Plotter Cut Stencil Masks for the Deposition of Organic and Inorganic Materials and a New Rapid, Cost Effective Technique for Antimicrobial Evaluations
Abstract
:1. Introduction
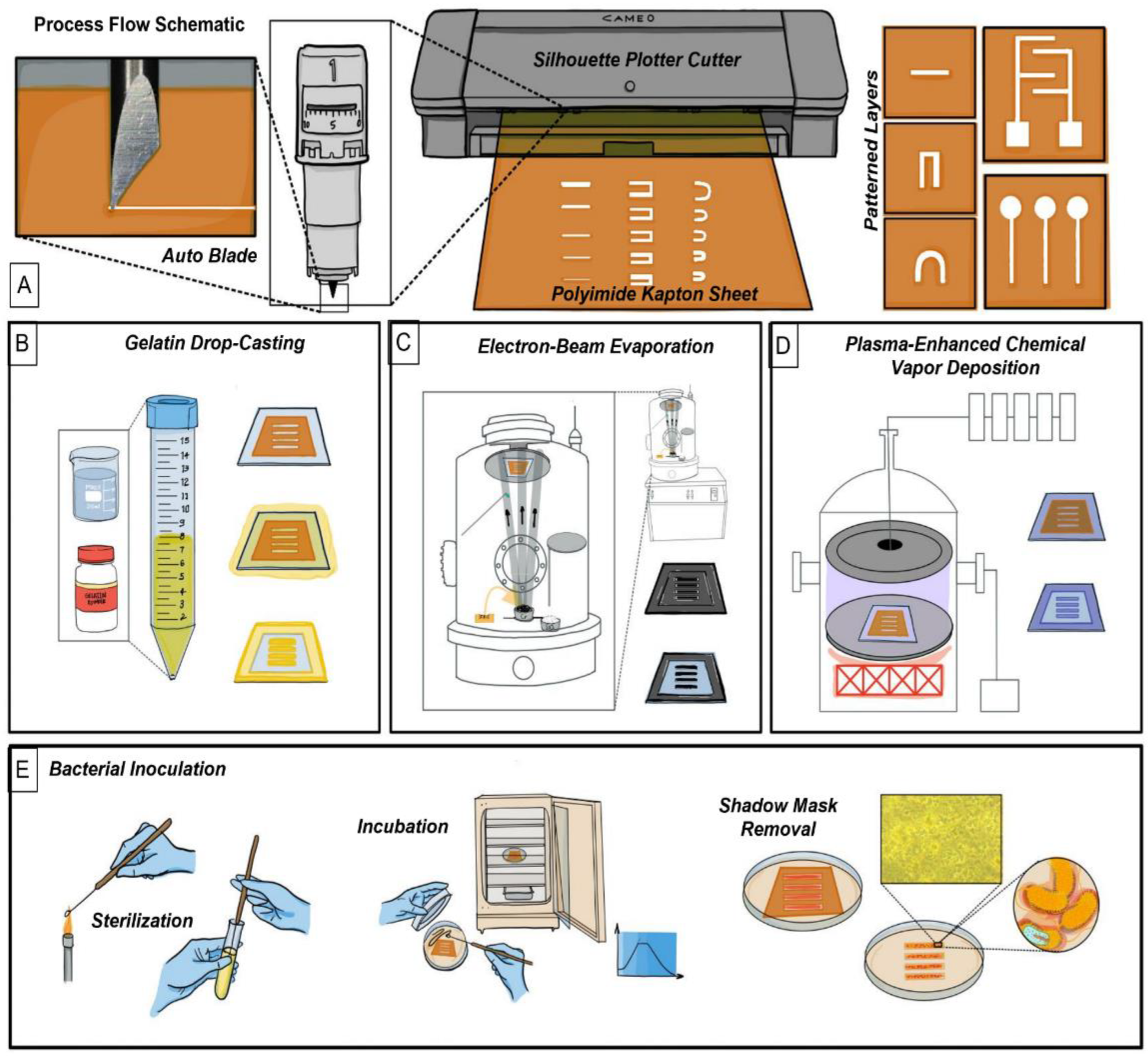
2. Materials and Methods
2.1. Methods of Imaging
2.2. Analysis of the Cutting Blade
2.3. Stencil Mask Designs
2.4. Gelatin Casting
2.5. Interdigitated Electrode Design (IDE) and Microfabrication
2.6. Silicon Dioxide Deposition
2.7. Bacteria Handling
2.8. Disk Diffusion Assay
3. Results
3.1. Analysis of the Cutting Blade
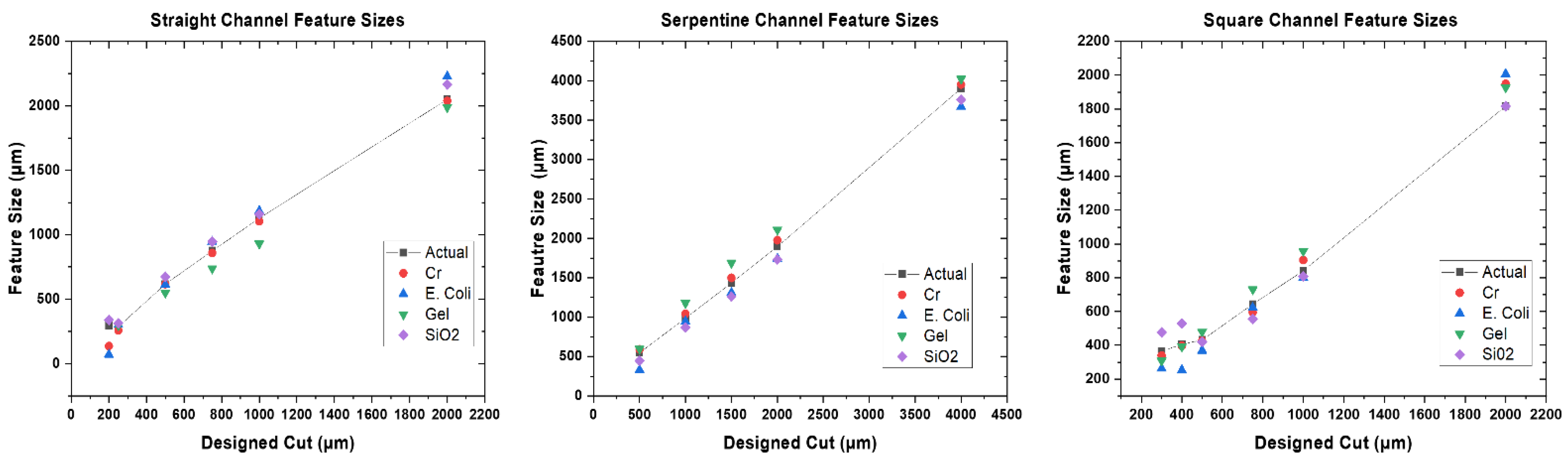
3.2. Design to Device Measurements
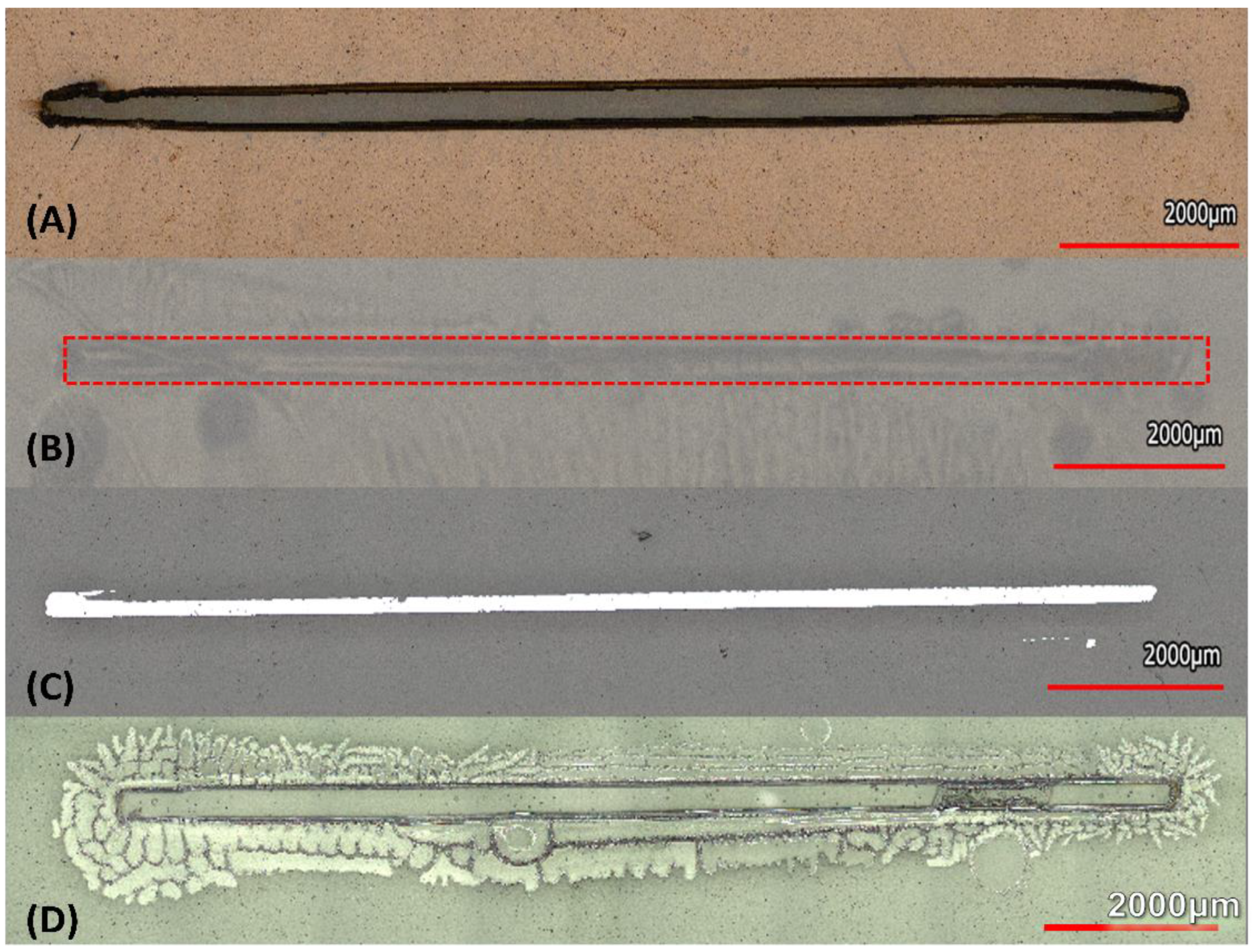
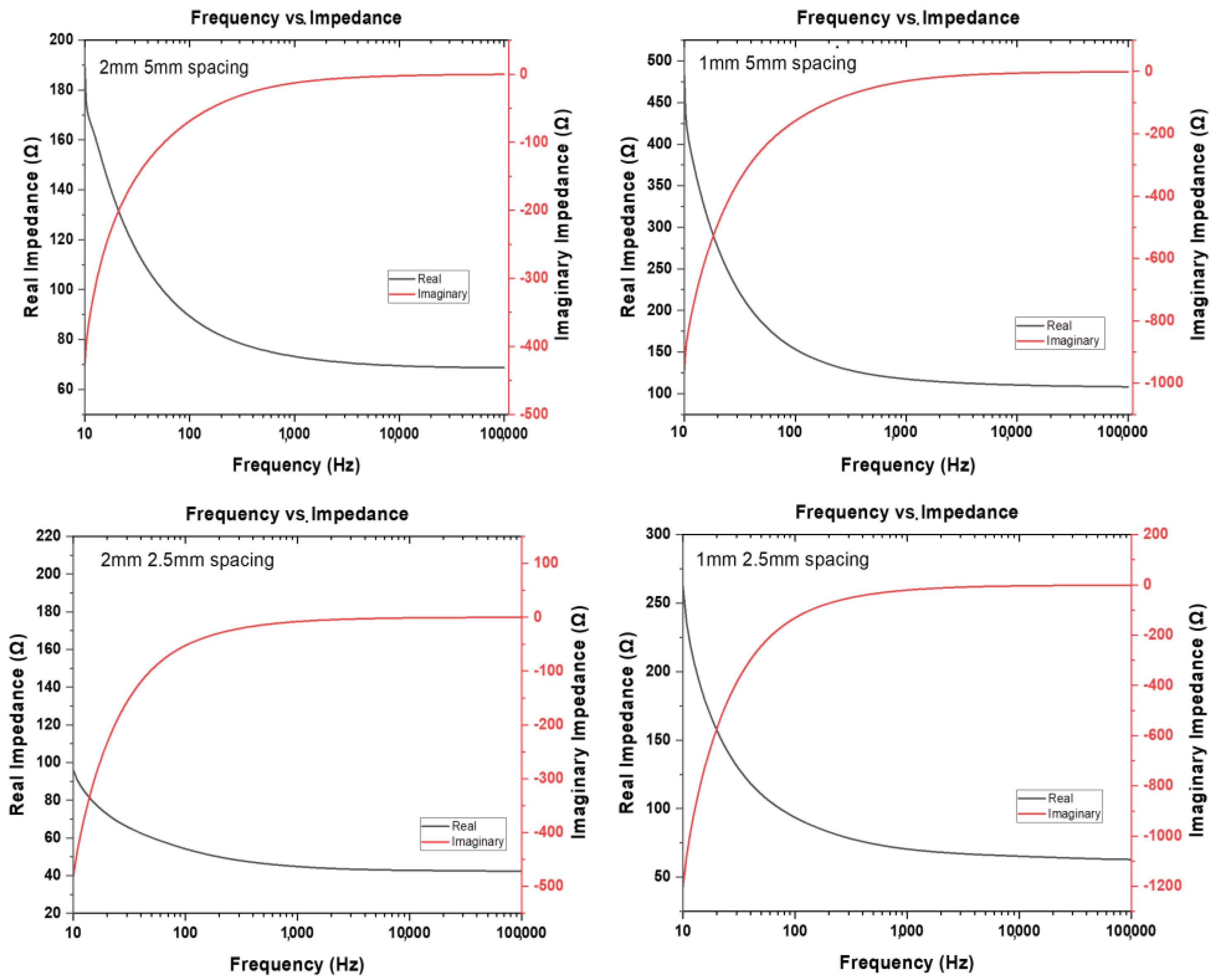
3.3. Interdigitated Electrodes Fabrication
3.4. Silicon Dioxide Deposition Results
3.5. Optimized Kirby Bauer Stencil Mask
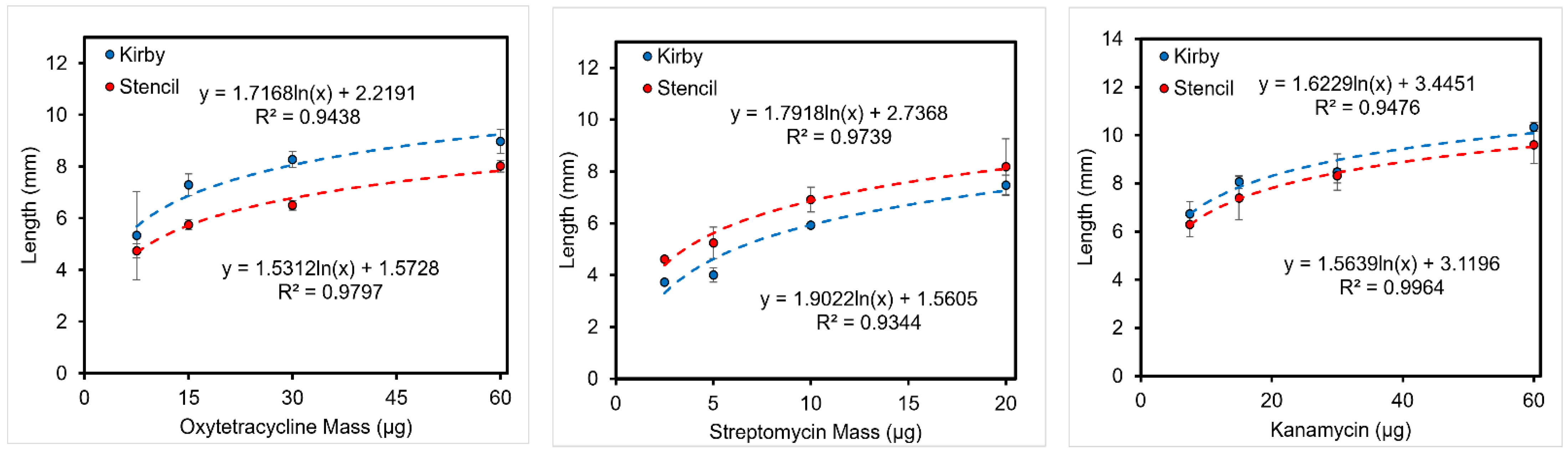
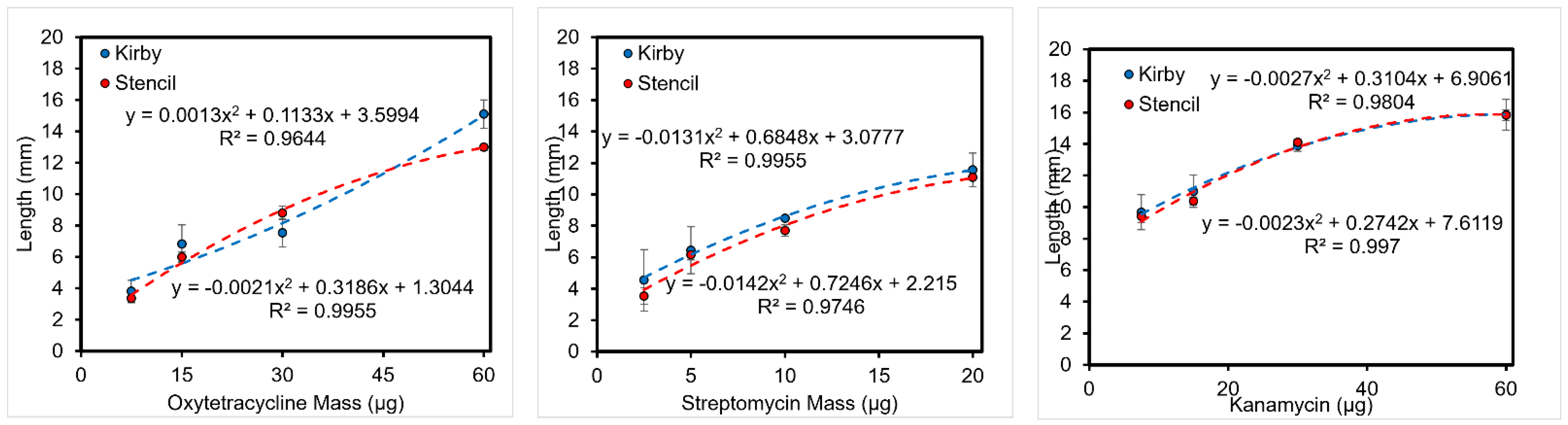
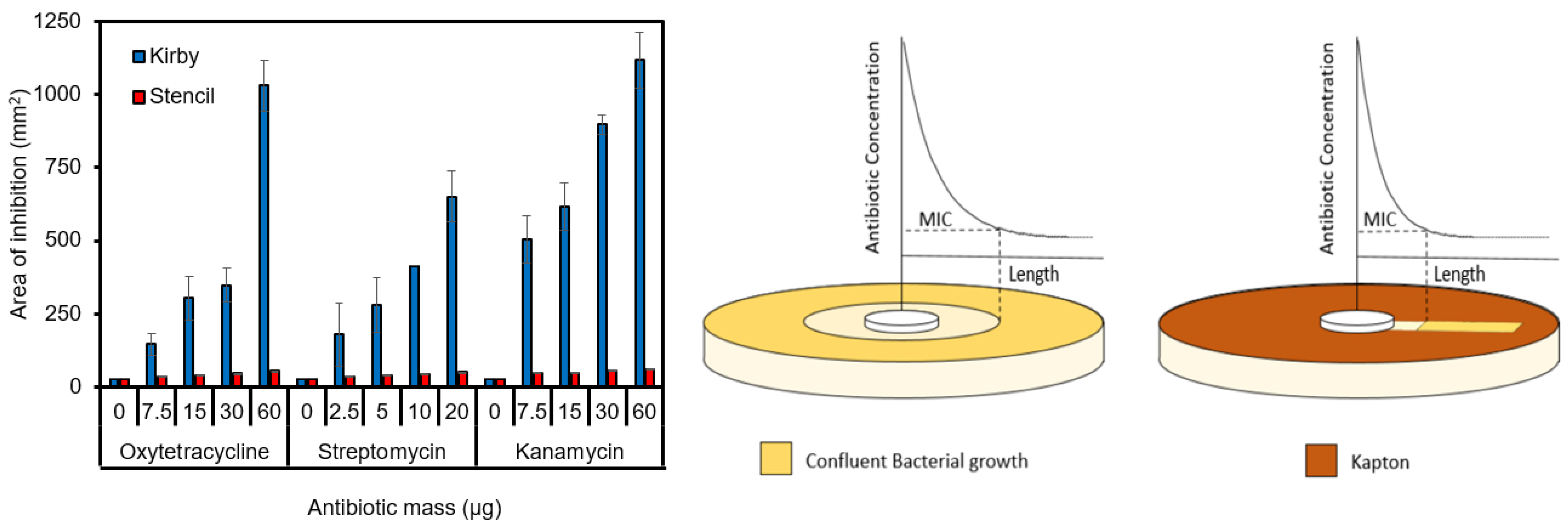
3.5.1. Length of Inhibition
3.5.2. Area of Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yesilkoy, F.; Flauraud, V.; Rüegg, M.; Kim, B.J.; Brugger, J. 3D nanostructures fabricated by advanced stencil lithography. Nanoscale 2016, 8, 4945–4950. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Brannon, J. Excimer Laser Ablation and Etching, 1st ed.; The Education Committee, American Vacuum Society: New York, NY, USA, 1993. [Google Scholar]
- Fourkas, J.T. Nanoscale Photolithography with Visible Light. J. Phys. Chem. Lett. 2010, 1, 1221–1227. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Xu, X.; Zhao, C.; Xu, X.; Weiss, P.S. Single-Step Dual-Layer Photolithography for Tunable and Scalable Nanopatterning. ACS Nano 2021, 15, 12180–12188. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.B.; Chen, S.C. Surface-plasmon-assisted nanoscale photolithography by polarized light. Appl. Phys. Lett. 2005, 86, 253107. [Google Scholar] [CrossRef] [Green Version]
- Pavel, E.; Prodan, G.; Marinescu, V.; Trusca, R. Recent advances in 3- to 10-nm quantum optical lithography. J. Micro Nanolithogr. MEMS MOEMS 2019, 18, 020501. [Google Scholar] [CrossRef]
- Hart, C.; Rajaraman, S. Low-Power, Multimodal Laser Micromachining of Materials for Applications in sub-5 µm Shadow Masks and sub-10 µm Interdigitated Electrodes (IDEs) Fabrication. Micromachines 2020, 11, 178. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.I.; Kong, D.S.; Murthy, S.K.; Carr, P.A. Enabling Microfluidics: From Clean Rooms to Makerspaces. Trends Biotechnol. 2017, 35, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Nath, P.; Maity, T.S.; Pettersson, F.; Resnick, J.; Kunde, Y.; Kraus, N.; Castano, N. Polymerase chain reaction compatibility of adhesive transfer tape based microfluidic platforms. Microsyst. Technol. 2013, 20, 1187–1193. [Google Scholar] [CrossRef]
- Islam, M.; Natu, R.; Martinez-Duarte, R. A study on the limits and advantages of using a desktop cutter plotter to fabricate microfluidic networks. Microfluid. Nanofluid. 2015, 19, 973–985. [Google Scholar] [CrossRef]
- Qamar, A.Z.; Shamsi, M.H. Desktop Fabrication of Lab-On-Chip Devices on Flexible Substrates: A Brief Review. Micromachines 2020, 11, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, A.; Ausaf, T.; Rajasekaran, P.; Rajaraman, S. Multimodal Microfluidic Biosensor with Interdigitated Electrodes (IDE) and Microelectrode Array (MEA) for Bacterial Detection and Identification. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019. [Google Scholar] [CrossRef]
- Johns, P.; Roseway, A.; Czerwinski, M. Tattio: Fabrication of Aesthetic and Functional Temporary Tattoos. In Proceedings of the 2016 CHI Conference Extended Abstracts on Human Factors in Computing Systems, San Jose, CA, USA, 7–12 May 2016; pp. 3699–3702. [Google Scholar]
- Kundu, A.; Ausaf, T.; Rajaraman, S. 3D Printing, Ink Casting and Micromachined Lamination (3D PICLµM): A Makerspace Approach to the Fabrication of Biological Microdevices. Micromachines 2018, 9, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartholomeusz, D.A.; Boutte, R.W.; Andrade, J.D. Xurography: Rapid prototyping of microstructures using a cutting plotter. J. Microelectromech. Syst. 2005, 14, 1364–1374. [Google Scholar] [CrossRef]
- Yuen, P.K.; Goral, V.N. Low-cost rapid prototyping of flexible microfluidic devices using a desktop digital craft cutter. Lab Chip 2009, 10, 384–387. [Google Scholar] [CrossRef]
- Glavan, A.C.; Martinez, R.V.; Maxwell, E.J.; Subramaniam, A.B.; Nunes, R.M.D.; Soh, S.; Whitesides, G.M. Rapid fabrication of pressure-driven open-channel microfluidic devices in omniphobic RF paper. Lab Chip 2013, 13, 2922–2930. [Google Scholar] [CrossRef] [Green Version]
- Samae, M.; Rodniam, C.; Chirasatitsin, S. Characterization of Microfluidic chips Fabricated by a low-cost technique using a vinyl cutter. In Proceedings of the 7th TSME Internationa Conference on Mechanical Engineering, Bali, Indonesia, 1–2 July 2015. [Google Scholar]
- DuPont de Nemours, Inc. DuPont™ Kapton® HN Polyimde Film; Dupont: Wilmington, DE, USA, 2021. [Google Scholar]
- Lindner, E.; Cosofret, V.V.; Ufer, S.; Buck, R.P.; Kusy, R.P.; Ash, R.B.; Nagle, H.T. Flexible (Kapton-based) microsensor arrays of high stability for cardiovascular applications. J. Chem. Soc. Faraday Trans. 1993, 89, 361–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Yuan, H.; Yan, W.; Chen, S.; Qiu, M.; Liao, B.; Chen, L.; Ouyang, X.; Zhang, X.; et al. Mechanically Robust Irradiation, Atomic Oxygen, and Static-Durable CrOx/CuNi Coatings on Kapton Serving as Space Station Solar Cell Arrays. ACS Appl. Mater. Interfaces 2022, 14, 21461–21473. [Google Scholar] [CrossRef]
- Masihi, S.; Panahi, M.; Maddipatla, D.; Hajian, S.; Bose, A.K.; Palaniappan, V.; Narakathu, B.B.; Bazuin, B.J.; Atashbar, M.Z. Cohesion Failure Analysis in a Bi-layered Copper/Kapton Structure for Flexible Hybrid Electronic Sensing Applications. In Proceedings of the 2021 IEEE International Conference on Electro Information Technology (EIT), Pleasant, MI, USA, 14–15 May 2021; pp. 409–412. [Google Scholar] [CrossRef]
- Yong, K.; Ashraf, A.; Kang, P.; Nam, S. Rapid Stencil Mask Fabrication Enabled One-Step Polymer-Free Graphene Patterning and Direct Transfer for Flexible Graphene Devices. Sci. Rep. 2016, 6, 24890. [Google Scholar] [CrossRef] [Green Version]
- Kinnamon, D.S.; Krishnan, S.; Brosler, S.; Sun, E.; Prasad, S. Screen Printed Graphene Oxide Textile Biosensor for Applications in Inexpensive and Wearable Point-of-Exposure Detection of Influenza for At-Risk Populations. J. Electrochem. Soc. 2018, 165, B3084–B3090. [Google Scholar] [CrossRef]
- Gnanasambanthan, H.; Nageswaran, S.; Maji, D. Fabrication of Flexible Thin Film Strain Sensors using Kapton Tape as Stencil Mask. In Proceedings of the 2019 International Conference on Vision towards Emerging Trends in Communication and Networking (ViTECoN), Vellore, India, 30–31 March 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Shim, H.; Jang, S.; Jang, J.G.; Rao, Z.; Hong, J.-I.; Sim, K.; Yu, C. Fully rubbery synaptic transistors made out of all-organic materials for elastic neurological electronic skin. Nano Res. 2021, 15, 758–764. [Google Scholar] [CrossRef]
- Alexander, F., Jr.; Price, D.T.; Bhansali, S. Optimization of interdigitated electrode (IDE) arrays for impedance based evaluation of Hs 578T cancer cells. J. Phys. Conf. Ser. 2010, 224, 012134. [Google Scholar] [CrossRef]
- Tandon, N.; Marolt, D.; Cimetta, E.; Vunjak-Novakovic, G. Bioreactor engineering of stem cell environments. Biotechnol. Adv. 2013, 31, 1020–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, G.L.; Hollingsworth, J.; Morris, J.J.G. Emerging Foodborne Pathogens: Escherichia coil O157:H7 as a Model of Entry of a New Pathogen into the Food Supply of the Developed World. Epidemiol. Rev. 1996, 18, 29–51. [Google Scholar] [CrossRef]
- Kaper, J.B. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Zarei, S.; Taghavi, S.M.; Hamzehzarghani, H.; Osdaghi, E.; Lamichhane, J. Epiphytic growth of Xanthomonas arboricola and Xanthomonas citri on non-host plants. Plant Pathol. 2017, 67, 660–670. [Google Scholar] [CrossRef]
- Ibrahim, M.; Claudel, J.; Kourtiche, D.; Nadi, M. Geometric parameters optimization of planar interdigitated electrodes for bioimpedance spectroscopy. J. Electr. Bioimpedance 2013, 4, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Timms, S.; Colquhoun, K.; Fricker, C. Detection of Escherichia coli in potable water using indirect impedance technology. J. Microbiol. Methods 1996, 26, 125–132. [Google Scholar] [CrossRef]
- Franks, W.; Schenker, I.; Schmutz, P.; Hierlemann, A. Impedance Characterization and Modeling of Electrodes for Biomedical Applications. IEEE Trans. Biomed. Eng. 2005, 52, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Yoon, D.S.; Kim, S.K.; Kim, T.S.; Kim, S.; Pak, E.Y.; No, K. AC frequency characteristics of coplanar impedance sensors as design parameters. Lab Chip 2005, 5, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez, H.; Pacio, M.; Diaz, T.; Rosendo, E.; Garcia, G.; Garcia, A.; Mora, F.; Escalante, G. Low temperature deposition: Properties of SiO2 films from TEOS and ozone by APCVD system. J. Phys. Conf. Ser. 2009, 167, 012020. [Google Scholar] [CrossRef] [Green Version]
- Fujino, K.; Nishimoto, Y.; Tokumasu, N.; Maeda, K. Silicon Dioxide Deposition by Atmospheric Pressure and Low-Temperature CVD Using TEOS and Ozone. J. Electrochem. Soc. 1990, 137, 2883. [Google Scholar] [CrossRef]
- Mannino, G.; Ruggeri, R.; Alberti, A.; Privitera, V.; Fortunato, G.; Maiolo, L. Electrical Properties of Ultrathin SiO2 Layer Deposited at 50 °C by Inductively Coupled Plasma-Enahnced Chemical Vapor Deposition. Appl. Phys. Express 2012, 5, 021103. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Bhargav, H.S.; Shastri, S.D.; Poornav, S.P.; Darshan, K.M.; Nayak, M.M. Measurement of the Zone of Inhibition of an Antibiotic. In Proceedings of the 2016 IEEE 6th International Conference on Advanced Computing (IACC), Bhimavaram, India, 27–28 February 2016; pp. 409–414. [Google Scholar] [CrossRef]
- Wanger, A.; Mills, K. Etest for susceptibility testing of Mycobacterium tuberculosis and Mycobacterium avium-intracellulare. Diagn. Microbiol. Infect. Dis. 1994, 19, 179–181. [Google Scholar] [CrossRef]
- Freixo, I.M.; Caldas, P.C.S.; Martins, F.; Brito, R.C.; Ferreira, R.M.C.; Fonseca, L.S.; Saad, M.H.F. Evaluation of Etest Strips for Rapid Susceptibility Testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 2002, 40, 2282–2284. [Google Scholar] [CrossRef] [Green Version]
- Hausdorfer, J.; Sompek, E.; Allerberger, F.; Dierich, M.P.; Rüsch-Gerdes, S. E-test for susceptibility testing of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 1998, 2, 751–755. [Google Scholar]
- Joyce, L.F.; Downes, J.; Stockman, K.; Andrew, J.H. Comparison of five methods, including the PDM Epsilometer test (E test), for antimicrobial susceptibility testing of Pseudomonas aeruginosa. J. Clin. Microbiol. 1992, 30, 2709–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, A.L.; Barchiesi, F.; A McGough, D.; Rinaldi, M.G. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J. Clin. Microbiol. 1995, 33, 535–540. [Google Scholar] [CrossRef] [PubMed]
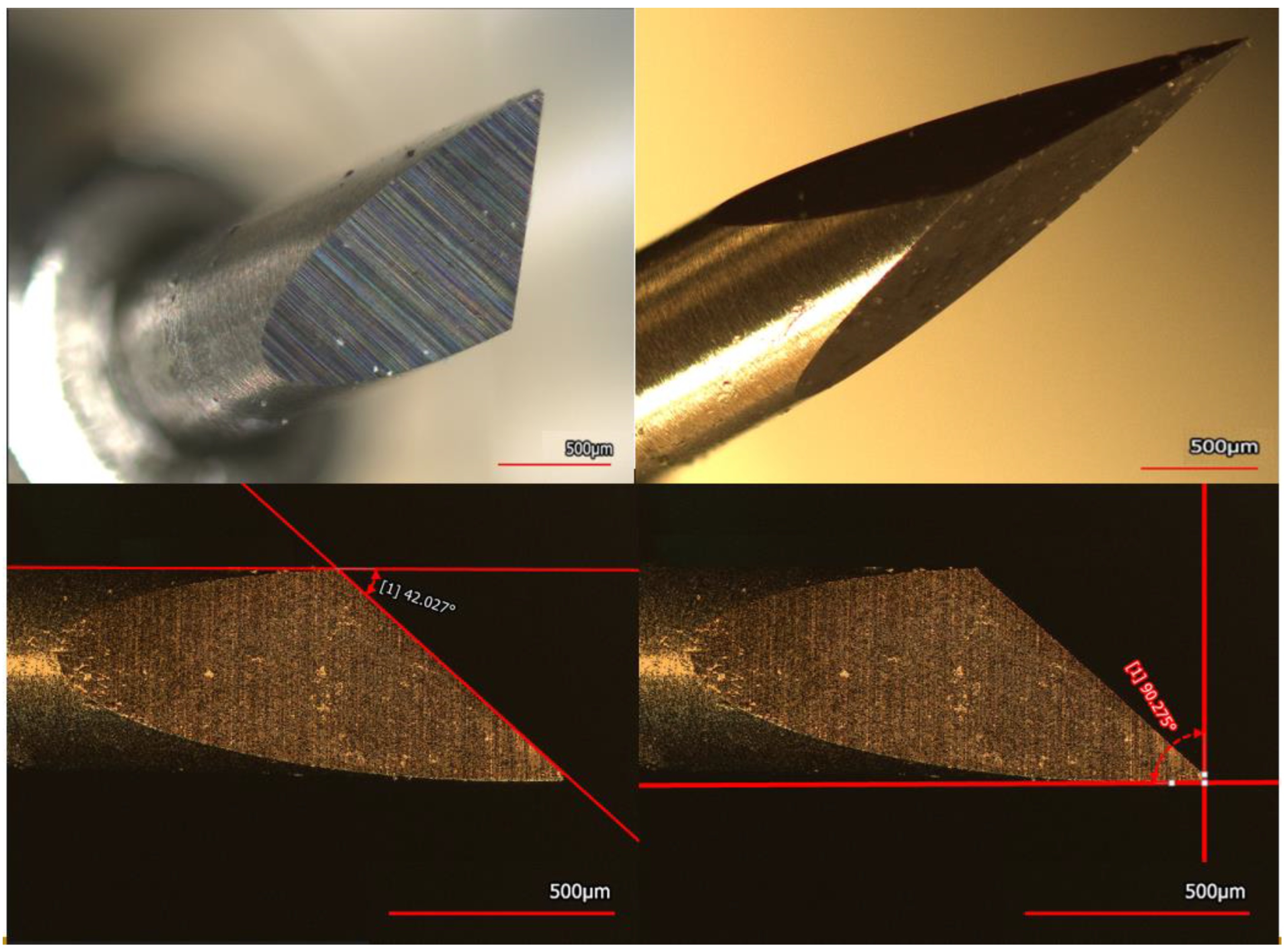


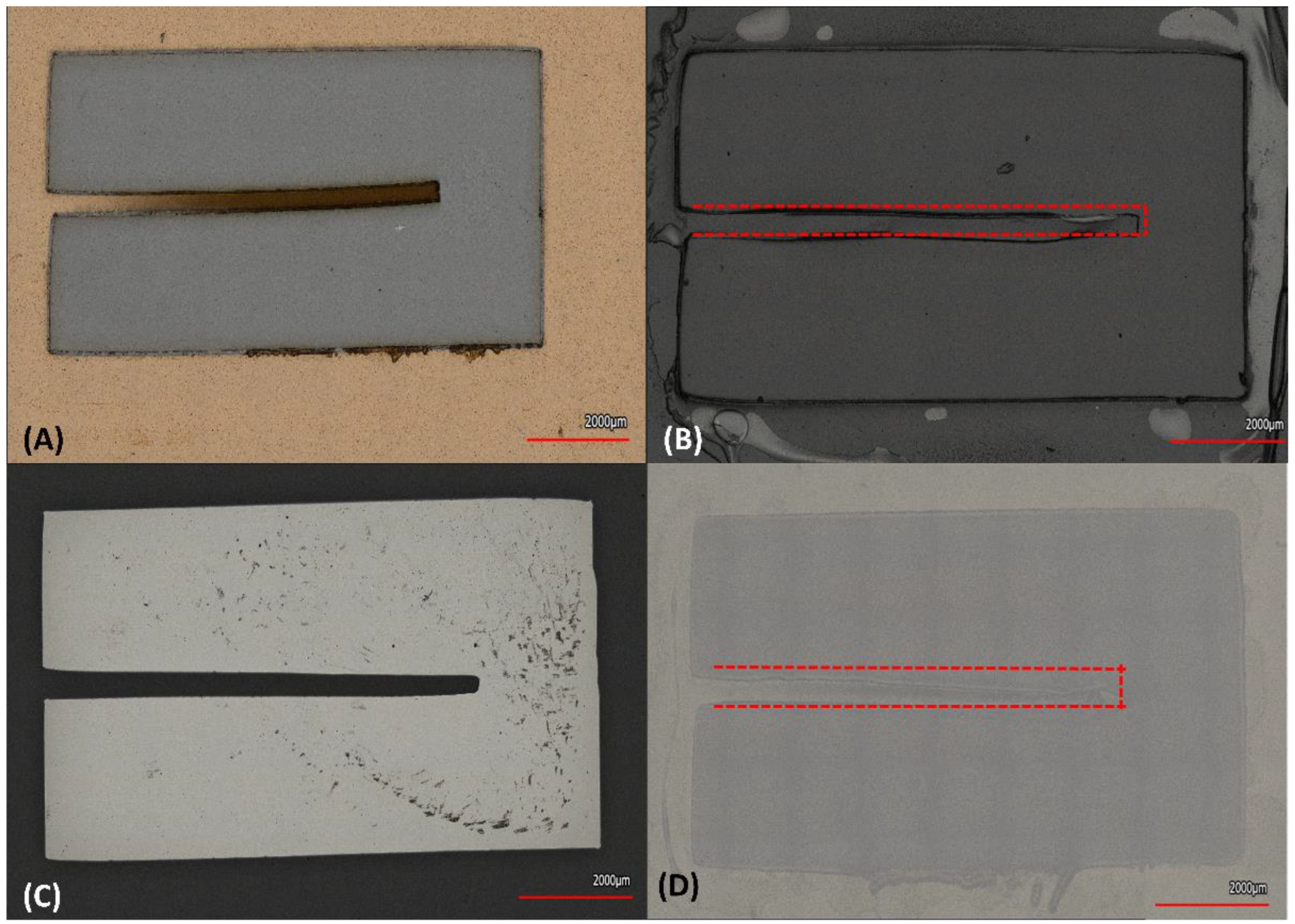

| Force | FSR (N) | Corners H Angles (°) | Corners V Angles (°) |
|---|---|---|---|
| F1 | 0.09415 | ~ | ~ |
| F10 | 0.1285 | ~ | ~ |
| F22 | 0.2962 | 92.48 ± 2.86 | 93.72 ± 1.02 |
| F24 | 0.3236 | 93.32 ± 1.98 | 95.44 ± 0.97 |
| F26 | 0.3433 | 91.18 ± 1.31 | 94.18 ± 1.31 |
| F33 | 0.3629 | ~ | ~ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Childs, A.; Pereira, J.; Didier, C.M.; Baksh, A.; Johnson, I.; Castro, J.M.; Davidson, E.; Santra, S.; Rajaraman, S. Plotter Cut Stencil Masks for the Deposition of Organic and Inorganic Materials and a New Rapid, Cost Effective Technique for Antimicrobial Evaluations. Micromachines 2023, 14, 14. https://doi.org/10.3390/mi14010014
Childs A, Pereira J, Didier CM, Baksh A, Johnson I, Castro JM, Davidson E, Santra S, Rajaraman S. Plotter Cut Stencil Masks for the Deposition of Organic and Inorganic Materials and a New Rapid, Cost Effective Technique for Antimicrobial Evaluations. Micromachines. 2023; 14(1):14. https://doi.org/10.3390/mi14010014
Chicago/Turabian StyleChilds, Andre, Jorge Pereira, Charles M. Didier, Aliyah Baksh, Isaac Johnson, Jorge Manrique Castro, Edwin Davidson, Swadeshmukul Santra, and Swaminathan Rajaraman. 2023. "Plotter Cut Stencil Masks for the Deposition of Organic and Inorganic Materials and a New Rapid, Cost Effective Technique for Antimicrobial Evaluations" Micromachines 14, no. 1: 14. https://doi.org/10.3390/mi14010014
APA StyleChilds, A., Pereira, J., Didier, C. M., Baksh, A., Johnson, I., Castro, J. M., Davidson, E., Santra, S., & Rajaraman, S. (2023). Plotter Cut Stencil Masks for the Deposition of Organic and Inorganic Materials and a New Rapid, Cost Effective Technique for Antimicrobial Evaluations. Micromachines, 14(1), 14. https://doi.org/10.3390/mi14010014






