Low-Grade Thermal Energy Harvesting and Self-Powered Sensing Based on Thermogalvanic Hydrogels
Abstract
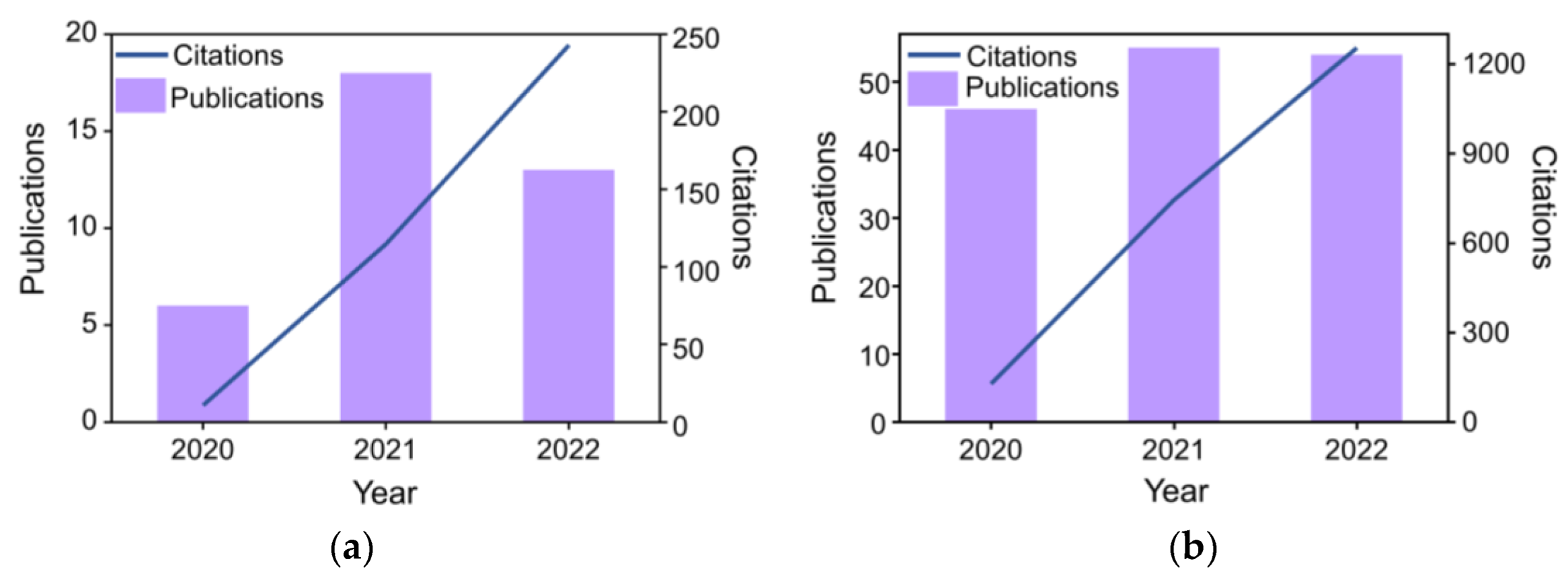
:1. Introduction
2. Improving the Efficiency of Single Thermocell
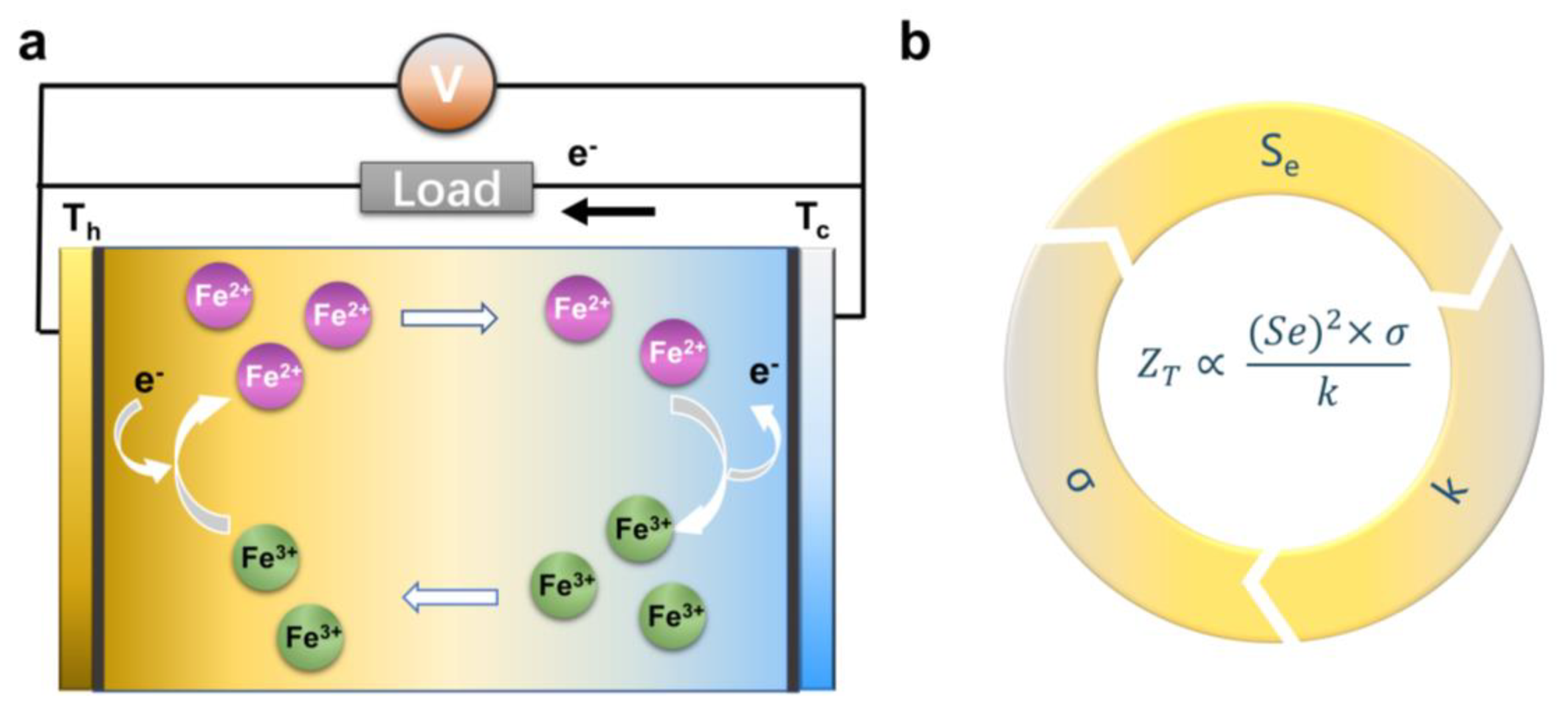
2.1. The Basic Principle of the Thermogalvanic Effect
2.2. Reaction Thermodynamics
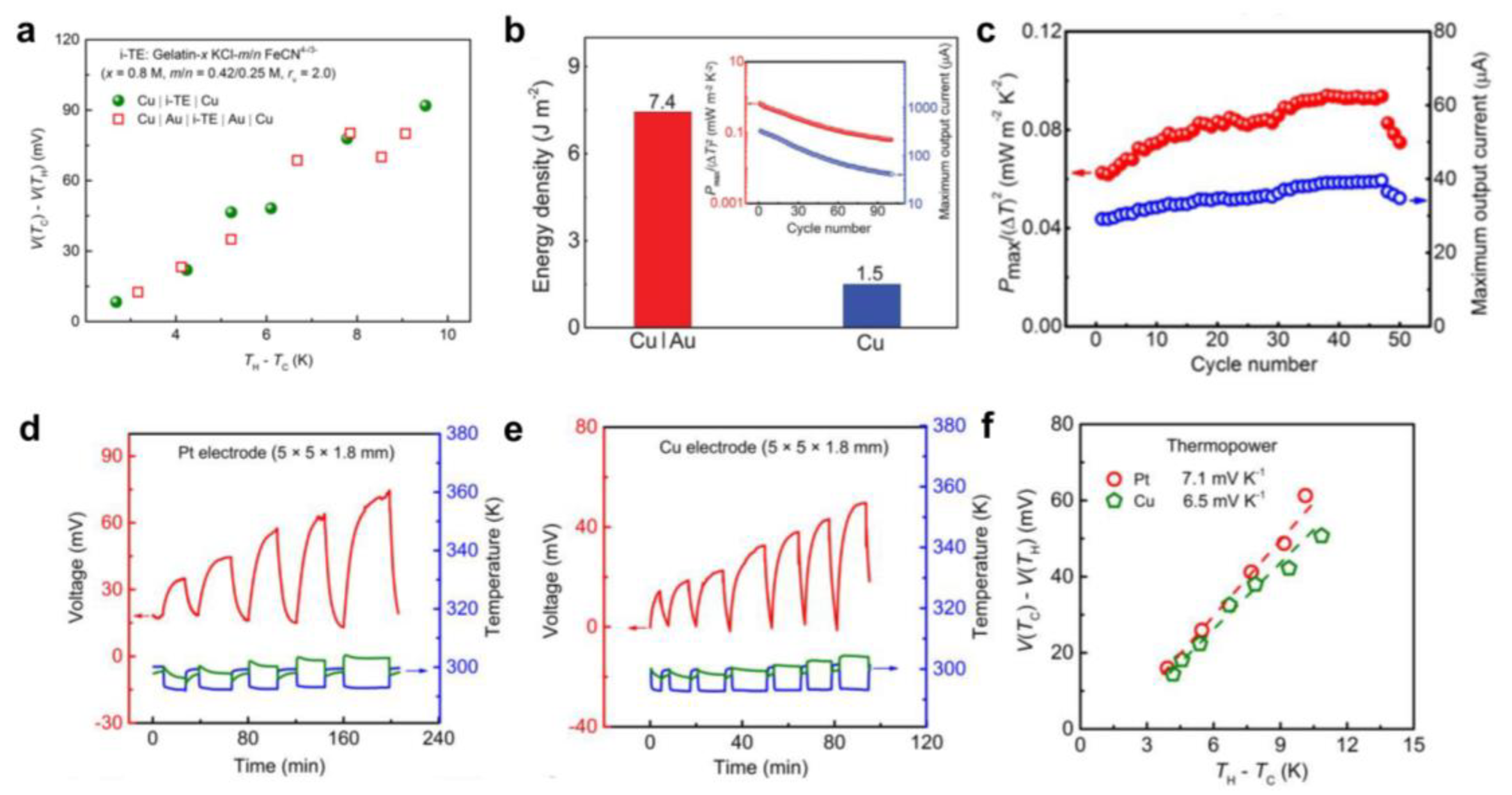
2.3. Thermopower Measurement and Calculation
2.4. Reaction Kinetics
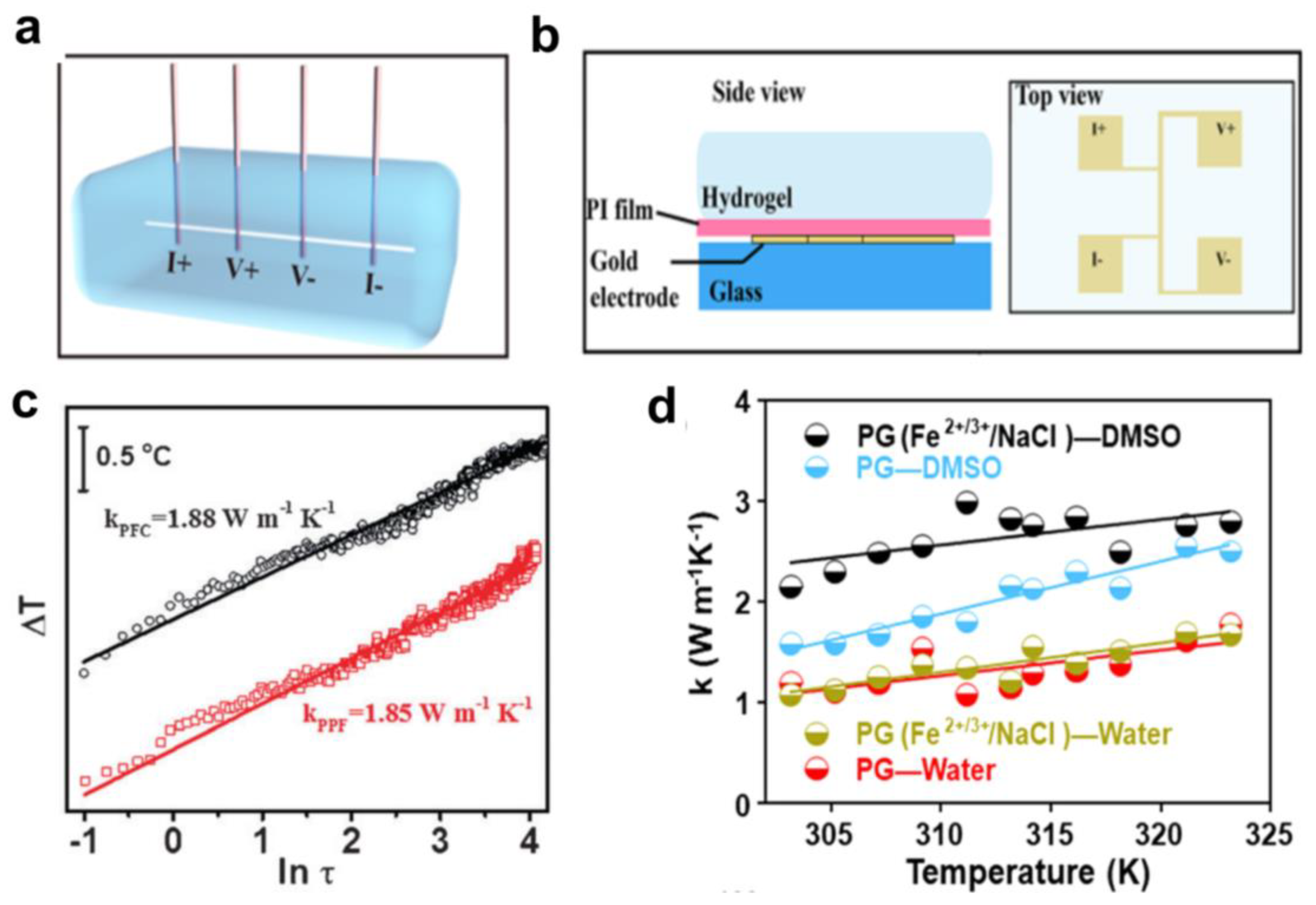
2.5. Thermal Transport in Hydrogels
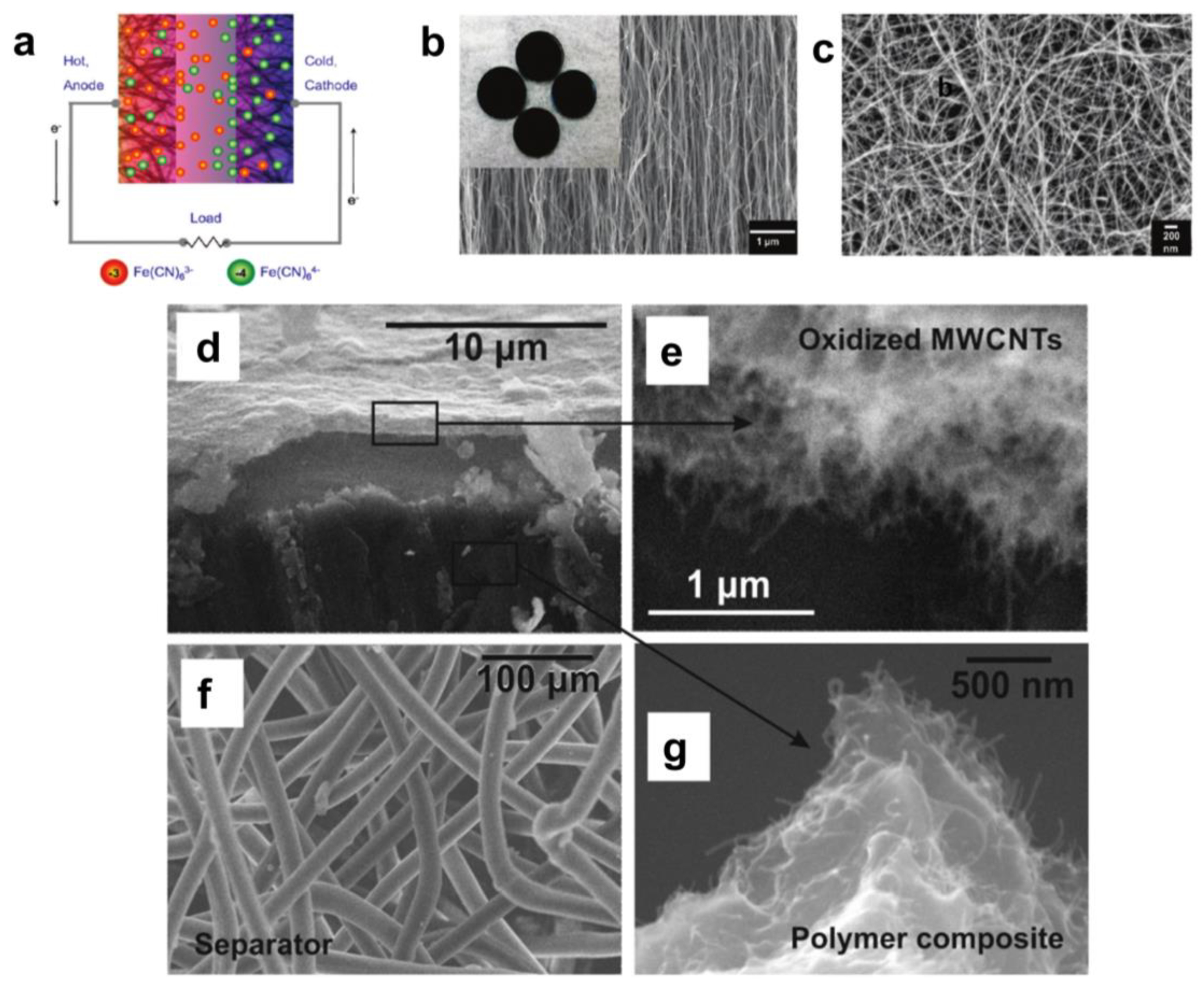
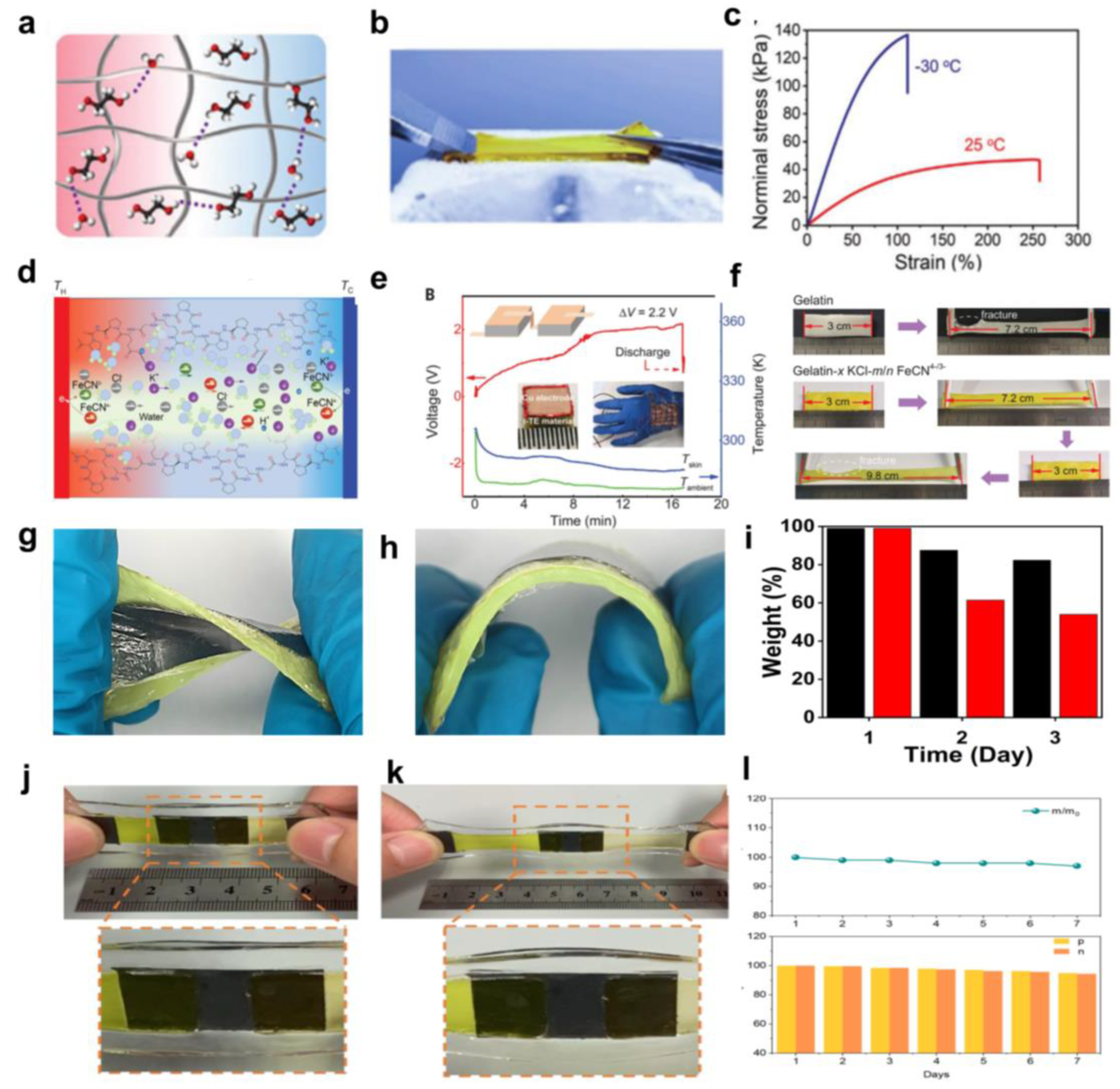
3. Selection of Polymer Networks and Encapsulation Methods
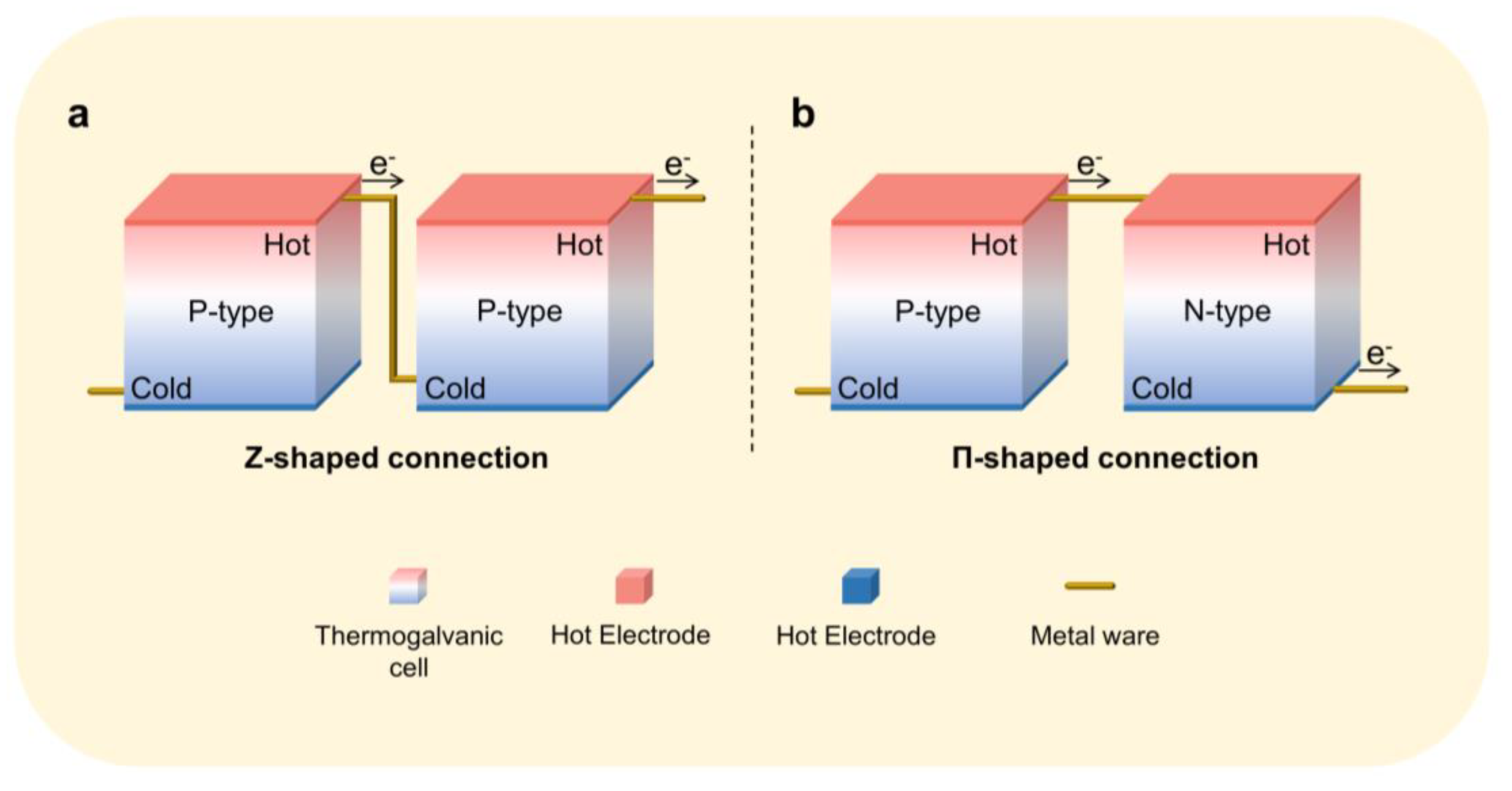
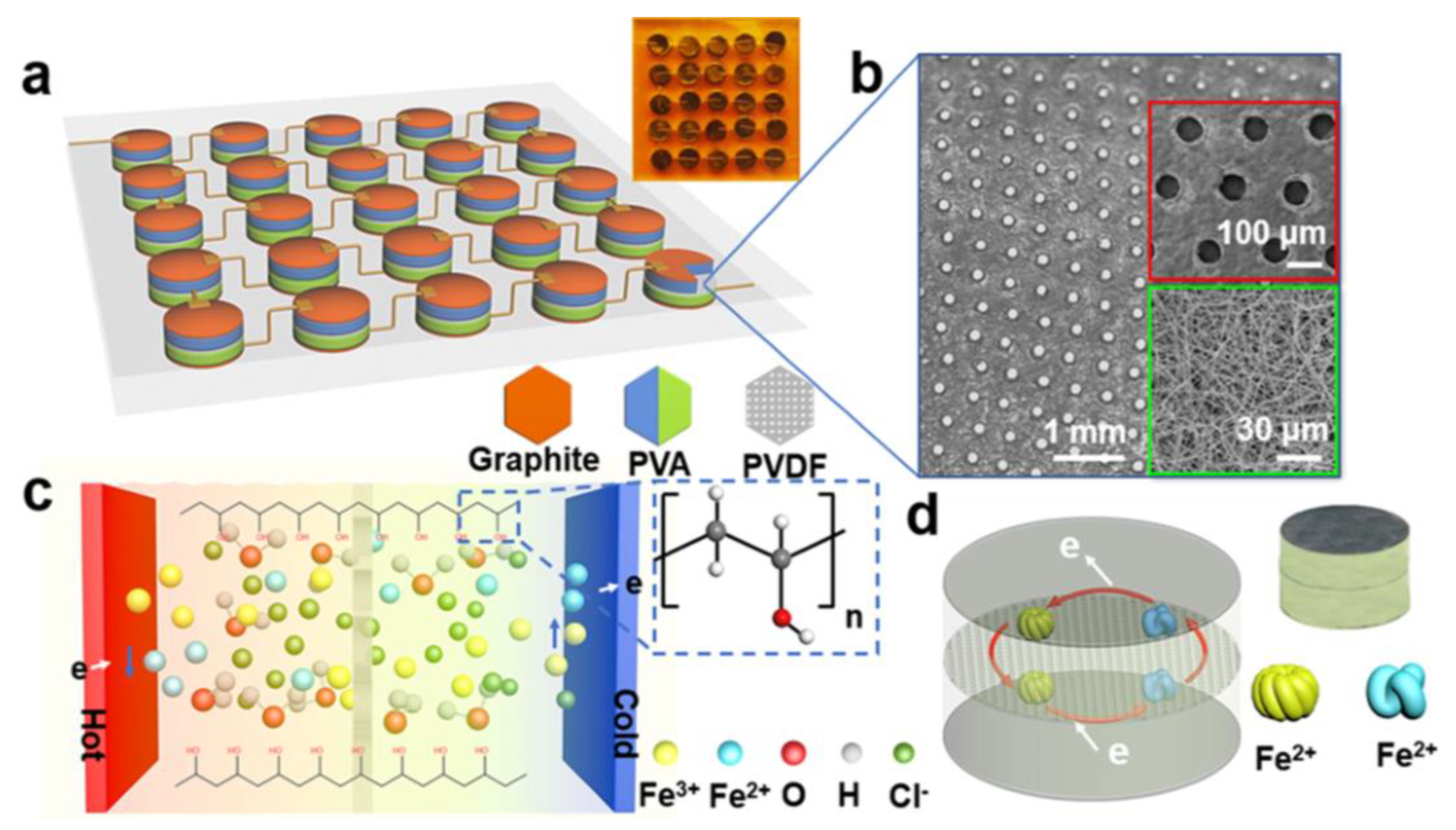
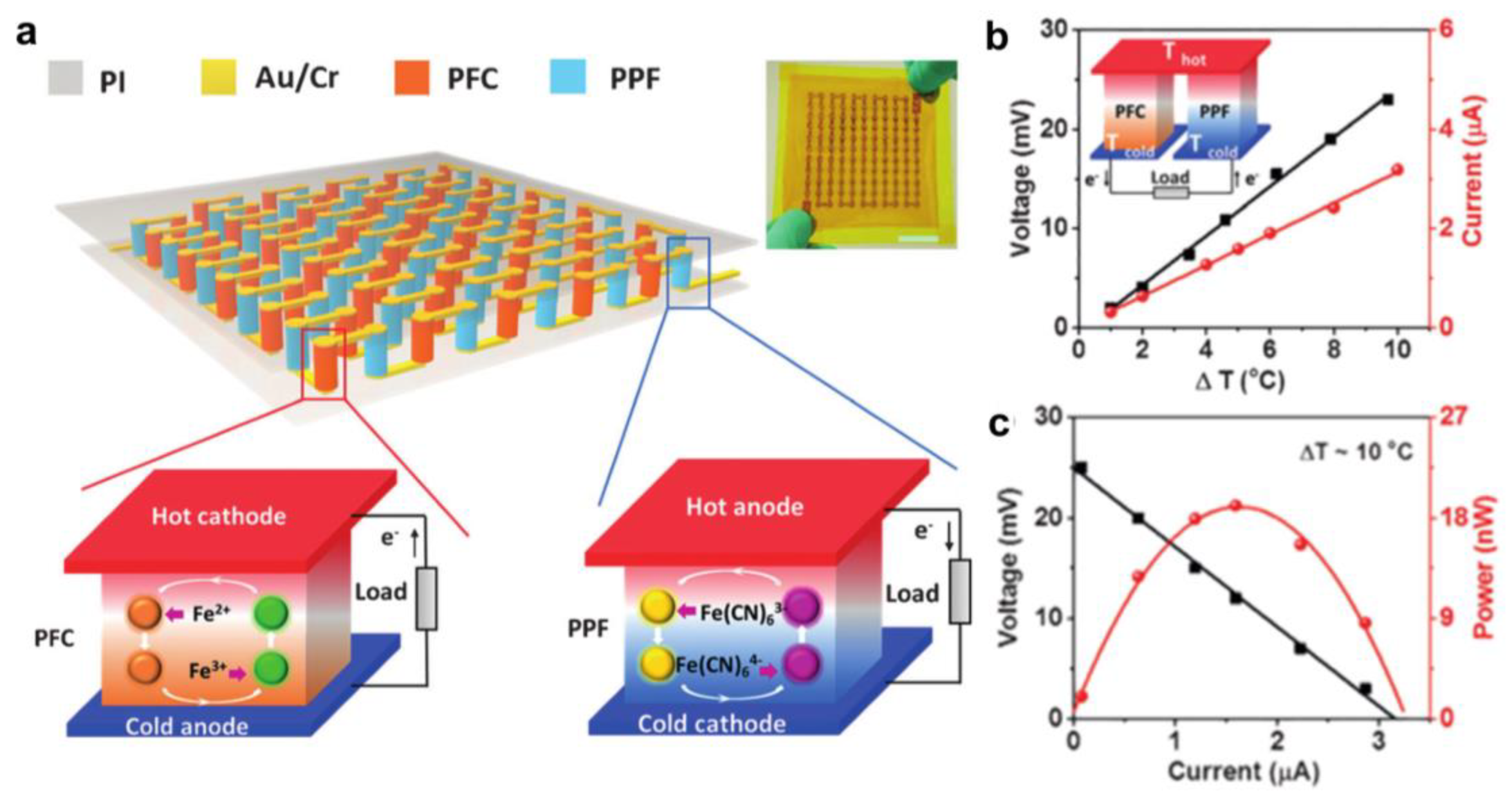
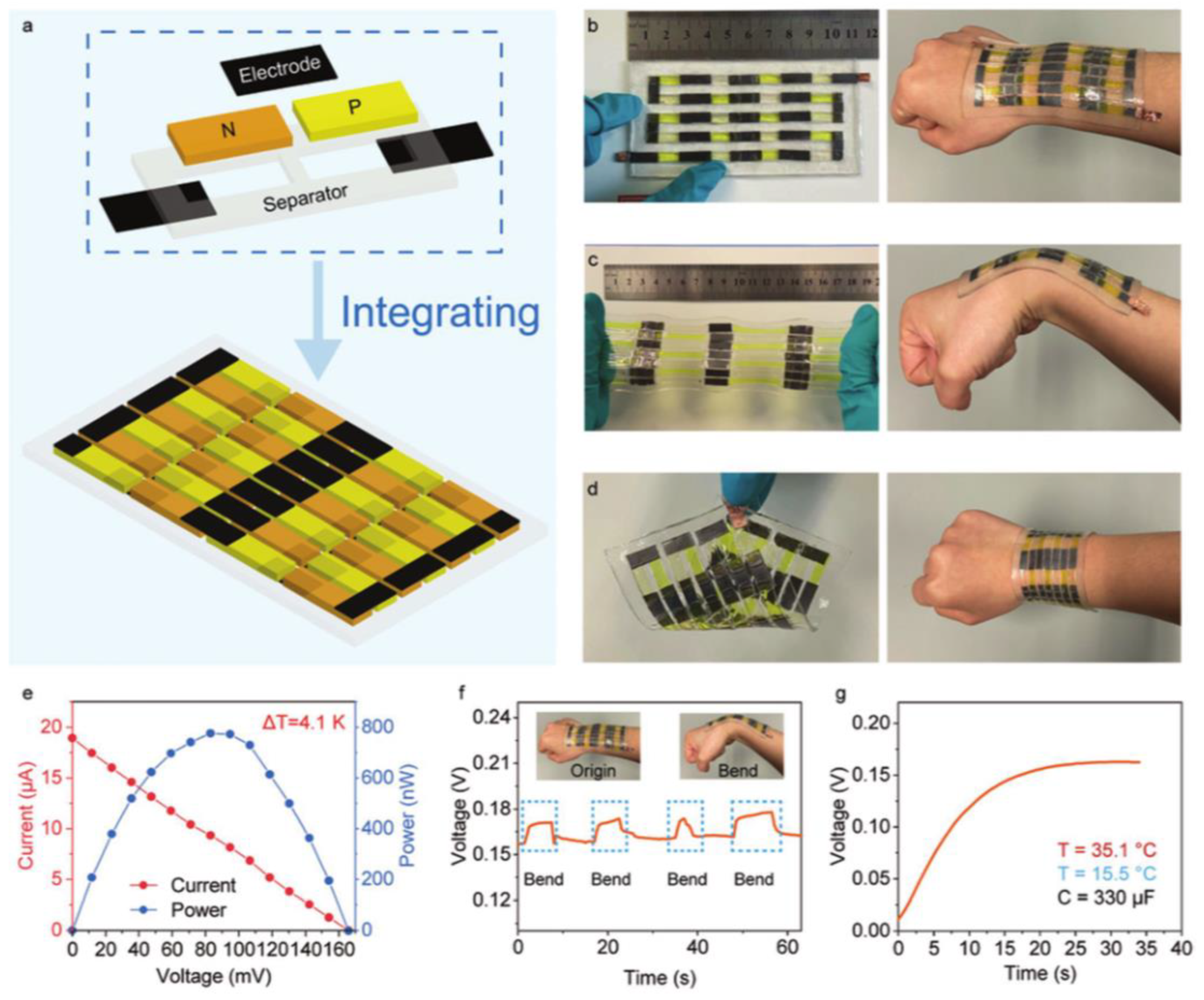
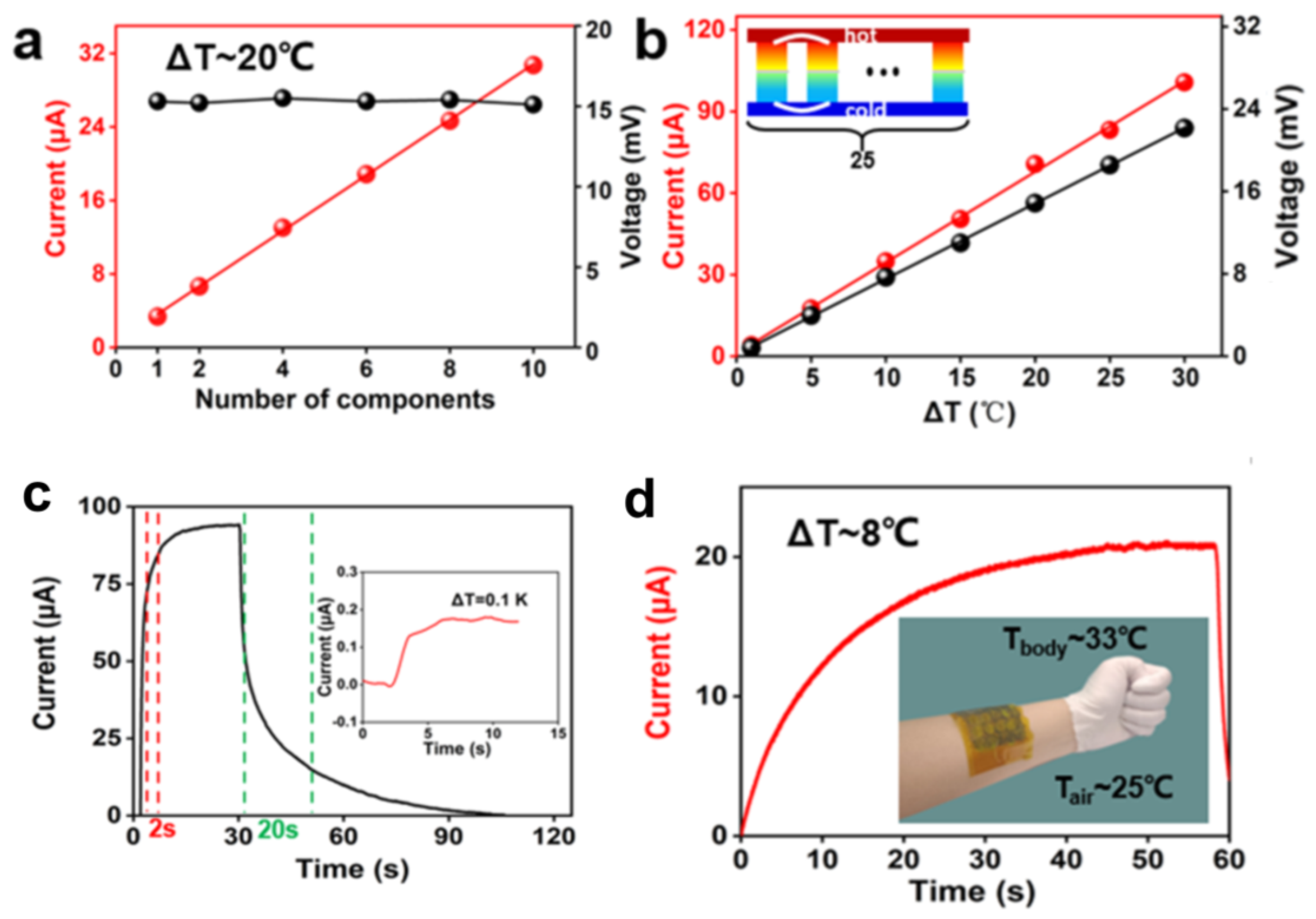
4. Integration of Thermoelectric Hydrogels
Cell Connection Methods
5. Potential Applications for Thermogalvanic Hydrogel Devices
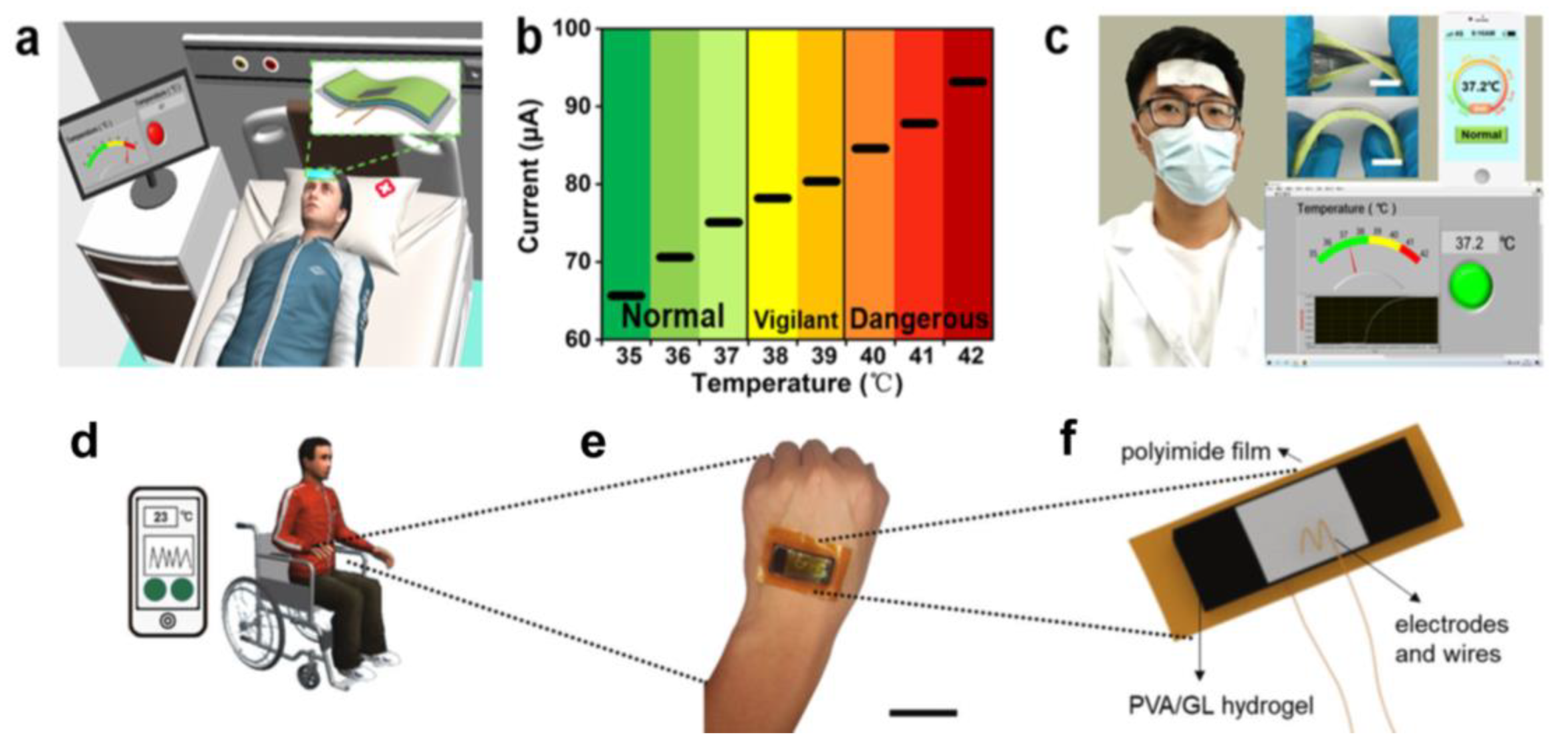
5.1. Body Temperature Sensors
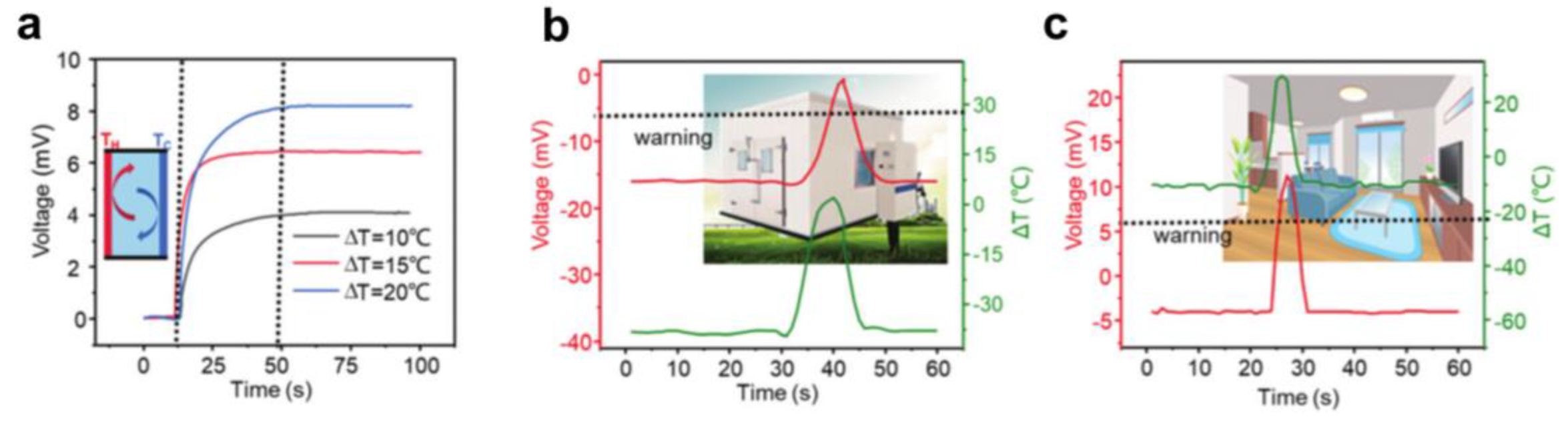
5.2. Environmental Temperature Monitoring
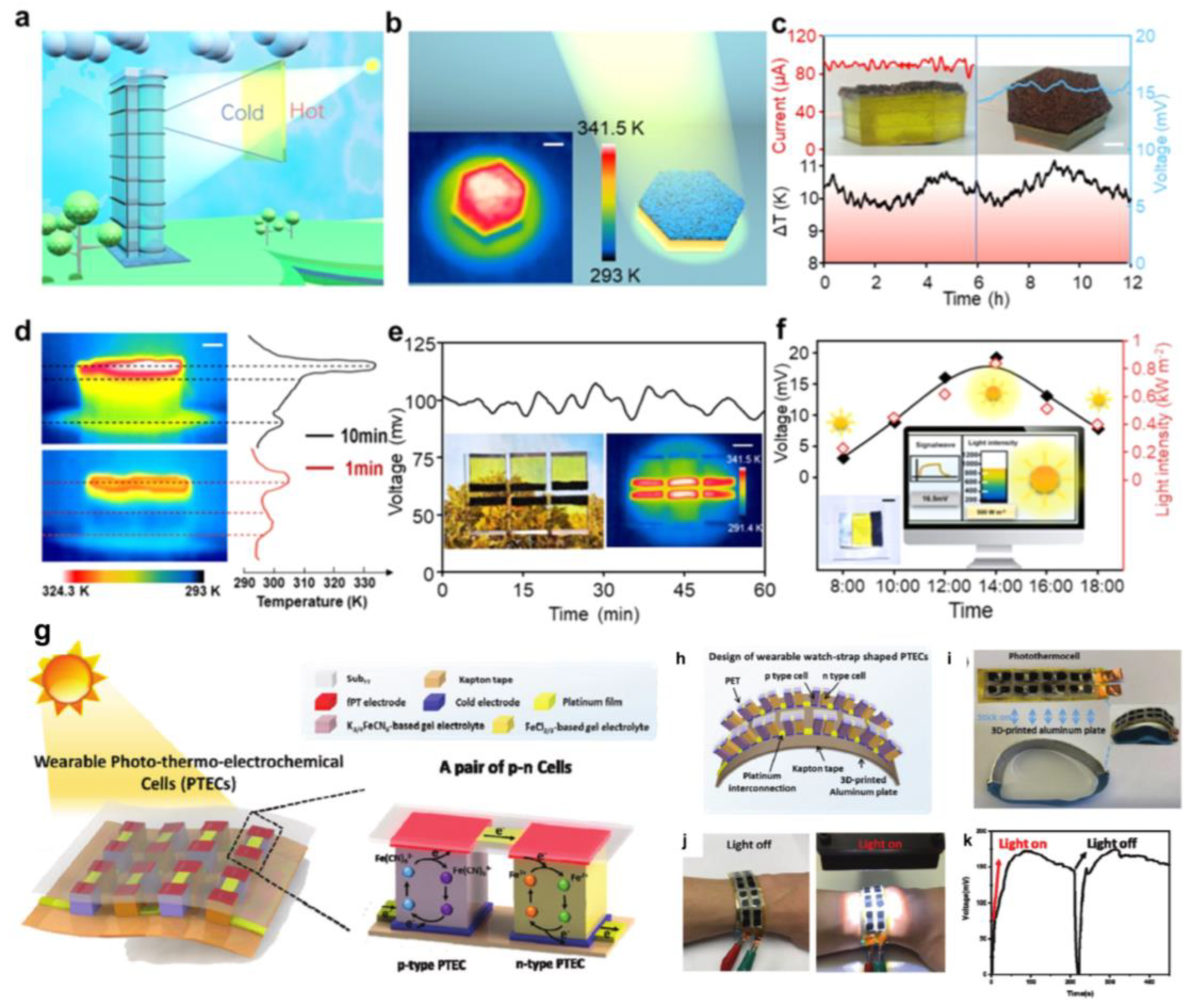
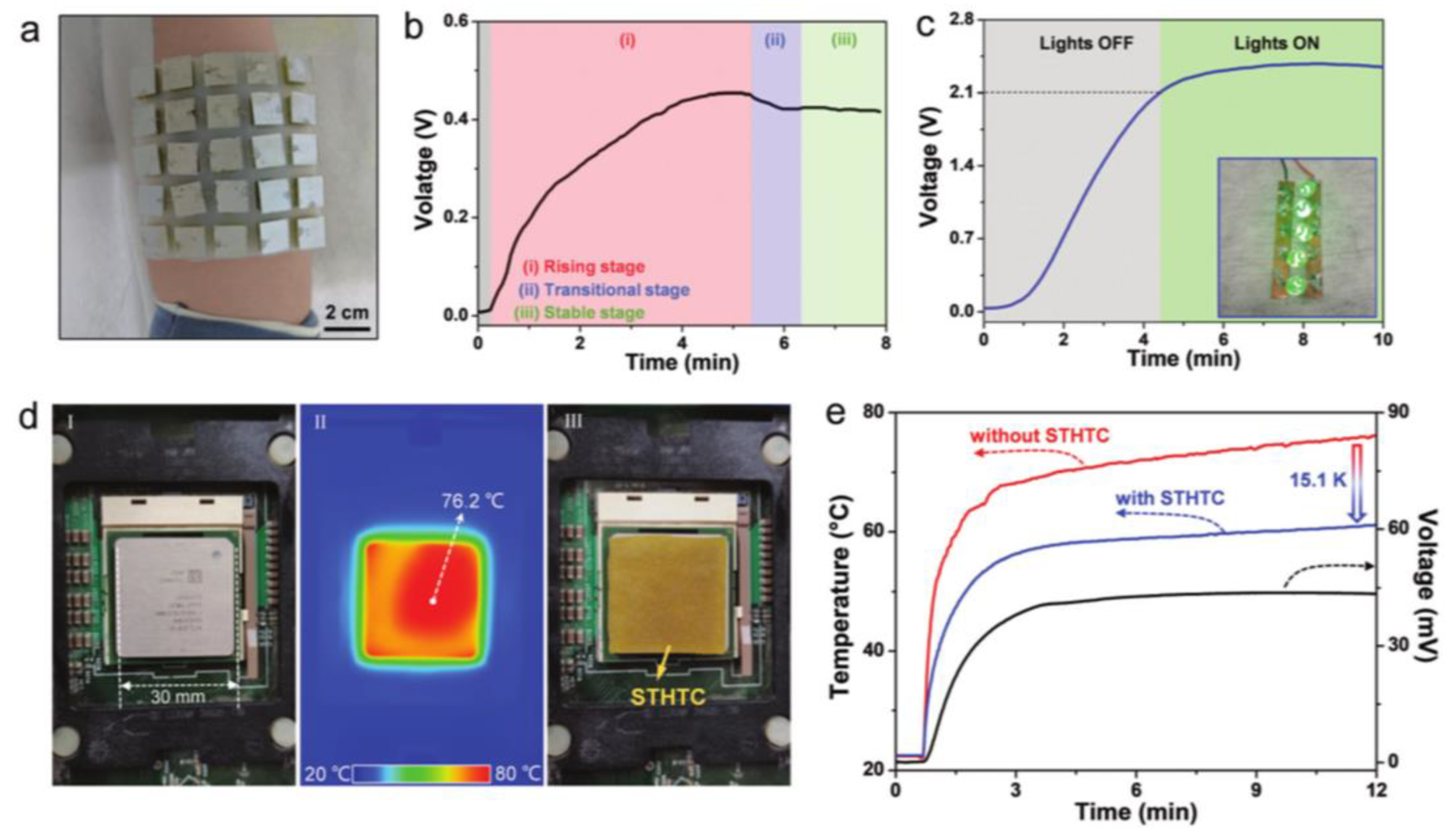
5.3. Solar Energy Collection
5.4. Body Heat Harvesting
5.5. Heat Generation Device Heat Harvesting
5.6. Integration with Other Systems
6. Opportunities, Challenges, and Future Directions
6.1. Opportunities
6.2. Challenges
6.3. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Black, J.J.; Aldous, L. Thermoelectrochemistry using conventional and novel gelled electrolytes in heat-to-current thermocells. Electrochim. Acta 2017, 225, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Abramovitz, A.; Shmilovitz, D. Short Survey of Architectures of Photovoltaic Arrays for Solar Power Generation Systems. Energies 2021, 14, 4917. [Google Scholar] [CrossRef]
- Ansu-Mensah, P.; Bein, M.A. Towards sustainable consumption: Predicting the impact of social-psychological factors on energy conservation intentions in Northern Cyprus. Nat. Resour. Forum 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Celik, A.N.; Ozgur, E. Review of Turkey’s photovoltaic energy status: Legal structure, existing installed power and comparative analysis. Renew. Sustain. Energy Rev. 2020, 134, 110344. [Google Scholar] [CrossRef]
- Demirbas, A.; Hashem, A.A.; Bakhsh, A.A. The cost analysis of electric power generation in Saudi Arabia. Energy Sources Part B Econ. Plan. Policy 2017, 12, 591–596. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Are wood pellets a green fuel? Science 2018, 359, 1328–1329. [Google Scholar] [CrossRef]
- Xiang, D.; Yang, S.; Li, X.; Qian, Y. Life cycle assessment of energy consumption and GHG emissions of olefins production from alternative resources in China. Energy Convers. Manag. 2015, 90, 12–20. [Google Scholar] [CrossRef]
- Opeyemi, B.M. Path to sustainable energy consumption: The possibility of substituting renewable energy for non-renewable energy. Energy 2021, 228, 120519. [Google Scholar] [CrossRef]
- Liu, X.; Elgowainy, A.; Wang, M. Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products. Green Chem. 2020, 22, 5751–5761. [Google Scholar] [CrossRef]
- Iqbal, S.; Wang, Y.; Shaikh, P.A.; Maqbool, A.; Hayat, K. Exploring the asymmetric effects of renewable energy production, natural resources, and economic progress on CO2 emissions: Fresh evidence from Pakistan. Environ. Sci. Pollut. Res. 2022, 29, 7067–7078. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Sherrell, P.C.; Liu, L.; Wang, Y.; Chen, J. Potentially Wearable Thermo-Electrochemical Cells for Body Heat Harvesting: From Mechanism, Materials, Strategies to Applications. Adv. Sci. 2021, 8, 2100669. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Yan, J.; Sun, J. Roles of Ionic Liquids in Adjusting Nature of Ionogels: A Mini Review. Adv. Funct. Mater. 2022, 32, 2203988. [Google Scholar] [CrossRef]
- Wu, B.; Guo, Y.; Hou, C.; Zhang, Q.; Li, Y.; Wang, H. High-Performance Flexible Thermoelectric Devices Based on All-Inorganic Hybrid Films for Harvesting Low-Grade Heat. Adv. Funct. Mater. 2019, 29, 1900304. [Google Scholar] [CrossRef]
- Duan, J.; Yu, B.; Huang, L.; Hu, B.; Xu, M.; Feng, G.; Zhou, J. Liquid-state thermocells: Opportunities and challenges for low-grade heat harvesting. Joule 2021, 5, 768–779. [Google Scholar] [CrossRef]
- Dupont, M.F.; MacFarlane, D.R.; Pringle, J.M. Thermo-electrochemical cells for waste heat harvesting—Progress and perspectives. Chem. Commun. 2017, 53, 6288–6302. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, M.; Ling, W.; Cheng, L.; Lei, H.; Li, W.; Huang, Y. Thermo-electrochemical cells for heat to electricity conversion: From mechanisms, materials, strategies to applications. Energy Environ. Sci. 2022, 15, 3670–3687. [Google Scholar] [CrossRef]
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef] [Green Version]
- Fathabadi, H. Replacing commercial thermoelectric generators with a novel electrochemical device in low-grade heat applications. Energy 2019, 174, 932–937. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Chu, C.; Ni, Y.; Neisiany, R.E.; You, Z. Biodegradable Elastomers and Gels for Elastic Electronics. Adv. Sci. 2022, 9, 2105146. [Google Scholar] [CrossRef]
- Rahimi, M.; Straub, A.P.; Zhang, F.; Zhu, X.; Elimelech, M.; Gorski, C.A.; Logan, B.E. Emerging electrochemical and membrane-based systems to convert low-grade heat to electricity. Energy Environ. Sci. 2018, 11, 276–285. [Google Scholar] [CrossRef]
- Battistel, A.; Peljo, P. Recent trends in thermoelectrochemical cells and thermally regenerative batteries. Curr. Opin. Electrochem. 2021, 30, 100853. [Google Scholar] [CrossRef]
- Burmistrov, I.; Khanna, R.; Gorshkov, N.; Kiselev, N.; Artyukhov, D.; Boychenko, E.; Yudin, A.; Konyukhov, Y.; Kravchenko, M.; Gorokhovsky, A.; et al. Advances in Thermo-Electrochemical (TEC) Cell Performances for Harvesting Low-Grade Heat Energy: A Review. Sustainability 2022, 14, 9483. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.-J.; Bocklund, B.; Shang, S.-L.; Zhou, B.-C.; Liu, Z.-K.; Chen, L.-Q. First-principles thermodynamic theory of Seebeck coefficients. Phys. Rev. B 2018, 98, 224101. [Google Scholar] [CrossRef] [Green Version]
- Baliban, R.C.; Elia, J.A.; Weekman, V.; Floudas, C.A. Process synthesis of hybrid coal, biomass, and natural gas to liquids via Fischer-Tropsch synthesis, ZSM-5 catalytic conversion, methanol synthesis, methanol-to-gasoline, and methanol-to-olefins/distillate technologies. Comput. Chem. Eng. 2012, 47, 29–56. [Google Scholar] [CrossRef]
- Moioli, E.; Schildhauer, T. Negative CO2 emissions from flexible biofuel synthesis: Concepts, potentials, technologies. Renew. Sustain. Energy Rev. 2022, 158, 112120. [Google Scholar] [CrossRef]
- Ohno, H.; Ikhlayel, M.; Tamura, M.; Nakao, K.; Suzuki, K.; Morita, K.; Kato, Y.; Tomishige, K.; Fukushima, Y. Direct dimethyl carbonate synthesis from CO2 and methanol catalyzed by CeO2 and assisted by 2-cyanopyridine: A cradle-to-gate greenhouse gas emission study. Green Chem. 2021, 23, 457–469. [Google Scholar] [CrossRef]
- Timmerman, J.; Hennen, M.; Bardow, A.; Lodewijks, P.; Vandevelde, L.; Van Eetvelde, G. Towards low carbon business park energy systems: A holistic techno-economic optimisation model. Energy 2017, 125, 747–770. [Google Scholar] [CrossRef]
- Villarroel-Schneider, J.; Hoglund-Isaksson, L.; Mainali, B.; Marti-Herrero, J.; Cardozo, E.; Malmquist, A.; Martin, A. Energy self-sufficiency and greenhouse gas emission reductions in Latin American dairy farms through massive implementation of biogas-based solutions. Energy Convers. Manag. 2022, 261, 115670. [Google Scholar] [CrossRef]
- Buckingham, M.A.; Stoffel, F.; Zhang, S.; Liu, Y.; Marken, F.; Chen, J.; Aldous, L. Nanostructuring Electrode Surfaces and Hydrogels for Enhanced Thermocapacitance. ACS Appl. Nano Mater. 2022, 5, 438–445. [Google Scholar] [CrossRef]
- Liu, H.; Lai, W.-H.; Lei, Y.; Yang, H.; Wang, N.; Chou, S.; Liu, H.K.; Dou, S.X.; Wang, Y.-X. Electrolytes/Interphases: Enabling Distinguishable Sulfur Redox Processes in Room-Temperature Sodium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103304. [Google Scholar] [CrossRef]
- Pang, L.; Wang, H. Inorganic Aqueous Anionic Redox Liquid Electrolyte for Supercapacitors. Adv. Mater. Technol. 2022, 7, 2100501. [Google Scholar] [CrossRef]
- Yang, N.; Yu, S.; Zhang, W.; Cheng, H.-M.; Simon, P.; Jiang, X. Electrochemical Capacitors with Confined Redox Electrolytes and Porous Electrodes. Adv. Mater. 2022, 34, 2202380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Bai, P.; Ji, X.; Yang, J.; Wang, C.; Xu, Y. Electrolytes and Interphases in Potassium Ion Batteries. Adv. Mater. 2021, 33, 2003741. [Google Scholar] [CrossRef]
- Mondal, H.; Karmakar, M.; Chattopadhyay, P.K.; Halder, A.; Singha, N.R. Scale-up one-pot synthesis of waste collagen and apple pomace pectin incorporated pentapolymer biocomposites: Roles of waste collagen for elevations of properties and unary/ternary removals of Ti(IV), As(V), and V (V). J. Hazard. Mater. 2021, 409, 124873. [Google Scholar] [CrossRef]
- Mondal, H.; Karmakar, M.; Chattopadhyay, P.K.; Singha, N.R. New property-performance optimization of scalable alginate-g-terpolymer for Ce(IV), Mo(VI), and W(VI) exclusions. Carbohydr. Polym. 2020, 245, 116370. [Google Scholar] [CrossRef]
- Pu, S.; Liao, Y.; Chen, K.; Fu, J.; Zhang, S.; Ge, L.; Conta, G.; Bouzarif, S.; Cheng, T.; Hu, X.; et al. Thermogalvanic Hydrogel for Synchronous Evaporative Cooling and Low-Grade Heat Energy Harvesting. Nano Lett. 2020, 20, 3791–3797. [Google Scholar] [CrossRef]
- Shi, X.; He, J. Thermopower and harvesting heat. Science 2021, 371, 343–344. [Google Scholar] [CrossRef]
- Szuromi, P. Solar heat helps harvest humidity. Science 2017, 356, 392–394. [Google Scholar] [CrossRef]
- Yu, J.; Ma, T. Triboelectricity-based self-charging droplet capacitor for harvesting low-level ambient energy. Nano Energy 2020, 74, 104795. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Long, R.; Liu, Z.; Liu, W. Review of osmotic heat engines for low-grade heat harvesting. Desalination 2022, 527, 115571. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.; Moghaddam, M.A.E.; Asadi, A.; Mogharrebi, A.R.; Jafari, B.; Hasani, M.R.; Ganji, D.D. Effect of two different fins (longitudinal-tree like) and hybrid nano-particles (MoS2—TiO2) on solidification process in triplex latent heat thermal energy storage system. Alex. Eng. J. 2021, 60, 1967–1979. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, I.A. Pyroelectric nanogenerators (PyNGs) in converting thermal energy into electrical energy: Fundamentals and current status. Nano Energy 2021, 84, 105888. [Google Scholar] [CrossRef]
- Shi, W.; Pinto, B. Energy Savings Through Thermally Efficient Crucible Technology: Fundamentals, Process Modeling, and Applications. Jom 2017, 69, 2797–2802. [Google Scholar] [CrossRef]
- Trosheva, M.A.; Buckingham, M.A.; Aldous, L. Direct measurement of the genuine efficiency of thermogalvanic heat-to-electricity conversion in thermocells. Chem. Sci. 2022, 13, 4984–4998. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, H.; Wang, S.; Jiang, L.; Sun, G. A solid state thermogalvanic cell harvesting low-grade thermal energy. Int. J. Hydrogen Energy 2017, 42, 25877–25881. [Google Scholar] [CrossRef]
- Buckingham, M.A.; Zhang, S.; Liu, Y.; Chen, J.; Marken, F.; Aldous, L. Thermogalvanic and Thermocapacitive Behavior of Superabsorbent Hydrogels for Combined Low-Temperature Thermal Energy Conversion and Harvesting. ACS Appl. Energy Mater. 2021, 4, 11204–11214. [Google Scholar] [CrossRef]
- Khoury, A.; De Luca, A.; Sall, F.S.; Pazart, L.; Capellier, G. Performance of manual ventilation: How to define its efficiency in bench studies? A review of the literature. Anaesthesia 2015, 70, 985–992. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Yin, D.; Schiavone, P. Conversion efficiency and effective properties of particulate-reinforced thermoelectric composites. Z. Angew. Math. Phys. 2020, 71, 54. [Google Scholar] [CrossRef]
- Berardan, D.; Franger, S.; Dragoe, D.; Meena, A.K.; Dragoe, N. Colossal dielectric constant in high entropy oxides. Phys. Status Solidi-Rapid Res. Lett. 2016, 10, 328–333. [Google Scholar] [CrossRef]
- Carothers, K.J.; Norwood, R.A.; Pyun, J. High Verdet Constant Materials for Magneto-Optical Faraday Rotation: A Review. Chem. Mater. 2022, 34, 2531–2544. [Google Scholar] [CrossRef]
- Ghosh, G.; Chakraborty, A.; Pal, P.; Jana, B.; Ghosh, S. Direct Participation of Solvent Molecules in the Formation of Supramolecular Polymers. Chem. A Eur. J. 2022, 28, e202201082. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Muy, S.; Feng, S.; Katayama, Y.; Lu, Y.-C.; Chen, G.; Shao-Horn, Y. Non-covalent interactions in electrochemical reactions and implications in clean energy applications. Phys. Chem. Chem. Phys. 2018, 20, 15680–15686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renjith, A.; Lakshminarayanan, V. Electron-Transfer Studies of Model Redox-Active Species (Cationic, Anionic, and Neutral) in Deep Eutectic Solvents. J. Phys. Chem. C 2018, 122, 25411–25421. [Google Scholar] [CrossRef]
- Xu, C.; Sun, Y.; Zhang, J.; Xu, W.; Tian, H. Adaptable and Wearable Thermocell Based on Stretchable Hydrogel for Body Heat Harvesting. Adv. Energy Mater. 2022, 12, 2201542. [Google Scholar] [CrossRef]
- Yang, P.; Liu, K.; Chen, Q.; Mo, X.; Zhou, Y.; Li, S.; Feng, G.; Zhou, J. Wearable Thermocells Based on Gel Electrolytes for the Utilization of Body Heat. Angew. Chem. Int. Ed. 2016, 55, 12050–12053. [Google Scholar] [CrossRef]
- Kiran, S.R.; Basha, C.H.; Kumbhar, A.; Patil, N. A new design of single switch DC-DC converter for PEM fuel cell based EV system with variable step size RBFN controller. Sadhana Acad. Proc. Eng. Sci. 2022, 47, 128. [Google Scholar] [CrossRef]
- Kwan, T.H.; Wu, X.; Yao, Q. Multi-objective genetic optimization of the thermoelectric system for thermal management of proton exchange membrane fuel cells. Appl. Energy 2018, 217, 314–327. [Google Scholar] [CrossRef]
- Ressel, S.; Laube, A.; Fischer, S.; Chica, A.; Flower, T.; Struckmann, T. Performance of a vanadium redox flow battery with tubular cell design. J. Power Sources 2017, 355, 199–205. [Google Scholar] [CrossRef]
- Yeo, J.; Kim, T.; Jang, J.K.; Yang, Y. Practical Maximum-Power Extraction in Single Microbial Fuel Cell by Effective Delivery through Power Management System. Energies 2018, 11, 2312. [Google Scholar] [CrossRef]
- Basu, D.; Basu, S. Mathematical modeling of overpotentials of direct glucose alkaline fuel cell and experimental validation. J. Solid State Electrochem. 2013, 17, 2927–2938. [Google Scholar] [CrossRef]
- Bethune, K.; St-Pierre, J.; LaManna, J.M.; Hussey, D.S.; Jacobson, D.L. Contamination Mechanisms of Proton Exchange Membrane Fuel Cells—Mass Transfer Overpotential Origin. J. Phys. Chem. C 2020, 124, 24052–24065. [Google Scholar] [CrossRef]
- Feng, D.; Bao, C.; Gao, T. Prediction of overpotential and concentration profiles in solid oxide fuel cell based on improved analytical model of charge and mass transfer. J. Power Sources 2020, 449, 227499. [Google Scholar] [CrossRef]
- Bennett, S.; Vaquer-Sunyer, R.; Jorda, G.; Forteza, M.; Roca, G.; Marba, N. Thermal Performance of Seaweeds and Seagrasses Across a Regional Climate Gradient. Front. Mar. Sci. 2022, 9, 733315. [Google Scholar] [CrossRef]
- Qiao, H.W.; Yang, S.; Wang, Y.; Chen, X.; Wen, T.Y.; Tang, L.J.; Cheng, Q.; Hou, Y.; Zhao, H.; Yang, H.G. A Gradient Heterostructure Based on Tolerance Factor in High-Performance Perovskite Solar Cells with 0.84 Fill Factor. Adv. Mater. 2019, 31, 1804217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Patel, Y.; Zhang, T.; Offer, G.J. Modeling the Effects of Thermal Gradients Induced by Tab and Surface Cooling on Lithium Ion Cell Performance. J. Electrochem. Soc. 2018, 165, A3169–A3178. [Google Scholar] [CrossRef]
- An, Y.; Li, Z.; Sun, Y.; Li, S.; Xu, Y.; Dou, H.; Zhang, X. Thermally Chargeable Ammonium-Ion Capacitor for Energy Storage and Low-Grade Heat Harvesting. Batter. Supercaps 2022, 5, e202200036. [Google Scholar] [CrossRef]
- Gao, W.; Meng, H.; Chen, Y.; Liu, X. Quasi-solid n-type thermogalvanic thermocells with enhanced ionic conductivity for continuous low-grade heat harvesting. Appl. Phys. Lett. 2022, 121, 203902. [Google Scholar] [CrossRef]
- Lv, J.; Jia, H.; Chen, G.; Wang, Y.; Liu, M.; Ning, Y.; Wang, Y.; Yuan, L.; Lu, M.; Zhang, J. Pressure-Engineered Ti3C2Tx MXene with Enhanced Conductivity and Accelerated Reaction Kinetics of Lithium Storage. ACS Appl. Mater. Interfaces 2022, 14, 46056–46067. [Google Scholar] [CrossRef]
- Shen, J.; Ma, Y.; Yang, C.; Liu, S.; Li, J.; Chen, Z.; Tian, B.; Li, S. Boosting solar-thermal-electric conversion of thermoelectrochemical cells by construction of a carboxymethylcellulose-interpenetrated polyacrylamide network. J. Mater. Chem. A 2022, 10, 7785–7791. [Google Scholar] [CrossRef]
- Klaers, J.; Faelt, S.; Imamoglu, A.; Togan, E. Squeezed Thermal Reservoirs as a Resource for a Nanomechanical Engine beyond the Carnot Limit. Phys. Rev. X 2017, 7, 031044. [Google Scholar] [CrossRef]
- Lucia, U. Unreal perpetual motion machine, Rydberg constant and Carnot non-unitary efficiency as a consequence of the atomic irreversibility. Phys. A Stat. Mech. Its Appl. 2018, 492, 962–968. [Google Scholar] [CrossRef]
- Tonner, F.; Mahler, G. Quantum limit of the Carnot engine. Fortschr. Phys. Prog. Phys. 2006, 54, 939–956. [Google Scholar] [CrossRef]
- Yu, B.; Duan, J.; Cong, H.; Xie, W.; Liu, R.; Zhuang, X.; Wang, H.; Qi, B.; Xu, M.; Wang, Z.L.; et al. Thermosensitive crystallization-boosted liquid thermocells for low-grade heat harvesting. Science 2020, 370, 342–346. [Google Scholar] [CrossRef]
- Zhou, H.; Yamada, T.; Kimizuka, N. Supramolecular Thermo-Electrochemical Cells: Enhanced Thermoelectric Performance by Host-Guest Complexation and Salt-Induced Crystallization. J. Am. Chem. Soc. 2016, 138, 10502–10507. [Google Scholar] [CrossRef]
- Li, C.; Jiang, F.; Liu, C.; Liu, P.; Xu, J. Present and future thermoelectric materials toward wearable energy harvesting. Appl. Mater. Today 2019, 15, 543–557. [Google Scholar] [CrossRef]
- Rodriguez, J.C.; Nico, V.; Punch, J. A Vibration Energy Harvester and Power Management Solution for Battery-Free Operation of Wireless Sensor Nodes. Sensors 2019, 19, 3776. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Yao, H.; Cui, Y.; Yu, R.; Lin, Y.-W.; Chen, T.-W.; Xu, Y.; Qin, J.; Hsu, C.-S.; Ge, Z.; et al. Simultaneous Improvement of Efficiency and Stability of Organic Photovoltaic Cells by using a Cross-Linkable Fullerene Derivative. Small 2021, 17, 2101133. [Google Scholar] [CrossRef]
- Mbumba, M.T.; Malouangou, D.M.; Tsiba, J.M.; Akram, M.W.; Bai, L.; Yang, Y.; Guli, M. Compositional engineering solutions for decreasing trap state density and improving thermal stability in perovskite solar cells. J. Mater. Chem. C 2021, 9, 14047–14064. [Google Scholar] [CrossRef]
- Hirbodvash, Z.; Houache, M.S.E.; Krupin, O.; Khodami, M.; Northfield, H.; Olivieri, A.; Baranova, E.A.; Berini, P. Electrochemical Performance of Lithographically-Defined Micro-Electrodes for Integration and Device Applications. Chemosensors 2021, 9, 277. [Google Scholar] [CrossRef]
- Lee, D.; Kwak, M.; Moon, K.; Choi, W.; Park, J.; Yoo, J.; Song, J.; Lim, S.; Sung, C.; Banerjee, W.; et al. Various Threshold Switching Devices for Integrate and Fire Neuron Applications. Adv. Electron. Mater. 2019, 5, 1800866. [Google Scholar] [CrossRef]
- Gao, W.; Lei, Z.; Zhang, C.; Liu, X.; Chen, Y. Stretchable and Freeze-Tolerant Organohydrogel Thermocells with Enhanced Thermoelectric Performance Continually Working at Subzero Temperatures. Adv. Funct. Mater. 2021, 31, 2104071. [Google Scholar] [CrossRef]
- Angeles Mejia, S.; Baiza Gutman, L.A.; Ortega Camarillo, C.; Medina Navarro, R.; Sanchez Becerra, M.C.; Damasio Santana, L.; Cruz, M.; Hernandez Perez, E.; Diaz Flores, M. Nicotinamide prevents sweet beverage-induced hepatic steatosis in rats by regulating the G6PD, NADPH/NADP(+) and GSH/GSSG ratios and reducing oxidative and inflammatory stress. Eur. J. Pharmacol. 2018, 818, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, E.I.; Padilla-Loma, C.; Gomez-Hernandez, N.; Lopez-Lozano, N.E.; Casas-Flores, S.; Cervantes, F.J. Humic Substances Mediate Anaerobic Methane Oxidation Linked to Nitrous Oxide Reduction in Wetland Sediments. Front. Microbiol. 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.-G.; Qian, X.; Li, Q.; Deng, B.; Zhu, Y.; Han, Z.; Zhang, W.; Wang, W.; Feng, S.-P.; Chen, G.; et al. Giant thermopower of ionic gelatin near room temperature. Science 2020, 368, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Khodami, S.; Kaniewska, K.; Stojek, Z.; Karbarz, M. Hybrid double-network dual-crosslinked hydrogel with self-healing ability and mechanical stability. Synthesis, characterization and application for motion sensors. Eur. Polym. J. 2022, 173, 111258. [Google Scholar] [CrossRef]
- Nikolaychuk, P.A.; Kuvaeva, A.O. Studying Equilibrium in the Chemical Reaction between Ferric and Iodide Ions in Solution Using a Simple and Inexpensive Approach. J. Chem. Educ. 2016, 93, 1267–1269. [Google Scholar] [CrossRef]
- Linford, P.A.; Xu, L.; Huang, B.; Shao-Horn, Y.; Thompson, C.V. Multi-cell thermogalvanic systems for harvesting energy from cyclic temperature changes. J. Power Sources 2018, 399, 429–435. [Google Scholar] [CrossRef]
- Yang, P.; Fan, H.J. Electrochemical Impedance Analysis of Thermogalvanic Cells. Chem. Res. Chin. Univ. 2020, 36, 420–424. [Google Scholar] [CrossRef]
- Chirila, C.; Botea, M.; Iuga, A.; Tomulescu, A.G.; Balescu, L.; Galca, A.C.; Boni, A.G.; Leonat, L.; Pintilie, I.; Pintilie, L. Carbon-based sprayed electrodes for pyroelectric applications. PLoS ONE 2019, 14, e0221108. [Google Scholar] [CrossRef]
- Devi, M.; Vomero, M.; Fuhrer, E.; Castagnola, E.; Gueli, C.; Nimbalkar, S.; Hirabayashi, M.; Kassegne, S.; Stieglitz, T.; Sharma, S. Carbon-based neural electrodes: Promises and challenges. J. Neural Eng. 2021, 18, 041007. [Google Scholar] [CrossRef]
- Lopez-Naranjo, E.J.; Gonzalez-Ortiz, L.J.; Apatiga, L.M.; Rivera-Munoz, E.M.; Manzano-Ramirez, A. Transparent Electrodes: A Review of the Use of Carbon-Based Nanomaterials. J. Nanomater. 2016, 2016, 4928365. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Bilteanu, L.; Serban, A.I. Antioxidant Determination with the Use of Carbon-Based Electrodes. Chemosensors 2021, 9, 72. [Google Scholar] [CrossRef]
- Bogachuk, D.; Zouhair, S.; Wojciechowski, K.; Yang, B.; Babu, V.; Wagner, L.; Xu, B.; Lim, J.; Mastroianni, S.; Pettersson, H.; et al. Low-temperature carbon-based electrodes in perovskite solar cells. Energy Environ. Sci. 2020, 13, 3880–3916. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Hejazi, M.; Tong, W.; Ibbotson, M.R.; Prawer, S.; Garrett, D.J. Advances in Carbon-Based Microfiber Electrodes for Neural Interfacing. Front. Neurosci. 2021, 15, 658703. [Google Scholar] [CrossRef]
- Huang, Q.; Ni, S.; Jiao, M.; Zhong, X.; Zhou, G.; Cheng, H.-M. Aligned Carbon-Based Electrodes for Fast-Charging Batteries: A Review. Small 2021, 17, 2007676. [Google Scholar] [CrossRef]
- Hu, R.; Cola, B.A.; Haram, N.; Barisci, J.N.; Lee, S.; Stoughton, S.; Wallace, G.; Too, C.; Thomas, M.; Gestos, A.; et al. Harvesting Waste Thermal Energy Using Carbon-Nanotube-Based Thermo-Electrochemical Cell. Nano Lett. 2010, 10, 838–846. [Google Scholar] [CrossRef]
- Shpekina, V.; Burmistrov, I.; Gorshkov, N.; Artyukhov, D.; Kiselev, N.; Kovyneva, N.; Smirnova, Y. Development of thermo-electrochemical cells based on flexible nanocomposite electrodes with oxidized multi-walled carbon nanotubes coating. In Proceedings of the IOP Conference Series: Materials Science and Engineering 2019, Tambov, Russia, 13–15 November 2019. [Google Scholar] [CrossRef]
- Bazgaou, A.; Fatnassi, H.; Bouharroud, R.; Ezzaeri, K.; Gourdo, L.; Wifaya, A.; Demrati, H.; Elame, F.; Carreno-Ortega, A.; Bekkaoui, A.; et al. Effect of active solar heating system on microclimate, development, yield and fruit quality in greenhouse tomato production. Renew. Energy 2021, 165, 237–250. [Google Scholar] [CrossRef]
- Lugo, S.; Garcia-Valladares, O.; Best, R.; Hernandez, J.; Hernandez, F. Numerical simulation and experimental validation of an evacuated solar collector heating system with gas boiler backup for industrial process heating in warm climates. Renew. Energy 2019, 139, 1120–1132. [Google Scholar] [CrossRef]
- Zeh, R.; Ohlsen, B.; Philipp, D.; Bertermann, D.; Kotz, T.; Jocic, N.; Stockinger, V. Large-Scale Geothermal Collector Systems for 5th Generation District Heating and Cooling Networks. Sustainability 2021, 13, 6035. [Google Scholar] [CrossRef]
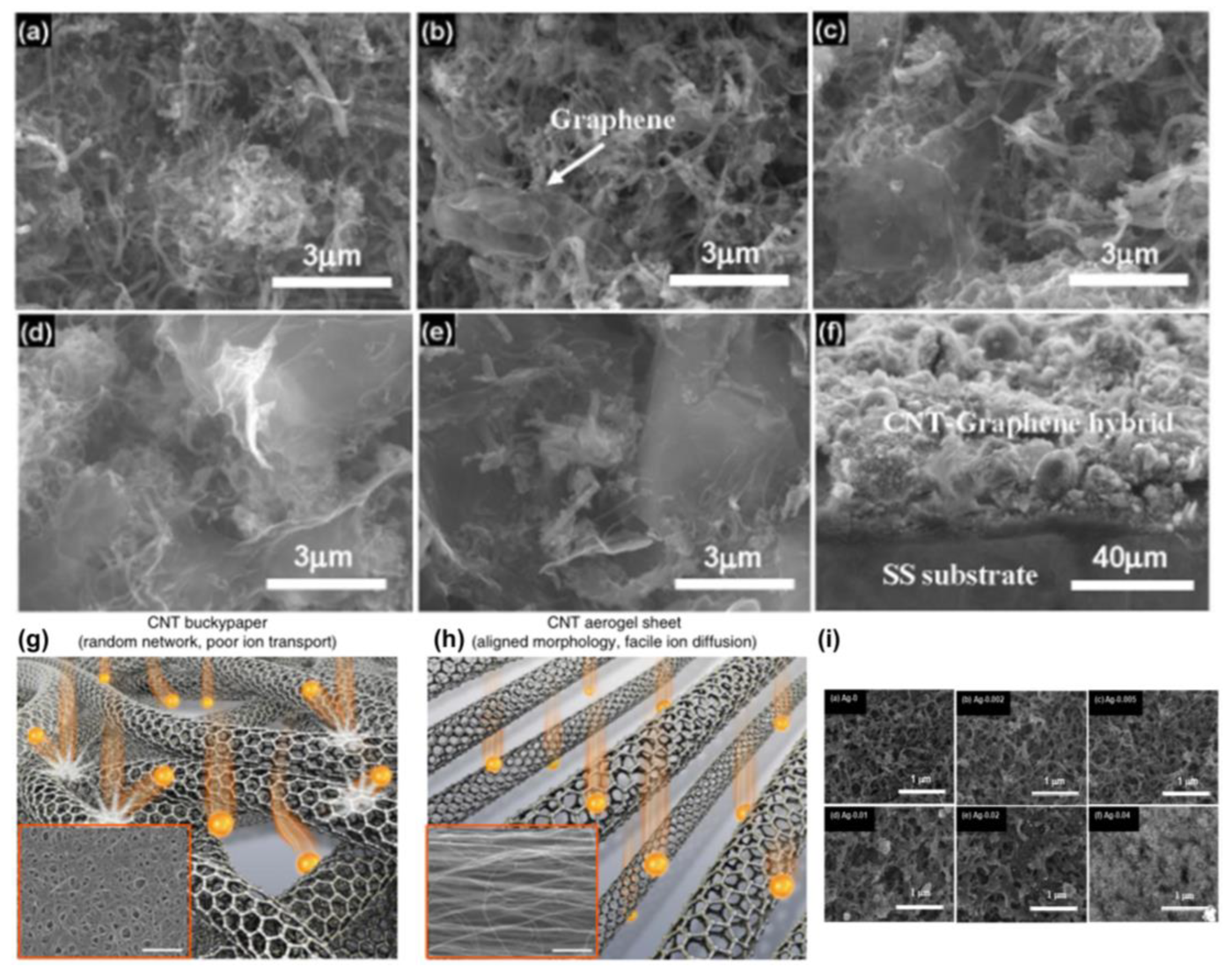
- Zhou, Y.; Qian, W.; Huang, W.; Liu, B.; Lin, H.; Dong, C. Carbon Nanotube-Graphene Hybrid Electrodes with Enhanced Thermo-Electrochemical Cell Properties. Nanomaterials 2019, 9, 1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, H.; Kim, T.; Song, H.; Choi, J.; Park, J.S.; Ovalle-Robles, R.; Yang, H.D.; Kihm, K.D.; Baughman, R.H.; Lee, H.H.; et al. High-efficiency electrochemical thermal energy harvester using carbon nanotube aerogel sheet electrodes. Nat. Commun. 2016, 7, 10600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, W.; Li, M.; Chen, L.; Zhang, J.; Dong, C. Improving thermo-electrochemical cell performance by constructing Ag–MgO–CNTs nanocomposite electrodes. RSC Adv. 2015, 5, 97982–97987. [Google Scholar] [CrossRef]
- Burda, I.; Baechler, C.; Gardin, S.; Verma, A.; Terrasi, G.P.; Kovacs, G. Low-cost scalable printing of carbon nanotube electrodes on elastomeric substrates: Towards the industrial production of EAP transducers. Sens. Actuators A Phys. 2018, 279, 712–724. [Google Scholar] [CrossRef]
- Fikry, M.; Abbas, M.; Sayed, A.; Nouh, A.; Ibrahim, A.; Mansour, A.S. Using a novel graphene/carbon nanotubes composite for enhancement of the supercapacitor electrode capacitance. J. Mater. Sci. Mater. Electron. 2022, 33, 3914–3924. [Google Scholar] [CrossRef]
- Paradise, M.; Goswami, T. Carbon nanotubes—Production and industrial applications. Mater. Des. 2007, 28, 1477–1489. [Google Scholar] [CrossRef]
- Ma, C.; Wu, L.; Dirican, M.; Cheng, H.; Li, J.; Song, Y.; Shi, J.; Zhang, X. Carbon black-based porous sub-micron carbon fibers for flexible supercapacitors. Appl. Surf. Sci. 2021, 537, 147914. [Google Scholar] [CrossRef]
- Neto, D.B.d.F.; Matsubara, E.Y.; Dirican, M.; Salussolia, G.F.; Zhang, X.; Rosolen, J.M. Li intercalation in nonwoven carbon nanotube/carbon fiber felt electrode: Influence of carbon fiber type. Diam. Relat. Mater. 2021, 115, 108353. [Google Scholar] [CrossRef]
- Peixoto da Cunha, C.E.; Batista Rodrigues, E.S.; Alecrim, M.F.; Thomaz, D.V.; Lopes Macedo, I.Y.; Garcia, L.F.; de Oliveira Neto, J.R.; Goncalves Moreno, E.K.; Ballaminut, N.; Gil, E.d.S. Voltammetric Evaluation of Diclofenac Tablets Samples through Carbon Black-Based Electrodes. Pharmaceuticals 2019, 12, 83. [Google Scholar] [CrossRef]
- Wang, W.; Luo, J.; Chen, S. Carbon oxidation reactions could misguide the evaluation of carbon black-based oxygen- evolution electrocatalysts. Chem. Commun. 2017, 53, 11556–11559. [Google Scholar] [CrossRef] [PubMed]
- Artyukhov, D.; Kiselev, N.; Gorshkov, N.; Kovyneva, N.; Ganzha, O.; Vikulova, M.; Gorokhovsky, A.; Offor, P.; Boychenko, E.; Burmistrov, I. Harvesting Waste Thermal Energy Using a Surface-Modified Carbon Fiber-Based Thermo-Electrochemical Cell. Sustainability 2021, 13, 1377. [Google Scholar] [CrossRef]
- Xu, T.; Li, W.; Ma, Z.; Qian, Y.; Jiang, Q.; Luo, Y.; Yang, J. High power generation from a new semi-solid thermo-electrochemical cell. Nano Energy 2022, 103, 107826. [Google Scholar] [CrossRef]
- Liu, J.; Ni, R.; Chau, Y. A self-assembled peptidic nanomillipede to fabricate a tuneable hybrid hydrogel. Chem. Commun. 2019, 55, 7093–7096. [Google Scholar] [CrossRef]
- Tang, N.; Peng, Z.; Guo, R.; An, M.; Chen, X.; Li, X.; Yang, N.; Zang, J. Thermal Transport in Soft PAAm Hydrogels. Polymers 2017, 9, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, C.; Li, X.; Cui, X.; Yang, X.; Zhang, X.; Yang, K.; Wang, T.; Zhang, H. Transparent stretchable thermogalvanic PVA/gelation hydrogel electrolyte for harnessing solar energy enabled by a binary solvent strategy. Nano Energy 2022, 100, 107449. [Google Scholar] [CrossRef]
- Bai, C.; Wang, Z.; Yang, S.; Cui, X.; Li, X.; Yin, Y.; Zhang, M.; Wang, T.; Sang, S.; Zhang, W.; et al. Wearable Electronics Based on the Gel Thermogalvanic Electrolyte for Self-Powered Human Health Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 37306–37312. [Google Scholar] [CrossRef]
- Ding, T.; Zhou, Y.; Wang, X.-Q.; Zhang, C.; Li, T.; Cheng, Y.; Lu, W.; He, J.; Ho, G.W. All-Soft and Stretchable Thermogalvanic Gel Fabric for Antideformity Body Heat Harvesting Wearable. Advanced Energy Mater. 2021, 11, 2102219. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Liu, X.; Lam, J.W.Y.; Tang, B.Z.; Chau, Y. Molecular logic operations from complex coacervation with aggregation-induced emission characteristics. Mater. Horiz. 2022, 9, 2443–2449. [Google Scholar] [CrossRef]
- Li, X.; Xiao, X.; Bai, C.; Mayer, M.; Cui, X.; Lin, K.; Li, Y.; Zhang, H.; Chen, J. Thermogalvanic hydrogels for self-powered temperature monitoring in extreme environments. J. Mater. Chem. C 2022, 10, 13789–13796. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Beirne, S.; Kim, K.; Qin, C.; Du, Y.; Zhou, Y.; Cheng, Z.; Wallace, G.; Chen, J. Wearable Photo-Thermo-Electrochemical Cells (PTECs) Harvesting Solar Energy. Macromol. Rapid Commun. 2022, 43, 2200001. [Google Scholar] [CrossRef]
- Zhang, D.; Mao, Y.; Ye, F.; Li, Q.; Bai, P.; He, W.; Ma, R. Stretchable thermogalvanic hydrogel thermocell with record-high specific output power density enabled by ion-induced crystallization. Energy Environ. Sci. 2022, 15, 2974–2982. [Google Scholar] [CrossRef]
- Fu, X.; Zhuang, Z.; Zhao, Y.; Liu, B.; Liao, Y.; Yu, Z.; Yang, P.; Liu, K. Stretchable and Self-Powered Temperature-Pressure Dual Sensing Ionic Skins Based on Thermogalvanic Hydrogels. ACS Appl. Mater. Interfaces 2022, 14, 44792–44798. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Adduru, J.; Rao, T.N.; Karthik, M. Conversion of Solar Energy into Electrical Energy Storage: Supercapacitor as an Ultrafast Energy-Storage Device Made from Biodegradable Agar-Agar as a Novel and Low-Cost Carbon Precursor. Glob. Chall. 2018, 2, 1800037. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hao, C.; Zhang, Q.; Meng, Q.; Liu, H. Research advances on photo-assisted CO2 conversion to methanol. Appl. Catal. Gen. 2022, 643, 118738. [Google Scholar] [CrossRef]
- Yaseen, S.; Tahir, M.B.; Wattoo, A.G. Photocorrosion inhibition of sulphide-based nanomaterials for energy production through photocatalytic water splitting. Int. J. Energy Res. 2022, 46, 634–666. [Google Scholar] [CrossRef]
- Yu, H.; Duan, J.; Du, W.; Xue, S.; Sun, J. China’s energy storage industry: Develop status, existing problems and countermeasures. Renew. Sustain. Energy Rev. 2017, 71, 767–784. [Google Scholar] [CrossRef]
- Dede, E.M.; Schmalenberg, P.; Wang, C.-M.; Zhou, F.; Nomura, T. Collection of low-grade waste heat for enhanced energy harvesting. AIP Adv. 2016, 6, 055113. [Google Scholar] [CrossRef] [Green Version]
- Reddick, C.; Sorin, M.; Bonhivers, J.-C.; Laperle, D. Waste heat and renewable energy integration in buildings. Energy Build. 2020, 211, 109803. [Google Scholar] [CrossRef]
- Bhatia, B.; Damodaran, A.R.; Cho, H.; Martin, L.W.; King, W.P. High-frequency thermal-electrical cycles for pyroelectric energy conversion. J. Appl. Phys. 2014, 116, 194509. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wang, Y.; Wen, J.; Chen, H.; Ma, W.; Fan, F.; Huang, Y.; Zhao, Z. Annealing Temperature-Dependent Terahertz Thermal-Electrical Conversion Characteristics of Three-Dimensional Microporous Graphene. ACS Appl. Mater. Interfaces 2019, 11, 6411–6420. [Google Scholar] [CrossRef]
- Rui, X.; Zheng, F.; Zheng, T. Feasibility analysis of booster cavity for solar thermal-electric conversion device. J. Renew. Sustain. Energy 2020, 12, 013702. [Google Scholar] [CrossRef]
- Tang, S.; Liu, X.; Wang, C.; Zhang, D.; Su, G.H.; Tian, W.; Qiu, S. Thermal-electrical coupling characteristic analysis of the heat pipe cooled reactor with static thermoelectric conversion. Ann. Nucl. Energy 2022, 168, 108870. [Google Scholar] [CrossRef]
- Xu, R.; Zhu, Q.; Xu, Z.; Feng, Y. Temperature-induced phase transition of PNZST ceramics near the morphotropic phase boundary for thermal-electrical energy conversion. J. Alloy. Compd. 2022, 905, 164140. [Google Scholar] [CrossRef]
- Zhang, Y.; Gurzadyan, G.G.; Umair, M.M.; Wang, W.; Lu, R.; Zhang, S.; Tang, B. Ultrafast and efficient photothermal conversion for sunlight-driven thermal-electric system. Chem. Eng. J. 2018, 344, 402–409. [Google Scholar] [CrossRef]
- Feistel, R.; Ebeling, W. Entropy and the Self-Organization of Information and Value. Entropy 2016, 18, 193. [Google Scholar] [CrossRef] [Green Version]
- Trucchi, D.M.; Bellucci, A.; Girolami, M.; Calvani, P.; Cappelli, E.; Orlando, S.; Polini, R.; Silvestroni, L.; Sciti, D.; Kribus, A. Solar Thermionic-Thermoelectric Generator (ST(2)G): Concept, Materials Engineering, and Prototype Demonstration. Adv. Energy Mater. 2018, 8, 1802310. [Google Scholar] [CrossRef]
- Wolf, M.; Rybakov, A.; Hinterding, R.; Feldhoff, A. Geometry Optimization of Thermoelectric Modules: Deviation of Optimum Power Output and Conversion Efficiency. Entropy 2020, 22, 1233. [Google Scholar] [CrossRef]
- Zianni, X. Thermoelectric Metamaterials: Nano-Waveguides for Thermoelectric Energy Conversion and Heat Management at the Nanoscale. Adv. Electron. Mater. 2021, 7, 2100176. [Google Scholar] [CrossRef]
- Hasan, S.; Kouzani, A.Z.; Adams, S.; Long, J.; Mahmud, M.A.P. Recent progress in hydrogel-based sensors and energy harvesters. Sens. Actuators A Phys. 2022, 335, 113382. [Google Scholar] [CrossRef]



| Redox Couple | Matrix | Se (mV K−1) | σ (S m−1) | Pmax/(ΔT)2 (mW m−2K−2) | Number of Charging–Discharging Cycles | Ref. |
|---|---|---|---|---|---|---|
| Fe3+/Fe2+ | PVA/Gelatin | 1.63 | 0.75 | 0.03 | 30 | [21] |
| Fe3+/Fe2+ | HCl/PVA | 1.02 | 1 | 0.01 | / | [56] |
| [Fe(CN)6]3−/[Fe(CN)6]4− | KCl/Gelatin | 17 | 1 | 0.66 | 50 | [23] |
| [Fe(CN)6]3/[Fe(CN)6]4− | PVA | 1.21 | 0.6 | 0.04 | / | [56] |
| I3−/I− | Cyclodextrins/aqueous | 1.9 | 2.4 | / | / | [75] |
| I3−/I− | PVA/Gelation | 0.63 | 0.06 | / | 5 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Bai, C.; Wang, Z.; Liu, X.; Li, X.; Cui, X. Low-Grade Thermal Energy Harvesting and Self-Powered Sensing Based on Thermogalvanic Hydrogels. Micromachines 2023, 14, 155. https://doi.org/10.3390/mi14010155
Zhang J, Bai C, Wang Z, Liu X, Li X, Cui X. Low-Grade Thermal Energy Harvesting and Self-Powered Sensing Based on Thermogalvanic Hydrogels. Micromachines. 2023; 14(1):155. https://doi.org/10.3390/mi14010155
Chicago/Turabian StyleZhang, Jiedong, Chenhui Bai, Zhaosu Wang, Xiao Liu, Xiangyu Li, and Xiaojing Cui. 2023. "Low-Grade Thermal Energy Harvesting and Self-Powered Sensing Based on Thermogalvanic Hydrogels" Micromachines 14, no. 1: 155. https://doi.org/10.3390/mi14010155





