suma, A.T. What is the role of supplementation with ascorbic acid, zinc, vitamin d, or n-acetylcysteine for prevention or treatment of COVID-19? Cleve. Clin. J. Med. 2020. [Google Scholar] [CrossRef]
- Charlotte, S.; Matías Leonel, A.; Pierre, B.; Christophe, R.; Cecile, B.; Daniel, J.; Yoshihiro, O.; Hiroshi, E.; Inti, G.; Gustavo Esteban Gergoff, G.; et al. The effect of low ascorbic acid content on tomato fruit ripening. Planta 2020, 252, 36. [Google Scholar]
- Ogunlesi, M.; Okiei, W.; Azeez, L.; Obakachi, V.; Osunsanmi, M.; Nkenchor, G. Vitamin c contents of tropical vegetables and foods determined by voltammetric and titrimetric methods and their relevance to the medicinal uses of the plants. Int. J. Electrochem. Sci. 2010, 5, 105–115. [Google Scholar]
- Jerzy, B.; Agnieszka, S.; Eulalia, J.B.; Ewa, C.; Henryk, Z. Content of selected bioactive components and antioxidant properties of broccoli (Brassica oleracea L.). Eur. Food Res. Technol. 2008, 226, 459–465. [Google Scholar]
- Li, F.; Zhang, W.Y.; Zhu, G.Y. Determination of ascorbic acid by flow injection chemiluminescence suppression method. Chin. J. Anal. Chem. 2000, 28, 1523–1526. [Google Scholar]
- Xiuyun, W.; Hiroaki, W.; Shunichi, U. Amperometric l-ascorbic acid biosensors equipped with enzyme micelle membrane. Talanta 2008, 74, 1681–1685. [Google Scholar]
- Luis Antonio, T.-G. Determination of l-ascorbic acid in tomato by capillary electrophoresis. J. Chem. Educ. 2012, 89, 1194–1197. [Google Scholar]
- Dan, W.; Shaojun, G.; Shaojun, D.; Erkang, W. Ultrathin pd nanowire as a highly active electrode material for sensitive and selective detection of ascorbic acid. Biosens. Bioelectron. 2010, 265, 1056–1061. [Google Scholar]
- Franco, F.F.; Hogg, R.A.; Manjakkal, L. Cu2o-based electrochemical biosensor for non-invasive and portable glucose detection. Biosensors 2022, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.; Barrett, C.; Laffir, F.R.; Thompson, M.; Galvin, P.; O’ Riordan, A. On-chip glucose detection based on glucose oxidase immobilized on a platinum-modified, gold microband electrode. Biosensors 2021, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Anoop, S.; Asha, S.; Aamir, A.; Ashok, K.S.; Hidemitsu, F.; Sandeep, A.; Ajit, K. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar]
- Kumar, S.; Pavelyev, V.; Tripathi, N.; Platonov, V.; Sharma, P.; Ahmad, R.; Mishra, P.; Khosla, A. Review—recent advances in the development of carbon nanotubes based flexible sensors. J. Electrochem. Soc. 2020, 167, 047506. [Google Scholar] [CrossRef]
- Li, L.; Zhang, P.; Li, Z.; Li, D.; Han, B.; Tu, L.; Li, B.; Wang, Y.; Ren, L.; Yang, P.; et al. Cus/prussian blue core-shell nanohybrid as electrochemical sensor for ascorbic acid detection. Nanotechnology 2019, 30, 325501. [Google Scholar]
- Mehmet Çetin, C.; Çiğdem Sayıklı, Ş.; Mustafa Kemal, S. Electrochemical biosensor based on self-assembled monolayers modified with gold nanoparticles for detection of her-3. Anal. Chim. Acta 2014, 814, 31–38. [Google Scholar]
- Rafiq, A.; Marya, K.; Nirmalya, T.; Khan, M.I.R.; Ajit, K. Hydrothermally synthesized nickel oxide nanosheets for non-enzymatic electrochemical glucose detection. J. Electrochem. Soc. 2020, 167, 107504. [Google Scholar]
- Asha, S.; Aamir, A.; Anoop, S.; Sai Kiran, O.; Ajit, K.; Sandeep, A. Review—recent advances in tin oxide nanomaterials as electrochemical/chemiresistive sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar]
- Dan, W.; Li, Y.; Zhang, Y.; Wang, P.; Wei, Q.; Du, B. Sensitive electrochemical sensor for simultaneous determination of dopamine, ascorbic acid, and uric acid enhanced by amino-group functionalized mesoporous Fe3O4@graphene sheets. Electrochim. Acta 2013, 116, 244–249. [Google Scholar]
- Kochaporn, C.; Proespichaya, K.; Warakorn, L.; Apon, N.; Panote, T. Ultra trace analysis of small molecule by label-free impedimetric immunosensor using multilayer modified electrode. Biosens. Bioelectron. 2011, 26, 4571–4578. [Google Scholar]
- Smart, A.; Westmacott, K.L.; Crew, A.; Doran, O.; Hart, J.P. An electrocatalytic screen-printed amperometric sensor for the selective measurement of thiamine (vitamin b1) in food supplements. Biosensors 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Grennan, K.; Killard, A.J.; Smyth, M.R. Physical characterizations of a screen-printed electrode for use in an amperometric biosensor system. Electroanalysis 2001, 13, 745–750. [Google Scholar] [CrossRef]
- Peter, Z.; Milan, S.; Daniele, R.; van den Jeroen, B.; Edsger, C.P.S. Screen-printed dry electrodes: Basic characterization and benchmarking. Adv. Eng. Mater. 2020, 22, 2000714. [Google Scholar]
- Alonso-Lomillo, M.A.; Olga, D.-R.; Abraham, S.-B.; Arcos-Martínez, M.J. Determination of ascorbic acid in serum samples by screen-printed carbon electrodes modified with gold nanoparticles. Talanta 2017, 174, 733–737. [Google Scholar] [CrossRef]
- Tabeling, P. Recent progress in the physics of microfluidics and related biotechnological applications. Curr. Opin. Biotechnol. 2014, 25, 129–134. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chang, C.-L.; Wang, Y.-N.; Fu, L.-M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Peng, J.; Wu, L.; Han, X.; Shaheen, N.; Zou, X. Hand-held zoom micro-imaging system based on microfluidic chip for point-of-care testing (poct) of vaginal inflammation. IEEE J. Transl. Eng. Health Med. 2021, 19, 2800109. [Google Scholar] [CrossRef]
- Surendran, V.; Chiulli, T.; Manoharan, S.; Knisley, S.; Packirisamy, M.; Chandrasekaran, A. Acoustofluidic micromixing enabled hybrid integrated colorimetric sensing, for rapid point-of-care measurement of salivary potassium. Biosensors 2019, 9, 73. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y. A digital microfluidic device integrated with electrochemical impedance spectroscopy for cell-based immunoassay. Biosensors 2022, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.-K. Integrated microfluidic pumps and valves operated by finger actuation. Lab Chip 2019, 19, 2973–2977. [Google Scholar] [CrossRef]
- Bonk, S.; Stubbe, M.; Buehler, S.; Tautorat, C.; Baumann, W.; Klinkenberg, E.-D.; Gimsa, J. Design and characterization of a sensorized microfluidic cell-culture system with electro-thermal micro-pumps and sensors for cell adhesion, oxygen, and ph on a glass chip. Biosensors 2015, 5, 513–536. [Google Scholar] [CrossRef]
- Wang, Y.; Toyoda, K.; Uesugi, K.; Morishima, K. A simple micro check valve using a photo-patterned hydrogel valve core. Sens. Actuator A Phys. 2020, 304, 111878. [Google Scholar] [CrossRef]
- Smith, Q.; Gerecht, S. Going with the flow: Microfluidic platforms in vascular tissue engineering. Curr. Opin. Chem. Eng. 2014, 3, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2014, 63, 218–231. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (pdms) properties for biomedical micro/nanosystems. Biomed. Microdevices 2006, 7, 281–293. [Google Scholar] [CrossRef]
- Kim, Y.; Salim, A.; Lim, S. Millimeter-wave-based spoof localized surface plasmonic resonator for sensing glucose concentration. Biosensors 2021, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Saggiomo, V.; Velders, A.H. Simple 3d printed scaffold-removal method for the fabrication of intricate microfluidic devices. Adv. Sci. 2015, 2, 1500125. [Google Scholar] [CrossRef]
- Salentijn, G.I.J.; Oomen, P.E.; Grajewski, M.; Verpoorte, E. Fused deposition modeling 3d printing for (bio)analytical device fabrication: Procedures, materials, and applications. Anal. Chem. 2017, 89, 7053–7061. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3d-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3d printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Zeng, H.; Hu, Z.; Fu, B. Numerical and experimental investigation on micromixers with serpentine microchannels. Int. J. Heat Mass Transf. 2016, 98, 131–140. [Google Scholar] [CrossRef]
- Venzac, B.; Deng, S.; Mahmoud, Z.; Lenferink, A.; Costa, A.; Bray, F.; Otto, C.; Rolando, C.; Le Gac, S. Pdms curing inhibition on 3d-printed molds: Why? Also, how to avoid it? Anal. Chem. 2021, 93, 7180–7187. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.N.; Chen, Y.; Shu, Y.; Chen, Y.; Tian, Q.; Wu, H. Direct, one-step molding of 3d-printed structures for convenient fabrication of truly 3d pdms microfluidic chips. Microfluid. Nanofluid. 2015, 19, 9–18. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Ravanini, A.E.; Figueiredo-Filho, L.C.S.; Iniesta, J.; Banks, C.E.; Fatibello-Filho, O. Imparting improvements in electrochemical sensors: Evaluation of different carbon blacks that give rise to significant improvement in the performance of electroanalytical sensing platforms. Electrochim. Acta 2015, 157, 125–133. [Google Scholar] [CrossRef]
- Mizuhata, H.; Nakao, S.-i.; Yamaguchi, T. Morphological control of pemfc electrode by graft polymerization of polymer electrolyte onto platinum-supported carbon black. J. Power Sources 2004, 138, 25–30. [Google Scholar] [CrossRef]
- Cao, G.; Hao, C.; Gao, X.; Lu, J.; Xue, W.; Meng, Y.; Cheng, C.; Tian, Y. Carbon nanotubes with carbon blacks as cofillers to improve conductivity and stability. ACS Omega 2019, 4, 4169–4175. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Zhou, L.; Li, X.; He, J.; Huang, W.; Cai, Y.; Wang, J.; Chen, T.; Du, Y.; Yao, Y. Au–nitrogen-doped graphene quantum dot composites as “on–off” nanosensors for sensitive photo-electrochemical detection of caffeic acid. Nanomaterials 2020, 10, 1972. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Sha, R.; Vishnu, N.; Kumar, R.; Badhulika, S. Disposable, efficient and highly selective electrochemical sensor based on cadmium oxide nanoparticles decorated screen-printed carbon electrode for ascorbic acid determination in fruit juices. Nano-Struct. Nano-Objects 2018, 16, 96–103. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, J.; Tricard, S.; Wang, Q.; Fang, J. Efficient detection of ascorbic acid utilizing molybdenum oxide@prussian blue/graphite felt composite electrodes. Electrochim. Acta 2019, 322, 134712. [Google Scholar] [CrossRef]
- Nandimalla, V.; Mansi, G.; Desikan, R.; Annamalai Senthil, K. Pencil graphite as an elegant electrochemical sensor for separation-free and simultaneous sensing of hypoxanthine, xanthine and uric acid in fish samples. Anal. Methods 2017, 9, 2265–2274. [Google Scholar]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef]
- Khairy, M.; Mahmoud, B.G. Copper oxide microstructures with hemisphere pineapple morphology for selective amperometric determination of vitamin c (l-ascorbic acid) in human fluids. Electroanalysis 2016, 28, 2606–2612. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Wang, H.; Zou, C.e.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuator B-Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Yan, S.; Li, X.; Xiong, Y.; Wang, M.; Yang, L.; Liu, X.; Li, X.; Alshahrani, L.A.M.; Liu, P.; Zhang, C. Simultaneous determination of ascorbic acid, dopamine and uric acid using a glassy carbon electrode modified with the nickel(ii)-bis(1,10-phenanthroline) complex and single-walled carbon nanotubes. Microchim. Acta 2016, 183, 1401–1408. [Google Scholar] [CrossRef]
- Li, J.; Shi, J.; Wen, L.; Shi, K.; Liu, H. Visual methods for determining vitamin c in real samples. Chin. J. Chem. Educ 2021, 42, 41–46. [Google Scholar]






| Electrode | Technology | Linear Range (µM) | Sensitivity (A M−1cm−2) | Reference |
|---|---|---|---|---|
| Fe3O4/r-GO/GCE 1 | CV/DPV | 160–7200 | 0.02 | [53] |
| CuO-SPE 2 | CV | 100–8000 | 0.11 | [54] |
| Au/RGO/GCE 3 | CV/DPV | 240–1500 | - | [55] |
| [Ni(phen)2] 2+/SWCNTs/GCE 4 | CV | 30–1547 | - | [56] |
| SPE | CV/DPV | 10–5000 | 0.067 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, M.; Zheng, J.; Zhang, X.; Zeng, J.; Liao, Y.; Chen, J.; Yang, J.; Zheng, X.; Hu, N. Electrochemical Detection of Ascorbic Acid in Finger-Actuated Microfluidic Chip. Micromachines 2022, 13, 1479. https://doi.org/10.3390/mi13091479
Liu X, Li M, Zheng J, Zhang X, Zeng J, Liao Y, Chen J, Yang J, Zheng X, Hu N. Electrochemical Detection of Ascorbic Acid in Finger-Actuated Microfluidic Chip. Micromachines. 2022; 13(9):1479. https://doi.org/10.3390/mi13091479
Chicago/Turabian StyleLiu, Xing, Mi Li, Jiahui Zheng, Xiaoling Zhang, Junyi Zeng, Yanjian Liao, Jian Chen, Jun Yang, Xiaolin Zheng, and Ning Hu. 2022. "Electrochemical Detection of Ascorbic Acid in Finger-Actuated Microfluidic Chip" Micromachines 13, no. 9: 1479. https://doi.org/10.3390/mi13091479
APA StyleLiu, X., Li, M., Zheng, J., Zhang, X., Zeng, J., Liao, Y., Chen, J., Yang, J., Zheng, X., & Hu, N. (2022). Electrochemical Detection of Ascorbic Acid in Finger-Actuated Microfluidic Chip. Micromachines, 13(9), 1479. https://doi.org/10.3390/mi13091479







