Type 1 Diabetes Mellitus: A Review on Advances and Challenges in Creating Insulin Producing Devices
Abstract
:1. Introduction
2. Type 1 Diabetes Mellitus
2.1. Causes of T1DM
2.1.1. Genetic Triggers of T1DM
2.1.2. Environmental Triggers of T1DM
2.2. Mechanism of T1DM Autoimmunity
2.3. Treatment Options for Type 1 Diabetes
2.4. In Vivo Research on Diabetes Mellitus
2.4.1. The Non-Obese Diabetic (NOD) Mouse
2.4.2. The Biobreeding (BB) Rat
2.5. In Vitro Research on Diabetes Mellitus
2.5.1. Requirements for the Establishment of In Vitro Models
Cell Sources
In Vitro or Ex Vivo Cell Culture Technology
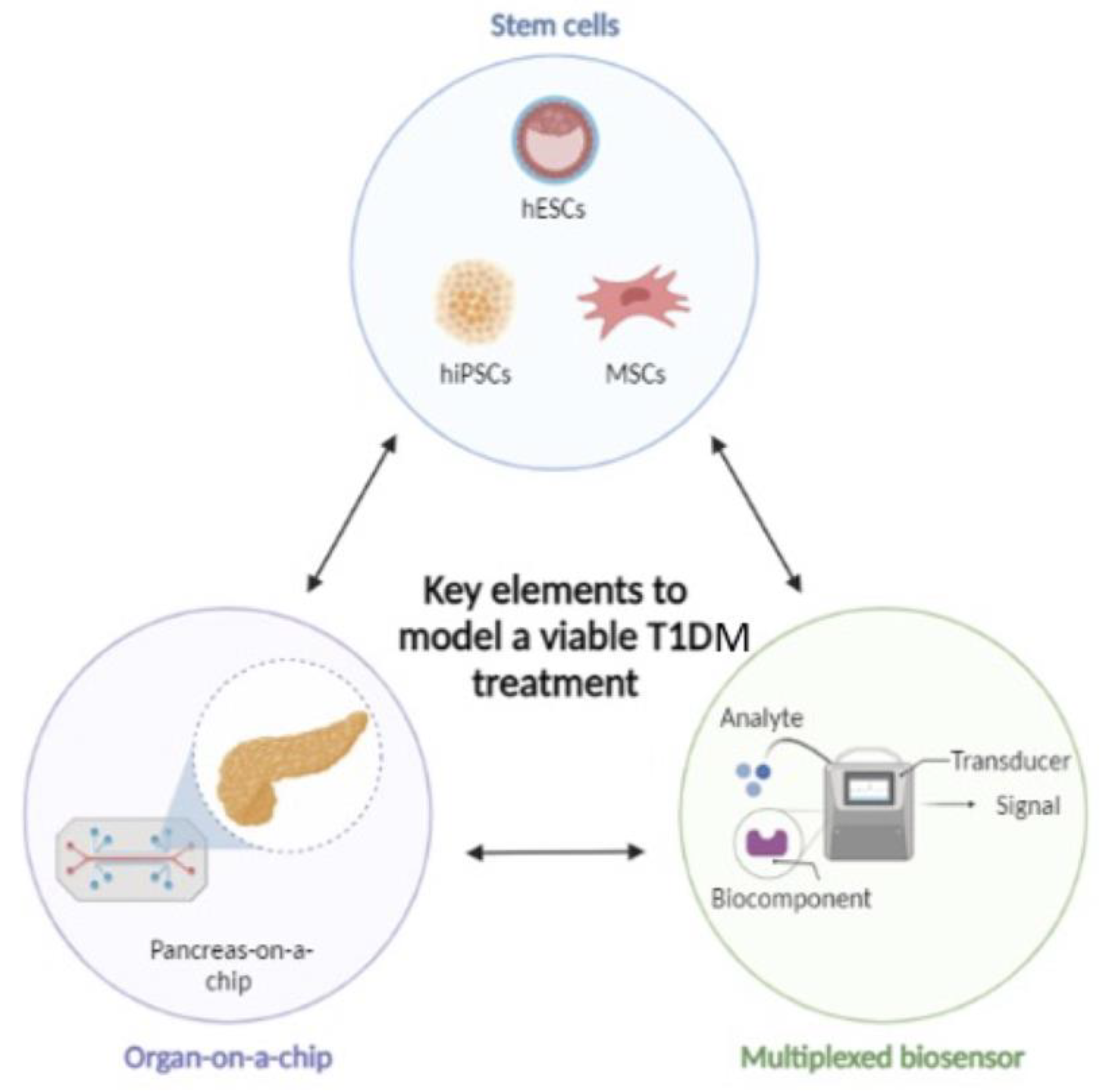
3. Novel In Vitro Models–Microfluidic Technologies Applications in T1DM
3.1. Pancreas-on-a-Chip
3.2. Microfluidic Perfusion Systems for Pancreatic Islet Research
3.3. Potential Analytical Tools for Islet Secretory Fingerprint (SF) Analysis
3.3.1. Label-Free Electrical Biosensors
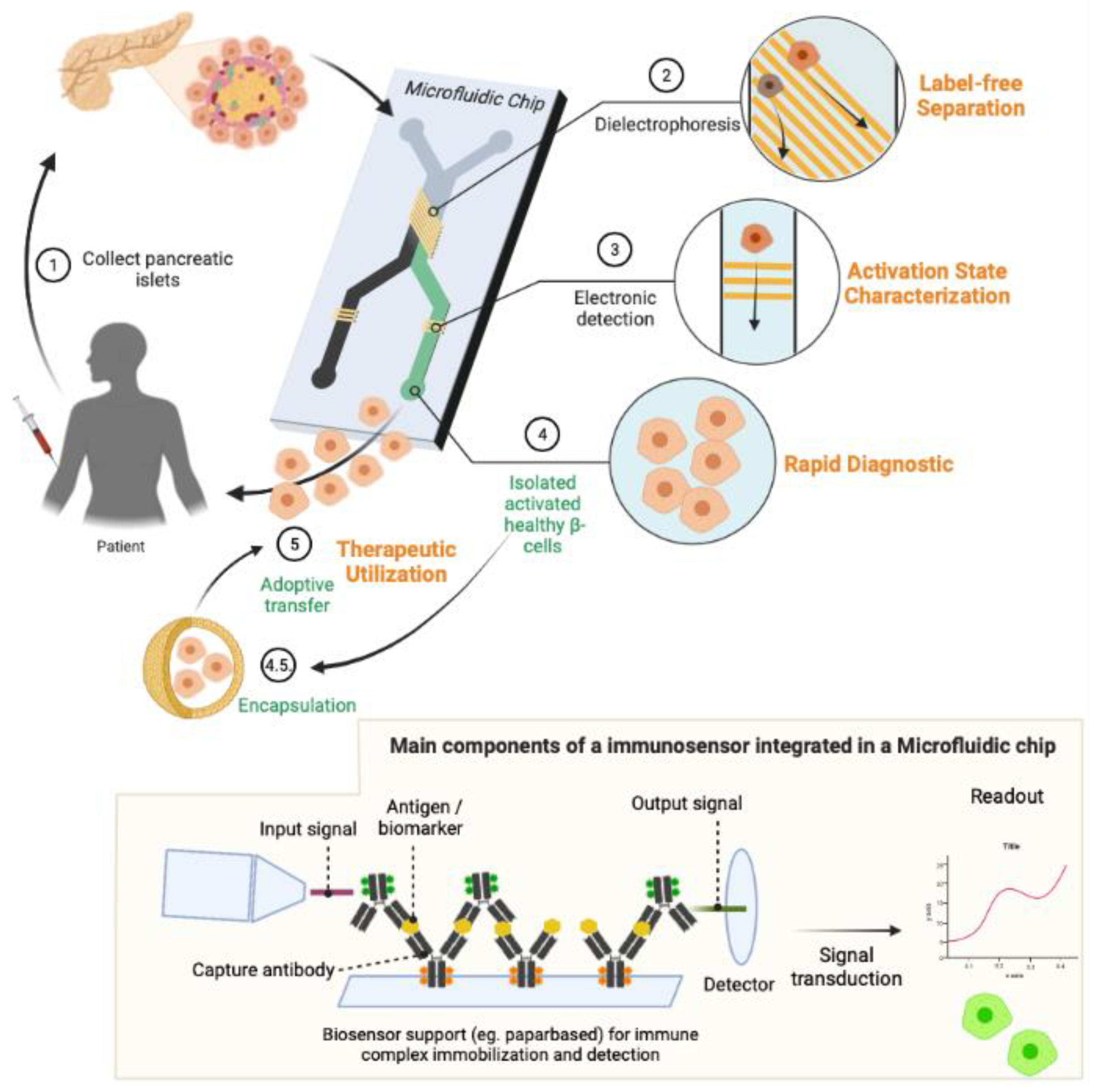

3.3.2. Surface Plasmon Resonance Imaging (SPRi)
3.3.3. On-Chip Applications of Islet Secretory Fingerprint (SF) Monitoring
4. In Vitro Research and Future T1DM Related Studies
Multi-Organ-on-a-Chip
5. Beta-Cell Replacement (Islet Transplantation)
Encapsulation Strategies
6. Stem Cell-Based Therapies
6.1. Stem Cell-Based Approaches
6.1.1. Human Embryonic Stem Cells (hESCs)
6.1.2. Human Induced Pluripotent Stem Cells (hiPSCs)
6.1.3. Mesenchymal Stem Cells (MSCs)
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF International Diabetes Federation. Diabetes Atlas 10th Edition. Global Diabetes Data Report 2000—2045. 2021. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 30 November 2022).
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational Diabetes Mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Gaál, Z.; Balogh, I. Monogenic Forms of Diabetes Mellitus. In Genetics of Endocrine Diseases and Syndromes; Igaz, P., Patócs, A., Eds.; Experientia Supplementum; Springer International Publishing: Cham, Switzerland, 2019; Volume 111, pp. 385–416. ISBN 978-3-030-25904-4. [Google Scholar]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic Regulation of Glucose Homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maahs, D.M.; West, N.A.; Lawrence, J.M.; Mayer-Davis, E.J. Epidemiology of Type 1 Diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 481–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and Incidence of Type 1 Diabetes in the World: A Systematic Review and Meta-Analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A. The Pathogenesis and Natural History of Type 1 Diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marré, M.L.; Piganelli, J.D. Environmental Factors Contribute to β Cell Endoplasmic Reticulum Stress and Neo-Antigen Formation in Type 1 Diabetes. Front. Endocrinol. 2017, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Boldison, J.; Wong, F.S. Immune and Pancreatic β Cell Interactions in Type 1 Diabetes. Trends Endocrinol. Metab. 2016, 27, 856–867. [Google Scholar] [CrossRef] [Green Version]
- Parkkola, A.; Härkönen, T.; Ryhänen, S.J.; Ilonen, J.; Knip, M. The Finnish Pediatric Diabetes Register Extended Family History of Type 1 Diabetes and Phenotype and Genotype of Newly Diagnosed Children. Diabetes Care 2013, 36, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Tandon, N. Understanding Type 1 Diabetes through Genetics: Advances and Prospects. Indian J. Endocr. Metab. 2015, 19, 39. [Google Scholar] [CrossRef]
- Kennedy, A.E.; Ozbek, U.; Dorak, M.T. What Has GWAS Done for HLA and Disease Associations? Int. J. Immunogenet. 2017, 44, 195–211. [Google Scholar] [CrossRef]
- Pociot, F. Type 1 Diabetes Genome-Wide Association Studies: Not to Be Lost in Translation. Clin. Trans. Immunol. 2017, 6, e162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakay, M.; Pandey, R.; Grant, S.F.A.; Hakonarson, H. The Genetic Contribution to Type 1 Diabetes. Curr. Diab. Rep. 2019, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Takahashi, A.; Matsunami, M.; Horikoshi, M.; Iwata, M.; Araki, S.; Toyoda, M.; Susarla, G.; Ahn, J.; Park, K.H.; et al. Genome-Wide Association Studies Identify Two Novel Loci Conferring Susceptibility to Diabetic Retinopathy in Japanese Patients with Type 2 Diabetes. Hum. Mol. Genet. 2021, 30, 716–726. [Google Scholar] [CrossRef]
- Xie, Z.; Chang, C.; Zhou, Z. Molecular Mechanisms in Autoimmune Type 1 Diabetes: A Critical Review. Clin. Rev. Allerg. Immunol. 2014, 47, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Sticht, J.; Álvaro-Benito, M.; Konigorski, S. Type 1 Diabetes and the HLA Region: Genetic Association Besides Classical HLA Class II Genes. Front. Genet. 2021, 12, 683946. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class I and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- El-Ella, S.S.A.; Shaltout, A.A.; Tawfik, M.A.M.; Deeb, M.; EL-Lahony, D.M.; Khatab, E.S.; Barseem, N.F. Non HLA Genetic Markers Association with Type-1 Diabetes Mellitus. Egypt. J. Med. Hum. Genet. 2011, 12, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Laine, A.-P.; Valta, M.; Toppari, J.; Knip, M.; Veijola, R.; Ilonen, J.; Lempainen, J. Non-HLA Gene Polymorphisms in the Pathogenesis of Type 1 Diabetes: Phase and Endotype Specific Effects. Front. Immunol. 2022, 13, 909020. [Google Scholar] [CrossRef]
- Regnell, S.E.; Lernmark, Å. Early Prediction of Autoimmune (Type 1) Diabetes. Diabetologia 2017, 60, 1370–1381. [Google Scholar] [CrossRef] [Green Version]
- Primavera, M.; Giannini, C.; Chiarelli, F. Prediction and Prevention of Type 1 Diabetes. Front. Endocrinol. 2020, 11, 248. [Google Scholar] [CrossRef]
- Redondo, M.J.; Geyer, S.; Steck, A.K.; Sharp, S.; Wentworth, J.M.; Weedon, M.N.; Antinozzi, P.; Sosenko, J.; Atkinson, M.; Pugliese, A.; et al. A Type 1 Diabetes Genetic Risk Score Predicts Progression of Islet Autoimmunity and Development of Type 1 Diabetes in Individuals at Risk. Diabetes Care 2018, 41, 1887–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, A.H.; Eff, C.; Leslie, R.D.G.; Pyke, D.A. Diabetes in Identical Twins: A Study of 200 Pairs. Diabetologia 1981, 20, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knip, M.; Simell, O. Environmental Triggers of Type 1 Diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007690. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Calvo, T.; Sabouri, S.; Anquetil, F.; von Herrath, M.G. The Viral Paradigm in Type 1 Diabetes: Who Are the Main Suspects? Autoimmun. Rev. 2016, 15, 964–969. [Google Scholar] [CrossRef]
- Richardson, S.J.; Morgan, N.G. Enteroviral Infections in the Pathogenesis of Type 1 Diabetes: New Insights for Therapeutic Intervention. Curr. Opin. Pharmacol. 2018, 43, 11–19. [Google Scholar] [CrossRef]
- Quinn, L.M.; Wong, F.S.; Narendran, P. Environmental Determinants of Type 1 Diabetes: From Association to Proving Causality. Front. Immunol. 2021, 12, 737964. [Google Scholar] [CrossRef] [PubMed]
- Traversi, D.; Rabbone, I.; Scaioli, G.; Vallini, C.; Carletto, G.; Racca, I.; Ala, U.; Durazzo, M.; Collo, A.; Ferro, A.; et al. Risk Factors for Type 1 Diabetes, Including Environmental, Behavioural and Gut Microbial Factors: A Case–Control Study. Sci. Rep. 2020, 10, 17566. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Qiao, Y.; Ling, W.; Pan, Y.; Geng, L.; Xiao, J.; Zhang, X.; Zhao, H. Climates on Incidence of Childhood Type 1 Diabetes Mellitus in 72 Countries. Sci. Rep. 2017, 7, 12810. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, I.M.; Joner, G.; Jenum, P.A.; Eskild, A.; Brunborg, C.; Torjesen, P.A.; Stene, L.C. Vitamin D-Binding Protein and 25-Hydroxyvitamin D during Pregnancy in Mothers Whose Children Later Developed Type 1 Diabetes: Maternal DBP/25-OH D, Childhood Diabetes. Diabetes Metab. Res. Rev. 2016, 32, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.M.; Hart, P.H.; de Klerk, N.H.; Davis, E.A.; Lucas, R.M. Are Low Sun Exposure and/or Vitamin D Risk Factors for Type 1 Diabetes? Photochem. Photobiol. Sci. 2017, 16, 381–398. [Google Scholar] [CrossRef]
- Miller, K.M.; Hart, P.H.; Lucas, R.M.; Davis, E.A.; de Klerk, N.H. Higher Ultraviolet Radiation during Early Life Is Associated with Lower Risk of Childhood Type 1 Diabetes among Boys. Sci. Rep. 2021, 11, 18597. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Garland, F.C. The Association between Ultraviolet B Irradiance, Vitamin D Status and Incidence Rates of Type 1 Diabetes in 51 Regions Worldwide. Diabetologia 2008, 51, 1391–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahadi, M.; Tabatabaeiyan, M.; Moazzami, K. Association between Environmental Factors and Risk of Type 1 Diabetes—A Case-Control Study. Endokrynol. Pol. 2011, 62, 134–137. [Google Scholar] [PubMed]
- Moltchanova, E.V.; Schreier, N.; Lammi, N.; Karvonen, M. Seasonal Variation of Diagnosis of Type 1 Diabetes Mellitus in Children Worldwide. Diabet. Med. 2009, 26, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Delovitch, T.L. Immune Mechanisms That Regulate Susceptibility to Autoimmune Type I Diabetes. CRIAI 2000, 19, 247–264. [Google Scholar] [CrossRef]
- Han, S.; Donelan, W.; Wang, H.; Reeves, W.; Yang, L.-J. Novel Autoantigens in Type 1 Diabetes. Am. J. Transl. Res. 2013, 5, 379–392. [Google Scholar]
- Ounissi-Benkalha, H.; Polychronakos, C. The Molecular Genetics of Type 1 Diabetes: New Genes and Emerging Mechanisms. Trends Mol. Med. 2008, 14, 268–275. [Google Scholar] [CrossRef]
- Engin, F. ER Stress and Development of Type 1 Diabetes. J. Investig. Med. 2016, 64, 2–6. [Google Scholar] [CrossRef]
- Knip, M.; Siljander, H. Autoimmune Mechanisms in Type 1 Diabetes. Autoimmun. Rev. 2008, 7, 550–557. [Google Scholar] [CrossRef]
- Harrison, L.C. The Dark Side of Insulin: A Primary Autoantigen and Instrument of Self-Destruction in Type 1 Diabetes. Mol. Metab. 2021, 52, 101288. [Google Scholar] [CrossRef]
- Roep, B.O.; Peakman, M. Antigen Targets of Type 1 Diabetes Autoimmunity. Cold Spring Harb. Perspect. Med. 2012, 2, a007781. [Google Scholar] [CrossRef]
- Mathieu, C.; Lahesmaa, R.; Bonifacio, E.; Achenbach, P.; Tree, T. Immunological Biomarkers for the Development and Progression of Type 1 Diabetes. Diabetologia 2018, 61, 2252–2258. [Google Scholar] [CrossRef] [Green Version]
- Insel, R.A.; Dunne, J.L.; Atkinson, M.A.; Chiang, J.L.; Dabelea, D.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Krischer, J.P.; Lernmark, Å.; et al. Staging Presymptomatic Type 1 Diabetes: A Scientific Statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015, 38, 1964–1974. [Google Scholar] [CrossRef] [Green Version]
- Sosenko, J.M. Staging the Progression to Type 1 Diabetes with Prediagnostic Markers. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Peters, A. Screening for Autoantibodies in Type 1 Diabetes: A Call to Action. J. Fam. Pract. 2021, 70, S47–S52. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Liu, D.; Yang, L.; Xu, J.; Wang, Q. Antigen-Specific Immunotherapies in Type 1 Diabetes. J. Trace Elem. Med. Biol. 2022, 73, 127040. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Wise, J. Type 1 Diabetes Still Shortens Life Span, Scottish Study Finds. BMJ 2015, 350, h59. [Google Scholar] [CrossRef]
- Gamble, A.; Pepper, A.R.; Bruni, A.; Shapiro, A.M.J. The Journey of Islet Cell Transplantation and Future Development. Islets 2018, 10, 80–94. [Google Scholar] [CrossRef] [Green Version]
- Chellappan, D.K.; Sivam, N.S.; Teoh, K.X.; Leong, W.P.; Fui, T.Z.; Chooi, K.; Khoo, N.; Yi, F.J.; Chellian, J.; Cheng, L.L.; et al. Gene Therapy and Type 1 Diabetes Mellitus. Biomed. Pharmacother. 2018, 108, 1188–1200. [Google Scholar] [CrossRef]
- Di Lorenzo, T.P.; Peakman, M.; Roep, B.O. Translational Mini-Review Series on Type 1 Diabetes: Systematic Analysis of T Cell Epitopes in Autoimmune Diabetes. Clin. Exp. Immunol. 2007, 148, 1–16. [Google Scholar] [CrossRef]
- Farney, A.C.; Sutherland, D.E.R.; Opara, E.C. Evolution of Islet Transplantation for the Last 30 Years. Pancreas 2016, 45, 8–20. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals From Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef]
- Rambaran, T.F. Nanopolyphenols: A Review of Their Encapsulation and Anti-Diabetic Effects. SN Appl. Sci. 2020, 2, 1335. [Google Scholar] [CrossRef]
- Rahmig, S.; Bornstein, S.; Chavakis, T.; Jaeckel, E.; Waskow, C. Humanized Mouse Models for Type 1 Diabetes Including Pancreatic Islet Transplantation. Horm. Metab. Res. 2014, 47, 43–47. [Google Scholar] [CrossRef]
- Luce, S.; Guinoiseau, S.; Gadault, A.; Letourneur, F.; Blondeau, B.; Nitschke, P.; Pasmant, E.; Vidaud, M.; Lemonnier, F.; Boitard, C. Humanized Mouse Model to Study Type 1 Diabetes. Diabetes 2018, 67, 1816–1829. [Google Scholar] [CrossRef] [Green Version]
- King, A.; Bowe, J. Animal Models for Diabetes: Understanding the Pathogenesis and Finding New Treatments. Biochem. Pharmacol. 2016, 99, 1–10. [Google Scholar] [CrossRef]
- Leiter, E.H.; Prochazka, M.; Coleman, D.L. The Non-Obese Diabetic (NOD) Mouse. Am. J. Pathol. 1987, 128, 380–383. [Google Scholar]
- Reed, J.C.; Herold, K.C. Thinking Bedside at the Bench: The NOD Mouse Model of T1DM. Nat. Rev. Endocrinol. 2015, 11, 308–314. [Google Scholar] [CrossRef]
- Geoffrey, R.; Jia, S.; Kwitek, A.E.; Woodliff, J.; Ghosh, S.; Lernmark, Å.; Wang, X.; Hessner, M.J. Evidence of a Functional Role for Mast Cells in the Development of Type 1 Diabetes Mellitus in the BioBreeding Rat. J. Immunol. 2006, 177, 7275–7286. [Google Scholar] [CrossRef] [Green Version]
- Chatzigeorgiou, A.; Halapas, A.; Kalafatakis, K.; Kamper, E. The Use of Animal Models in the Study of Diabetes Mellitus. In Vivo 2009, 23, 245–258. [Google Scholar]
- King, A.J. The Use of Animal Models in Diabetes Research: Animal Models of Diabetes. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Mordes, J.P.; Bortell, R.; Blankenhorn, E.P.; Rossini, A.A.; Greiner, D.L. Rat Models of Type 1 Diabetes: Genetics, Environment, and Autoimmunity. ILAR J. 2004, 45, 278–291. [Google Scholar] [CrossRef] [Green Version]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental Animal Models for Diabetes and Its Related Complications—A Review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef]
- Aldrich, V.R.; Hernandez-Rovira, B.B.; Chandwani, A.; Abdulreda, M.H. NOD Mice—Good Model for T1D but Not Without Limitations. Cell Transpl. 2020, 29, 096368972093912. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Leiter, E.H. The NOD Mouse Model of Type 1 Diabetes: As Good as It Gets? Nat. Med. 1999, 5, 601–604. [Google Scholar] [CrossRef]
- Chen, Y.-G.; Mathews, C.E.; Driver, J.P. The Role of NOD Mice in Type 1 Diabetes Research: Lessons from the Past and Recommendations for the Future. Front. Endocrinol. 2018, 9, 51. [Google Scholar] [CrossRef]
- Whalen, B.J.; Mordes, J.P.; Rossini, A.A. The BB Rat as a Model of Human Insulin-Dependent Diabetes Mellitus. Curr. Protoc. Immunol. 2001, 19, 1–15. [Google Scholar] [CrossRef]
- Verhaeghe, J.; Bouillon, R. Effects of Diabetes and Insulin on Bone Physiology. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 983–999. ISBN 978-0-12-373884-4. [Google Scholar]
- Gottlieb, P.A.; Rossini, A.A. The BB Rat Models of IDDM. In Autoimmune Disease Models; Elsevier: Amsterdam, The Netherlands, 1994; pp. 163–174. ISBN 978-0-08-091736-8. [Google Scholar]
- Yoon, J.-W.; Jun, H.-S. Insulin-Dependent Diabetes Mellitus, Experimental Models. In Encyclopedia of Immunology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 1390–1398. ISBN 978-0-12-226765-9. [Google Scholar]
- van den Brandt, J.; Fischer, H.J.; Walter, L.; Hünig, T.; Klöting, I.; Reichardt, H.M. Type 1 Diabetes in BioBreeding Rats Is Critically Linked to an Imbalance between Th17 and Regulatory T Cells and an Altered TCR Repertoire. J. Immunol. 2010, 185, 2285–2294. [Google Scholar] [CrossRef] [Green Version]
- Immunology of the rat. In Handbook of Vertebrate Immunology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 137–222. ISBN 978-0-12-546401-7.
- Mullen, Y. Development of the Nonobese Diabetic Mouse and Contribution of Animal Models for Understanding Type 1 Diabetes. Pancreas 2017, 46, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benam, K.H.; Dauth, S.; Hassell, B.; Herland, A.; Jain, A.; Jang, K.-J.; Karalis, K.; Kim, H.J.; MacQueen, L.; Mahmoodian, R.; et al. Engineered In Vitro Disease Models. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 195–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, J.N. Cell Lines Battle Cancer. Nature 2012, 483, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Martín, F.; Sánchez-Gilabert, A.; Tristán-Manzano, M.; Benabdellah, K. Stem Cells for Modeling Human Disease. In Pluripotent Stem Cells—From the Bench to the Clinic; Tomizawa, M., Ed.; IntechOpen: Rijeka, Croatia, 2016; ISBN 978-953-51-2471-9. [Google Scholar]
- Saha, K.; Jaenisch, R. Technical Challenges in Using Human Induced Pluripotent Stem Cells to Model Disease. Cell Stem Cell 2009, 5, 584–595. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Burdick, J. Cellular Encapsulation in 3D Hydrogels for Tissue Engineering. JoVE 2009, 32, 1590. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, A.; Akbari, E.; Imani, R. An Overview of Engineered Hydrogel-Based Biomaterials for Improved β-Cell Survival and Insulin Secretion. Front. Bioeng. Biotechnol. 2021, 9, 662084. [Google Scholar] [CrossRef]
- Velasco, V.; Shariati, S.A.; Esfandyarpour, R. Microtechnology-Based Methods for Organoid Models. Microsyst. Nanoeng. 2020, 6, 76. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Romitti, M.; Tourneur, A.; de Faria da Fonseca, B.; Doumont, G.; Gillotay, P.; Liao, X.-H.; Eski, S.E.; Van Simaeys, G.; Chomette, L.; Lasolle, H.; et al. Transplantable Human Thyroid Organoids Generated from Embryonic Stem Cells to Rescue Hypothyroidism. Nat. Commun. 2022, 13, 7057. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering Organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Abadpour, S.; Aizenshtadt, A.; Olsen, P.A.; Shoji, K.; Wilson, S.R.; Krauss, S.; Scholz, H. Pancreas-on-a-Chip Technology for Transplantation Applications. Curr. Diab. Rep. 2020, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Baptista, L.S.; Porrini, C.; Kronemberger, G.S.; Kelly, D.J.; Perrault, C.M. 3D Organ-on-a-Chip: The Convergence of Microphysiological Systems and Organoids. Front. Cell Dev. Biol. 2022, 10, 1043117. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Comas, J.; Ramón-Azcón, J. Islet-on-a-Chip for the Study of Pancreatic β-Cell Function. In Vitro Models 2022, 1, 41–57. [Google Scholar] [CrossRef]
- Castiello, F.R.; Heileman, K.; Tabrizian, M. Microfluidic Perfusion Systems for Secretion Fingerprint Analysis of Pancreatic Islets: Applications, Challenges and Opportunities. Lab Chip 2016, 16, 409–431. [Google Scholar] [CrossRef]
- Jun, Y.; Lee, J.; Choi, S.; Yang, J.H.; Sander, M.; Chung, S.; Lee, S.-H. In Vivo–Mimicking Microfluidic Perfusion Culture of Pancreatic Islet Spheroids. Sci. Adv. 2019, 5, eaax4520. [Google Scholar] [CrossRef] [Green Version]
- Rivera, K.R.; Yokus, M.A.; Erb, P.D.; Pozdin, V.A.; Daniele, M. Measuring and Regulating Oxygen Levels in Microphysiological Systems: Design, Material, and Sensor Considerations. Analyst 2019, 144, 3190–3215. [Google Scholar] [CrossRef]
- Shackman, J.G.; Reid, K.R.; Dugan, C.E.; Kennedy, R.T. Dynamic Monitoring of Glucagon Secretion from Living Cells on a Microfluidic Chip. Anal. Bioanal. Chem. 2012, 402, 2797–2803. [Google Scholar] [CrossRef] [Green Version]
- Dishinger, J.F.; Kennedy, R.T. Serial Immunoassays in Parallel on a Microfluidic Chip for Monitoring Hormone Secretion from Living Cells. Anal. Chem. 2007, 79, 947–954. [Google Scholar] [CrossRef]
- Dishinger, J.F.; Reid, K.R.; Kennedy, R.T. Quantitative Monitoring of Insulin Secretion from Single Islets of Langerhans in Parallel on a Microfluidic Chip. Anal. Chem. 2009, 81, 3119–3127. [Google Scholar] [CrossRef] [Green Version]
- Roper, M.G.; Shackman, J.G.; Dahlgren, G.M.; Kennedy, R.T. Microfluidic Chip for Continuous Monitoring of Hormone Secretion from Live Cells Using an Electrophoresis-Based Immunoassay. Anal. Chem. 2003, 75, 4711–4717. [Google Scholar] [CrossRef] [PubMed]
- Lomasney, A.R.; Yi, L.; Roper, M.G. Simultaneous Monitoring of Insulin and Islet Amyloid Polypeptide Secretion from Islets of Langerhans on a Microfluidic Device. Anal. Chem. 2013, 85, 7919–7925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritzel, R.A.; Meier, J.J.; Lin, C.-Y.; Veldhuis, J.D.; Butler, P.C. Human Islet Amyloid Polypeptide Oligomers Disrupt Cell Coupling, Induce Apoptosis, and Impair Insulin Secretion in Isolated Human Islets. Diabetes 2007, 56, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Haythorne, E.; Rohm, M.; van de Bunt, M.; Brereton, M.F.; Tarasov, A.I.; Blacker, T.S.; Sachse, G.; Silva dos Santos, M.; Terron Exposito, R.; Davis, S.; et al. Diabetes Causes Marked Inhibition of Mitochondrial Metabolism in Pancreatic β-Cells. Nat. Commun. 2019, 10, 2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocheleau, J.V.; Walker, G.M.; Head, W.S.; McGuinness, O.P.; Piston, D.W. Microfluidic Glucose Stimulation Reveals Limited Coordination of Intracellular Ca 2+ Activity Oscillations in Pancreatic Islets. Proc. Natl. Acad. Sci. USA 2004, 101, 12899–12903. [Google Scholar] [CrossRef] [Green Version]
- Adewola, A.F.; Lee, D.; Harvat, T.; Mohammed, J.; Eddington, D.T.; Oberholzer, J.; Wang, Y. Microfluidic Perifusion and Imaging Device for Multi-Parametric Islet Function Assessment. Biomed. Microdevices 2010, 12, 409–417. [Google Scholar] [CrossRef]
- Mohammed, J.S.; Wang, Y.; Harvat, T.A.; Oberholzer, J.; Eddington, D.T. Microfluidic Device for Multimodal Characterization of Pancreatic Islets. Lab Chip 2009, 9, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.N.; Green, B.J.; Altamentova, S.M.; Rocheleau, J.V. A Microfluidic Device Designed to Induce Media Flow throughout Pancreatic Islets While Limiting Shear-Induced Damage. Lab Chip 2013, 13, 4374. [Google Scholar] [CrossRef]
- Ernst, A.U.; Bowers, D.T.; Wang, L.-H.; Shariati, K.; Plesser, M.D.; Brown, N.K.; Mehrabyan, T.; Ma, M. Nanotechnology in Cell Replacement Therapies for Type 1 Diabetes. Adv. Drug Deliv. Rev. 2019, 139, 116–138. [Google Scholar] [CrossRef]
- Lai, X.; Yang, M.; Wu, H.; Li, D. Modular Microfluidics: Current Status and Future Prospects. Micromachines 2022, 13, 1363. [Google Scholar] [CrossRef]
- Liu, S.; Su, W.; Ding, X. A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection. Sensors 2016, 16, 2086. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mandal, K.; Hernandez, A.L.; Kawakita, S.; Huang, W.; Bandaru, P.; Ahadian, S.; Kim, H.-J.; Jucaud, V.; Dokmeci, M.R.; et al. State of the Art in Integrated Biosensors for Organ-on-a-Chip Applications. Curr. Opin. Biomed. Eng. 2021, 19, 100309. [Google Scholar] [CrossRef]
- Castiello, F.R.; Tabrizian, M. Multiplex Surface Plasmon Resonance Imaging-Based Biosensor for Human Pancreatic Islets Hormones Quantification. Anal. Chem. 2018, 90, 3132–3139. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, D.; Chen, P. Micro- and Nanotechnologies for Study of Cell Secretion. Anal. Chem. 2011, 83, 4393–4406. [Google Scholar] [CrossRef] [PubMed]
- Tsouti, V.; Boutopoulos, C.; Zergioti, I.; Chatzandroulis, S. Capacitive Microsystems for Biological Sensing. Biosens. Bioelectron. 2011, 27, 1–11. [Google Scholar] [CrossRef]
- Fang, Y. Label-Free Receptor Assays. Drug Discov. Today: Technol. 2010, 7, e5–e11. [Google Scholar] [CrossRef] [Green Version]
- Mazlan, N.S.; Ramli, M.M.; Abdullah, M.M.A.B.; Halin, D.S.C.; Isa, S.S.M.; Talip, L.F.A.; Danial, N.S.; Murad, S.A.Z. Interdigitated Electrodes as Impedance and Capacitance Biosensors: A Review; AIP Publishing LLC.: Krabi, Thailand, 2017; p. 020276. [Google Scholar]
- Fang, Y. Label-Free Biosensors for Cell Biology. Int. J. Electrochem. 2011, 2011, 460850. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Wang, Y.; Feng, Q.; Wei, Y.; Ji, J.; Zhang, W. Progress of New Label-Free Techniques for Biosensors: A Review. Crit. Rev. Biotechnol. 2016, 36, 465–481. [Google Scholar] [CrossRef]
- Jin, K.; Zhao, P.; Fang, W.; Zhai, Y.; Hu, S.; Ma, H.; Li, J. An Impedance Sensor in Detection of Immunoglobulin G with Interdigitated Electrodes on Flexible Substrate. Appl. Sci. 2020, 10, 4012. [Google Scholar] [CrossRef]
- Zou, Z.; Kai, J.; Rust, M.J.; Han, J.; Ahn, C.H. Functionalized Nano Interdigitated Electrodes Arrays on Polymer with Integrated Microfluidics for Direct Bio-Affinity Sensing Using Impedimetric Measurement. Sens. Actuators A Phys. 2007, 136, 518–526. [Google Scholar] [CrossRef]
- Luo, X.; Davis, J.J. Electrical Biosensors and the Label Free Detection of Protein Disease Biomarkers. Chem. Soc. Rev. 2013, 42, 5944. [Google Scholar] [CrossRef] [PubMed]
- Khristunova, E.; Dorozhko, E.; Korotkova, E.; Kratochvil, B.; Vyskocil, V.; Barek, J. Label-Free Electrochemical Biosensors for the Determination of Flaviviruses: Dengue, Zika, and Japanese Encephalitis. Sensors 2020, 20, 4600. [Google Scholar] [CrossRef] [PubMed]
- Nashruddin, S.; Abdullah, J.; Mohammad Haniff, M.; Mat Zaid, M.; Choon, O.; Mohd Razip Wee, M. Label Free Glucose Electrochemical Biosensor Based on Poly(3,4-Ethylenedioxy Thiophene):Polystyrene Sulfonate/Titanium Carbide/Graphene Quantum Dots. Biosensors 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Hatada, M.; Loew, N.; Okuda-Shimazaki, J.; Khanwalker, M.; Tsugawa, W.; Mulchandani, A.; Sode, K. Development of an Interdigitated Electrode-Based Disposable Enzyme Sensor Strip for Glycated Albumin Measurement. Molecules 2021, 26, 734. [Google Scholar] [CrossRef]
- Yoo, J.; Jeong, H.; Park, S.K.; Park, S.; Lee, J.S. Interdigitated Electrode Biosensor Based on Plasma-Deposited TiO2 Nanoparticles for Detecting DNA. Biosensors 2021, 11, 212. [Google Scholar] [CrossRef]
- Lara, S.; Perez-Potti, A. Applications of Nanomaterials for Immunosensing. Biosensors 2018, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Hiep, H.M.; Nakayama, T.; Saito, M.; Yamamura, S.; Takamura, Y.; Tamiya, E. A Microfluidic Chip Based on Localized Surface Plasmon Resonance for Real-Time Monitoring of Antigen–Antibody Reactions. Jpn. J. Appl. Phys. 2008, 47, 1337–1341. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Tong, H.; Huang, Z.; Liu, R.; Li, X.; Qiang, O.; Tang, C. Affinity Analysis of Somatostatin and Somatostatin Receptor by Surface Plasmon Resonance. Anal. Methods 2013, 5, 3201. [Google Scholar] [CrossRef]
- Lerch, M.; Kamimori, H.; Folkers, G.; Aguilar, M.-I.; Beck-Sickinger, A.G.; Zerbe, O. Strongly Altered Receptor Binding Properties in PP and NPY Chimeras Are Accompanied by Changes in Structure and Membrane Binding. Biochemistry 2005, 44, 9255–9264. [Google Scholar] [CrossRef]
- Patel, S.N.; Ishahak, M.; Chaimov, D.; Velraj, A.; LaShoto, D.; Hagan, D.W.; Buchwald, P.; Phelps, E.A.; Agarwal, A.; Stabler, C.L. Organoid Microphysiological System Preserves Pancreatic Islet Function within 3D Matrix. Sci. Adv. 2021, 7, eaba5515. [Google Scholar] [CrossRef]
- Pang, H.; Luo, S.; Xiao, Y.; Xia, Y.; Li, X.; Huang, G.; Xie, Z.; Zhou, Z. Emerging Roles of Exosomes in T1DM. Front. Immunol. 2020, 11, 593348. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, M.; Brooks, R.W.; Boccuzzi, L.; Robbins, P.D.; Ricordi, C. Ricordi Exosomes as Biomarkers and Therapeutic Tools for Type 1 Diabetes Mellitus. Eur. Rev. Med. Pharm. Sci 2017, 21, 2940–2956. [Google Scholar]
- Hussain, M.W.A.; Jahangir, S.; Ghosh, B.; Yesmin, F.; Anis, A.; Satil, S.N.; Anwar, F.; Rashid, M.H. Exosomes for Regulation of Immune Responses and Immunotherapy. J. Nanotheranostics 2022, 3, 55–85. [Google Scholar] [CrossRef]
- Bauer, S.; Wennberg Huldt, C.; Kanebratt, K.P.; Durieux, I.; Gunne, D.; Andersson, S.; Ewart, L.; Haynes, W.G.; Maschmeyer, I.; Winter, A.; et al. Functional Coupling of Human Pancreatic Islets and Liver Spheroids On-a-Chip: Towards a Novel Human Ex Vivo Type 2 Diabetes Model. Sci. Rep. 2017, 7, 14620. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Deng, P.; Wang, Y.; Zhang, X.; Guo, Y.; Chen, W.; Qin, J. Microengineered Multi-Organoid System from HiPSCs to Recapitulate Human Liver-Islet Axis in Normal and Type 2 Diabetes. Adv. Sci. 2022, 9, 2103495. [Google Scholar] [CrossRef] [PubMed]
- Essaouiba, A.; Okitsu, T.; Kinoshita, R.; Jellali, R.; Shinohara, M.; Danoy, M.; Legallais, C.; Sakai, Y.; Leclerc, E. Development of a Pancreas-Liver Organ-on-Chip Coculture Model for Organ-to-Organ Interaction Studies. Biochem. Eng. J. 2020, 164, 107783. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, S.H.; Choi, N.; Sung, J.H. Construction of Pancreas–Muscle–Liver Microphysiological System (MPS) for Reproducing Glucose Metabolism. Biotechnol. Bioeng. 2019, 116, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Dholakia, S.; Royston, E.; Quiroga, I.; Sinha, S.; Reddy, S.; Gilbert, J.; Friend, P.J. The Rise and Potential Fall of Pancreas Transplantation. Br. Med. Bull. 2017, 124, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, I.; Valiente, L.; Shiang, K.-D.; Ichii, H.; Kandeel, F.; Al-Abdullah, I.H. The Effects of Digestion Enzymes on Islet Viability and Cellular Composition. Cell Transpl. 2012, 21, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Ponte, G.M.; Pileggi, A.; Messinger, S.; Alejandro, A.; Ichii, H.; Baidal, D.A.; Khan, A.; Ricordi, C.; Goss, J.A.; Alejandro, R. Toward Maximizing the Success Rates of Human Islet Isolation: Influence of Donor and Isolation Factors. Cell Transpl. 2007, 16, 595–607. [Google Scholar] [CrossRef]
- Sabbah, S.; Liew, A.; Brooks, A.M.; Kundu, R.; Reading, J.L.; Flatt, A.; Counter, C.; Choudhary, P.; Forbes, S.; Rosenthal, M.J.; et al. Autoreactive T Cell Profiles Are Altered Following Allogeneic Islet Transplantation with Alemtuzumab Induction and Re-emerging Phenotype Is Associated with Graft Function. Am. J. Transplant. 2021, 21, 1027–1038. [Google Scholar] [CrossRef]
- Barshes, N.R.; Wyllie, S.; Goss, J.A. Inflammation-Mediated Dysfunction and Apoptosis in Pancreatic Islet Transplantation: Implications for Intrahepatic Grafts. J. Leukoc. Biol. 2005, 77, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Vaithilingam, V.; Bal, S.; Tuch, B.E. Encapsulated Islet Transplantation: Where Do We Stand? Rev. Diabet. Stud. 2017, 14, 51–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, B.; Reichel, A.; Steffen, A.; Zimerman, B.; Schally, A.V.; Block, N.L.; Colton, C.K.; Ludwig, S.; Kersting, S.; Bonifacio, E.; et al. Transplantation of Human Islets without Immunosuppression. Proc. Natl. Acad. Sci. USA 2013, 110, 19054–19058. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Montanucci, P.; Calafiore, R. Microencapsulation of Cells and Molecular Therapy of Type 1 Diabetes Mellitus: The Actual State and Future Perspectives between Promise and Progress. J. Diabetes Investig. 2021, 12, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Tomei, A.A.; Villa, C.; Ricordi, C. Development of an Encapsulated Stem Cell-Based Therapy for Diabetes. Expert Opin. Biol. Ther. 2015, 15, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Atkinson, M.; von Herrath, M. Satisfaction (Not) Guaranteed: Re-Evaluating the Use of Animal Models of Type 1 Diabetes. Nat. Rev. Immunol. 2004, 4, 989–997. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, E.; Hebrok, M. Stem Cell-Based Clinical Trials for Diabetes Mellitus. Front. Endocrinol. 2021, 12, 631463. [Google Scholar] [CrossRef]
- Päth, G.; Perakakis, N.; Mantzoros, C.S.; Seufert, J. Stem Cells in the Treatment of Diabetes Mellitus—Focus on Mesenchymal Stem Cells. Metabolism 2019, 90, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of Diabetes with Insulin-Producing Cells Derived in Vitro from Human Pluripotent Stem Cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of Functional Human Pancreatic β Cells In Vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velazco-Cruz, L.; Song, J.; Maxwell, K.G.; Goedegebuure, M.M.; Augsornworawat, P.; Hogrebe, N.J.; Millman, J.R. Acquisition of Dynamic Function in Human Stem Cell-Derived β Cells. Stem Cell Rep. 2019, 12, 351–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millman, J.R.; Xie, C.; Van Dervort, A.; Gürtler, M.; Pagliuca, F.W.; Melton, D.A. Generation of Stem Cell-Derived β-Cells from Patients with Type 1 Diabetes. Nat. Commun. 2016, 7, 11463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, T.; Wang, Y.; Chen, W.; Li, Z.; Su, W.; Guo, Y.; Deng, P.; Qin, J. Engineering Human Islet Organoids from IPSCs Using an Organ-on-Chip Platform. Lab Chip 2019, 19, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Meng, H.; Lin, J.; Ji, W.; Xu, T.; Liu, H. Pancreatic Islet Organoids-on-a-Chip: How Far Have We Gone? J. Nanobiotechnol. 2022, 20, 308. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Melton, D.A. How to Make a Functional β-Cell. Development 2013, 140, 2472–2483. [Google Scholar] [CrossRef] [Green Version]
- Kelly, O.G.; Chan, M.Y.; Martinson, L.A.; Kadoya, K.; Ostertag, T.M.; Ross, K.G.; Richardson, M.; Carpenter, M.K.; D’Amour, K.A.; Kroon, E.; et al. Cell-Surface Markers for the Isolation of Pancreatic Cell Types Derived from Human Embryonic Stem Cells. Nat. Biotechnol. 2011, 29, 750–756. [Google Scholar] [CrossRef]
- Schulz, T.C.; Young, H.Y.; Agulnick, A.D.; Babin, M.J.; Baetge, E.E.; Bang, A.G.; Bhoumik, A.; Cepa, I.; Cesario, R.M.; Haakmeester, C.; et al. A Scalable System for Production of Functional Pancreatic Progenitors from Human Embryonic Stem Cells. PLoS ONE 2012, 7, e37004. [Google Scholar] [CrossRef]
- Vazin, T.; Freed, W.J. Human Embryonic Stem Cells: Derivation, Culture, and Differentiation: A Review. Restor. Neurol. Neurosci. 2010, 28, 589–603. [Google Scholar] [CrossRef]
- Lo, B.; Parham, L. Ethical Issues in Stem Cell Research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Kondo, Y.; Toyoda, T.; Inagaki, N.; Osafune, K. IPSC Technology-Based Regenerative Therapy for Diabetes. J. Diabetes Investig. 2018, 9, 234–243. [Google Scholar] [CrossRef]
- Shang, L.; Hua, H.; Foo, K.; Martinez, H.; Watanabe, K.; Zimmer, M.; Kahler, D.J.; Freeby, M.; Chung, W.; LeDuc, C.; et al. β-Cell Dysfunction Due to Increased ER Stress in a Stem Cell Model of Wolfram Syndrome. Diabetes 2014, 63, 923–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishi, M.; Nanjo, K. Insulin Gene Mutations and Diabetes: Insulin Gene Mutations and Diabetes. J. Diabetes Investig. 2011, 2, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, A.K.; Vimalraj, S.; Nandakumar, M.; Abdelalim, E.M. Insulin Resistance in Diabetes: The Promise of Using Induced Pluripotent Stem Cell Technology. World J. Stem Cells 2021, 13, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, T.; Mae, S.-I.; Tanaka, H.; Kondo, Y.; Funato, M.; Hosokawa, Y.; Sudo, T.; Kawaguchi, Y.; Osafune, K. Cell Aggregation Optimizes the Differentiation of Human ESCs and IPSCs into Pancreatic Bud-like Progenitor Cells. Stem Cell Res. 2015, 14, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Itakura, G.; Kawabata, S.; Ando, M.; Nishiyama, Y.; Sugai, K.; Ozaki, M.; Iida, T.; Ookubo, T.; Kojima, K.; Kashiwagi, R.; et al. Fail-Safe System against Potential Tumorigenicity after Transplantation of IPSC Derivatives. Stem Cell Rep. 2017, 8, 673–684. [Google Scholar] [CrossRef]
- Gutierrez-Aranda, I.; Ramos-Mejia, V.; Bueno, C.; Munoz-Lopez, M.; Real, P.J.; Mácia, A.; Sanchez, L.; Ligero, G.; Garcia-Parez, J.L.; Menendez, P. Human Induced Pluripotent Stem Cells Develop Teratoma More Efficiently and Faster than Human Embryonic Stem Cells Regardless the Site of Injection. Stem Cells 2010, 28, 1568–1570. [Google Scholar] [CrossRef] [Green Version]
- Arzouni, A.A.; Vargas-Seymour, A.; Nardi, N.; King, A.J.F.; Jones, P.M. Using Mesenchymal Stromal Cells in Islet Transplantation. Stem Cells Transl. Med. 2018, 7, 559–563. [Google Scholar] [CrossRef]
- Deschepper, M.; Oudina, K.; David, B.; Myrtil, V.; Collet, C.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Survival and Function of Mesenchymal Stem Cells (MSCs) Depend on Glucose to Overcome Exposure to Long-Term, Severe and Continuous Hypoxia. J. Cell. Mol. Med. 2011, 15, 1505–1514. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal Stem Cells Are Short-Lived and Do Not Migrate beyond the Lungs after Intravenous Infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef] [PubMed]





| Dietary Factors, Life Events, Lifestyle, Climatic Condition | Consequence | Ref. |
|---|---|---|
| Number of sunshine hours and climate/air temperature. | Lower sunshine hours and lower temperatures could be linked with onset of T1DM. | [30] |
| Vitamin D deficiency in pregnancy. | Lower levels of calciferol (vitamin D) during the third trimester of pregnancy could be associated with a higher risk of developing diabetes. | [31] |
| Sun exposure/Vitamin D deficiency. | Higher sun exposure associates with lower risk of developing T1DM. | [32] |
| Intensity of UVB radiation and the number of sunshine hours per day. | Higher UV-B exposure in the third trimester and first year of life appears to interact with lower T1DM associated risk. | [33,34] |
| Association between environmental factors and risk of T1DM. | Maternal age > 35, duration of > 6 months of cow milk feeding, and cesarean delivery were associated with T1DM. | [35] |
| Seasonal variation of T1DM. | T1DM diagnosis show a predominance in the cold months of the year. | [36] |
| Insulin Injection | Pancreatic Islets Transplant | Stem Cell Therapy |
|---|---|---|
| Lifesaving | Improved glycemic control | Virtually illimited source of insulin-producing cells |
| Decreased quality of life | Release dependence from exogenous insulin | Can help to improve transplant techniques success (e.g., MSCs, encapsulation) |
| Expensive | Mostly, safe | Autologous transplant (no immune rejection risk; hiPSCs) |
| Not accurate | Limited number of available viable cells for transplant | Precision medicine with primary cell line cultures directly from the patient’s own cells (hiPSCs) |
| Associated comorbidities | High rates of transplanted cell death | Ethical questions (hESCs) |
| Difficult vascularization | Difficult cell culture maintenance (hESCs & hiPSCs) | |
| May require immunosuppression treatments (cytotoxic) | Requires new methods to improve glycose stimuli monitoring | |
| Formation of teratomas | ||
| Very expensive | ||
| Requires the development of platforms for functionality and safety assessments |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Oliveira, S.M.; Rebocho, A.; Ahmadpour, E.; Nissapatorn, V.; de Lourdes Pereira, M. Type 1 Diabetes Mellitus: A Review on Advances and Challenges in Creating Insulin Producing Devices. Micromachines 2023, 14, 151. https://doi.org/10.3390/mi14010151
Rodrigues Oliveira SM, Rebocho A, Ahmadpour E, Nissapatorn V, de Lourdes Pereira M. Type 1 Diabetes Mellitus: A Review on Advances and Challenges in Creating Insulin Producing Devices. Micromachines. 2023; 14(1):151. https://doi.org/10.3390/mi14010151
Chicago/Turabian StyleRodrigues Oliveira, Sonia M., António Rebocho, Ehsan Ahmadpour, Veeranoot Nissapatorn, and Maria de Lourdes Pereira. 2023. "Type 1 Diabetes Mellitus: A Review on Advances and Challenges in Creating Insulin Producing Devices" Micromachines 14, no. 1: 151. https://doi.org/10.3390/mi14010151
APA StyleRodrigues Oliveira, S. M., Rebocho, A., Ahmadpour, E., Nissapatorn, V., & de Lourdes Pereira, M. (2023). Type 1 Diabetes Mellitus: A Review on Advances and Challenges in Creating Insulin Producing Devices. Micromachines, 14(1), 151. https://doi.org/10.3390/mi14010151










