Study on the Deterioration Mechanism of Pb on TiO2 Oxygen Sensor
Abstract
:1. Introduction
2. Experiments
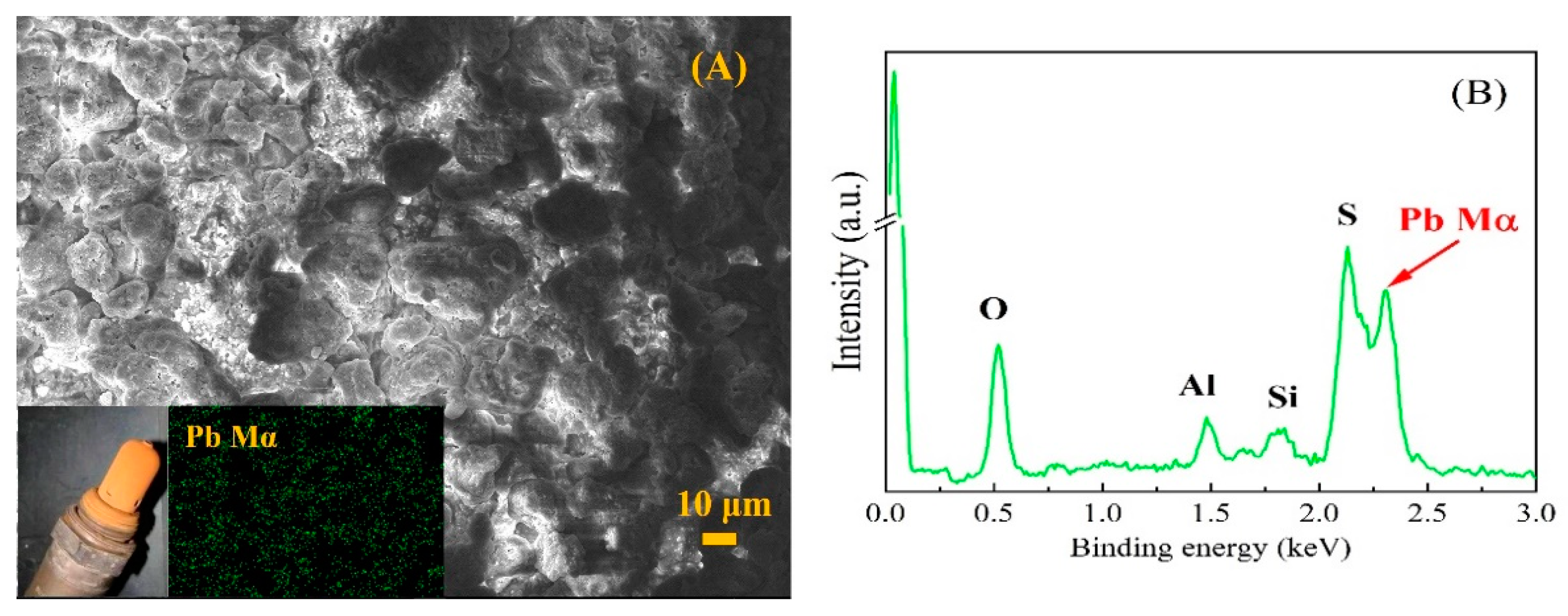
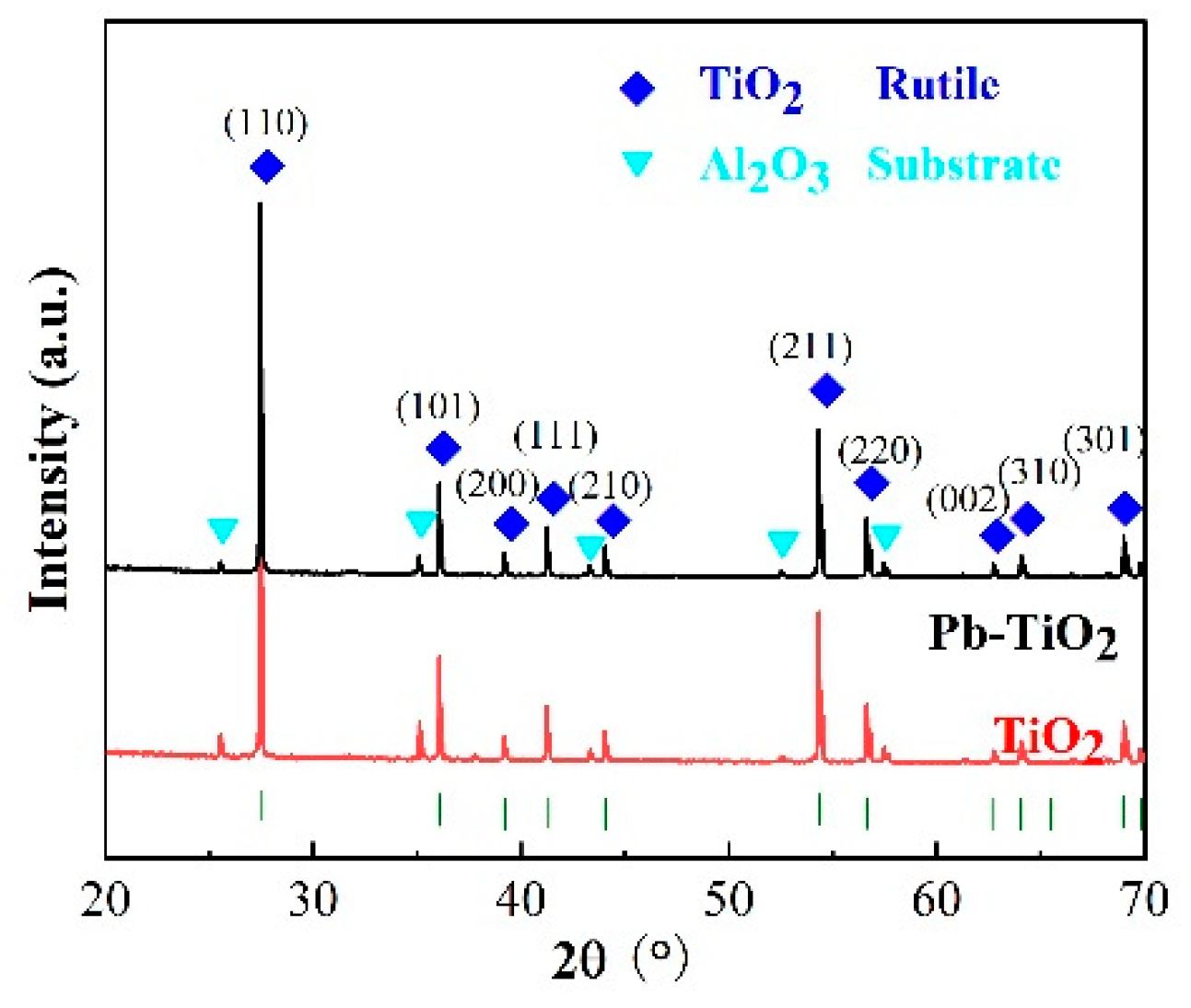
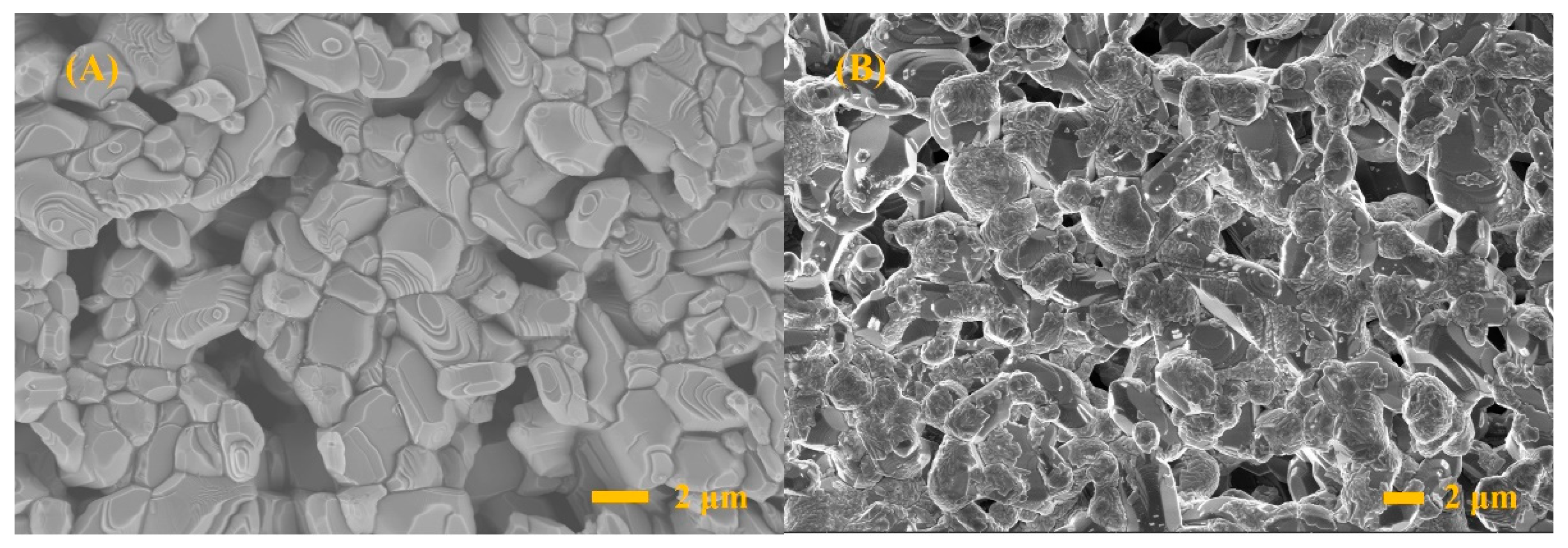
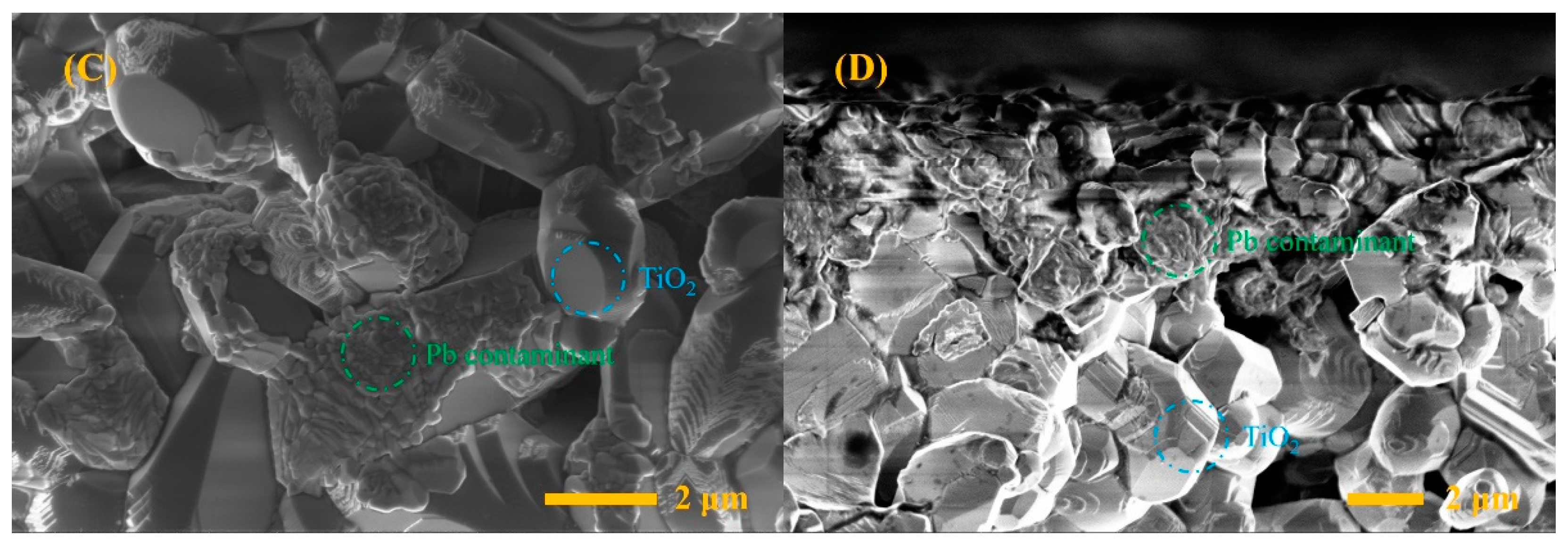
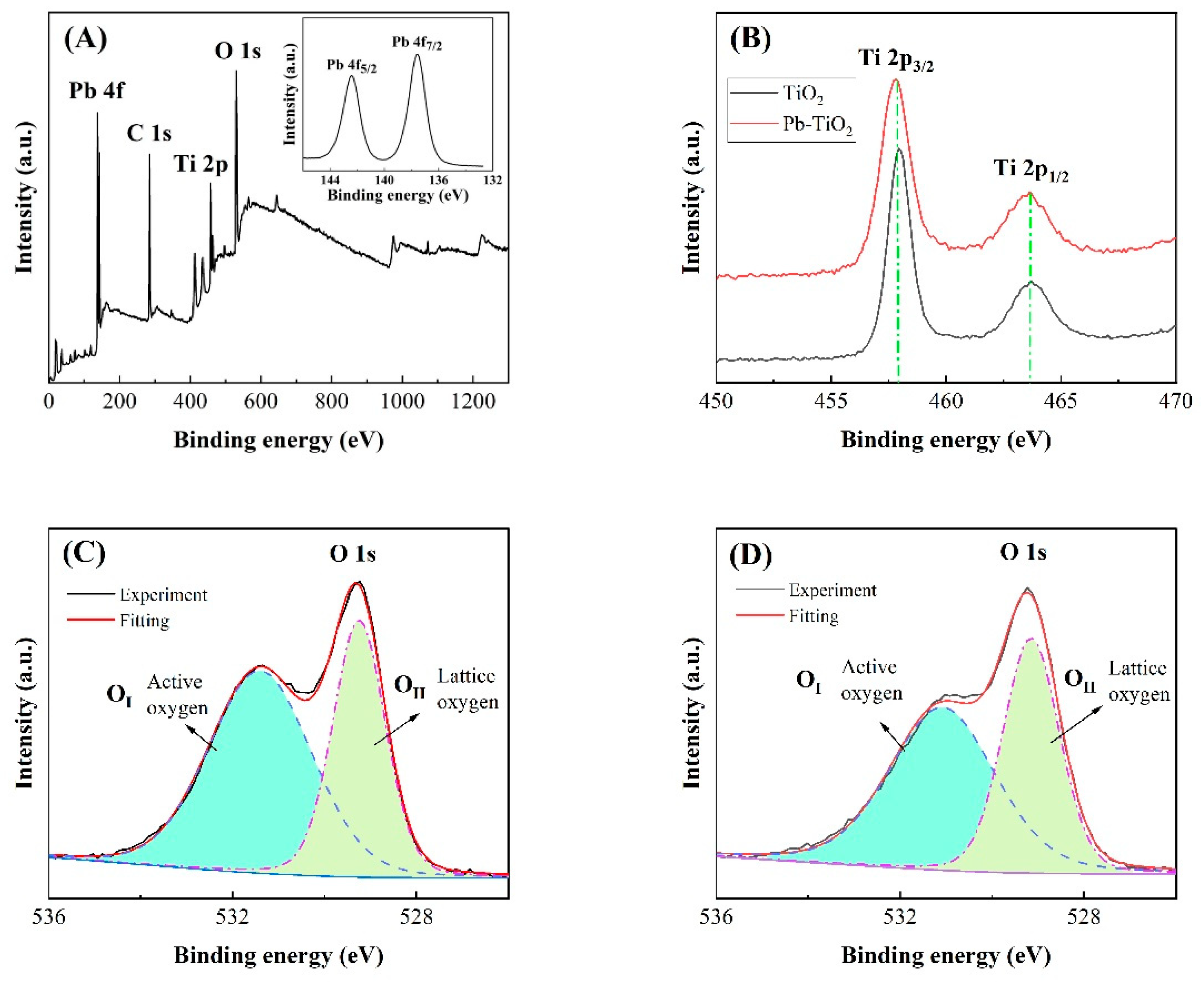
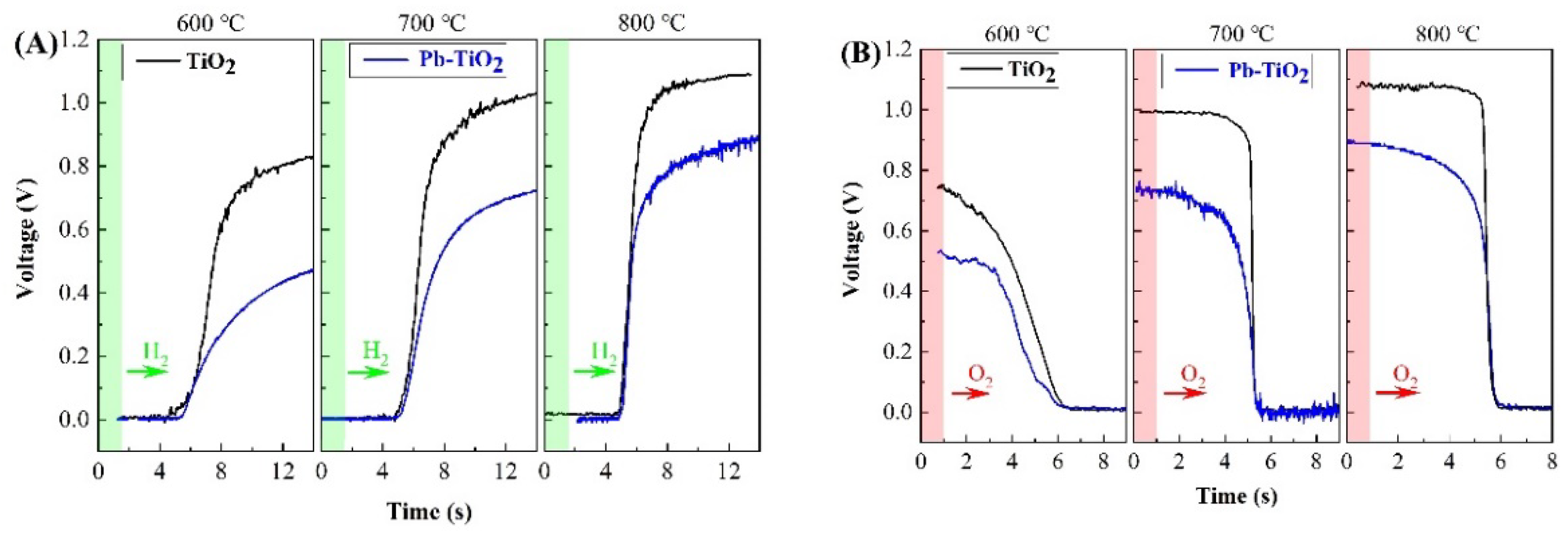
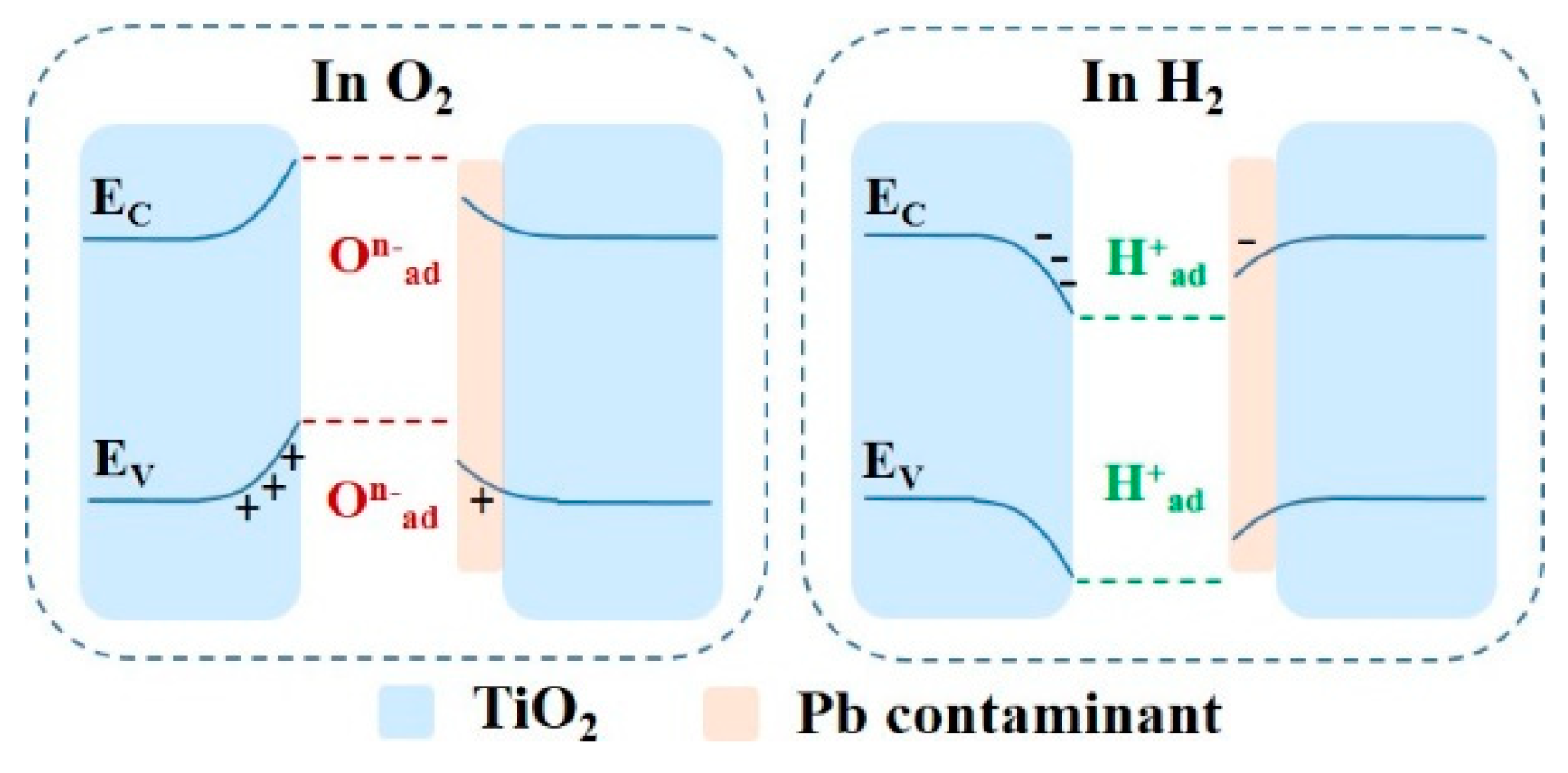
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, T.; Zhang, C.; Liu, C.; Hu, Q. Variability of PM2.5 and O3 concentrations and their driving forces over Chinese megacities during 2018–2020. J. Environ. Sci. 2022, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Rodríguez, M.; Villegas-Alcaraz, J.F.; Salazar-Hernández, C.; Mendoza-Miranda, J.M.; Jiménez-Islas, H.; Hernández, J.G.S.; Ortíz-Alvarado, J.D.D.; Juarez-Rios, H. Monitoring of oil lubrication limits, fuel consumption, and excess CO2 production on civilian vehicles in Mexico. Energy 2022, 257, 124765. [Google Scholar] [CrossRef]
- Halley, S.; Ramaiyan, K.P.; Tsui, L.-k.; Garzon, F. A review of zirconia oxygen, NOx, and mixed potential gas sensors-History and current trends. Sens. Actuators B Chem. 2022, 370, 132363. [Google Scholar] [CrossRef]
- Lee, J.-H. Review on zirconia air-fuel ratio sensors for automotive applications. J. Mater. Sci. 2003, 38, 4247–4257. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Miura, N. Development of zirconia-based potentiometric NOx sensors for automotive and energy industries in the early 21st century: What are the prospects for sensors? Sens. Actuators B Chem. 2007, 121, 639–651. [Google Scholar] [CrossRef]
- Akasaka, S.; Amamoto, Y.; Yuji, H.; Kanno, I. Limiting current type yttria-stabilized zirconia thin-film oxygen sensor with spiral Ta2O5 gas diffusion layer. Sens. Actuators B Chem. 2021, 327, 128932. [Google Scholar] [CrossRef]
- Sun, X.; Liu, F.; Wang, Y.; Liu, X.; Jian, J.; Zeng, D.; Liu, L.; Yuan, H.; Lu, G. A dense diffusion barrier limiting current oxygen sensor for detecting full concentration range. Sens. Actuators B Chem. 2019, 305, 127521. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature applications—A review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Moos, R.; Sahner, K.; Fleischer, M.; Guth, U.; Barsan, N.; Weimar, U. Solid state gas sensor research in Germany—A status report. Sensors 2009, 9, 4323. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, W.; Li, Y. Computational study of surface orientation effect of rutile TiO2 on H2S and CO sensing mechanism. Appl. Surf. Sci. 2019, 495, 143619. [Google Scholar] [CrossRef]
- Pan, M.; Shi, Z.; Liu, J.; Wang, Y. Study on the process of micro-arc oxidation TiO2 film for rapid detection of triethylamine. Mater. Sci. Semicond. Process. 2022, 146, 106679. [Google Scholar] [CrossRef]
- Wang, D.; Yang, J.; Bao, L.; Cheng, Y.; Tian, L.; Ma, Q.; Xu, J.; Li, H.-J.; Wang, X. Pd nanocrystal sensitization two-dimension porous TiO2 for instantaneous and high efficient H2 detection. J. Colloid Interface Sci. 2021, 597, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Haidry, A.A.; Wang, Z.; Ji, Y. Improved acetone sensing characteristics of TiO2 nanobelts with Ag modification. J. Alloys Compd. 2021, 887, 161312. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, M.; Nawaz, S.A.; Tian, X.; Wang, H.; Wang, J. TiO2 hierarchical nano blooming-flower decorated by Pt for formaldehyde detection. Nanotechnology 2021, 32, 365601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, L.; Huang, B.; Li, X. Enhanced sensing performance of Au-decorated TiO2 nanospheres with hollow structure for formaldehyde detection at room temperature. Sens. Actuators B Chem. 2022, 358, 131465. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Xu, Z.; Shen, W.; Wu, Y.; Corriou, J.-P. Computational assisted tuning of Co-doped TiO2 nanoparticles for ammonia detection at room temperatures. Appl. Surf. Sci. 2022, 601, 154214. [Google Scholar] [CrossRef]
- Wu, K.; Debliquy, M.; Zhang, C. Room temperature gas sensors based on Ce doped TiO2 nanocrystals for highly sensitive NH3 detection. Chem. Eng. J. 2022, 444, 136449. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Sen, S.; Kundu, S. Development of La-impregnated TiO2 based ethanol sensors for next generation automobile application. J. Mater. Sci. Mater. Electron. 2022, 33, 15296–15312. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Recent progress of nanostructured sensing materials from 0D to 3D: Overview of structure—Property—Application relationship for gas sensors. Small Methods 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Shi, Y.; Han, J.; Yang, Z.; Zhang, Y.; Long, C.; Guo, J.; Zhu, Y.; Qiu, X.; et al. Boosting CO2 conversion with terminal alkynes by molecular architecture of graphene oxide-supported Ag aanoparticles. Matter 2020, 3, 558–570. [Google Scholar] [CrossRef]
- Singh, A.; Sikarwar, S.; Verma, A.; Yadav, B.C. The recent development of metal oxide heterostructures based gas sensor, their future opportunities and challenges: A review. Sens. Actuators A Phys. 2021, 332, 113127. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.; Tervoort, E.; Zeng, G.; Liu, T.; Chen, X.; Sologubenko, A.; Niederberger, M. Nano-sized structurally disordered metal oxide composite aerogels as high-power anodes in hybrid supercapacitors. ACS Nano 2018, 12, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zeng, W.; Li, Y. A nest-like TiO2 nanostructures for excellent performance ethanol sensor. Mater. Lett. 2019, 248, 82–85. [Google Scholar] [CrossRef]
- GB17930-2016; Gasoline for Motor Vehicle. National Standard of the People’s Republic of China. Standardization Administration of China: Beijing, China, 2016.
- BS EN 228:2012; Automotive fFuels-Unleaded Petrol-Requirements and Test Methods, European Standard. European Committee for Standardization: Brussels, British, 2012.
- Irshad, M.; Afzal, A.; Mujahid, A.; Bajwa, S.Z.; Hussain, T.; Zaman, W.-U.; Latif, U.; Din, M.I.; Ali, F.; Iqbal, N. Preventing SOx emissions through process control: Interdigital capacitors for on-site monitoring of thiophene-based environmental pollutants in oil refineries. J. Electrochem. Soc. 2021, 168, 097505. [Google Scholar] [CrossRef]
- Chai, H.; Zheng, Z.; Liu, K.; Xu, J.; Wu, K.; Luo, Y.; Liao, H.; Debliquy, M.; Zhang, C. Stability of metal oxide semiconductor gas sensors: A review. IEEE Sens. J. 2022, 22, 5470–5481. [Google Scholar] [CrossRef]
- Binnig, S.; Fuchs, S.; Collantes, C.R.; Volpp, H.-R. Exhaust gas condensate—Formation, characterization and influence on platinum measuring electrodes in diesel vehicles. Sens. Actuators B Chem. 2017, 242, 1251–1258. [Google Scholar] [CrossRef]
- Kornely, M.; Menzler, N.H.; Weber, A.; Ivers-Tiffée, E. Degradation of a High Performance SOFC Cathode by Cr-Poisoning at OCV-Conditions. Fuel Cells 2013, 13, 506–510. [Google Scholar] [CrossRef]
- Rettig, F.; Moos, R.; Plog, C. Poisoning of Temperature independent resistive oxygen sensors by sulfur dioxide. J. Electroceramics 2004, 13, 733–738. [Google Scholar] [CrossRef]
- Zhang, M.; Ji, X.; Li, Z.; Yan, Y.; Huang, Y. Adverse effects on Pt/TiO2 lambda oxygen sensor contaminated with sulfur. Sens. Actuators B Chem. 2017, 248, 119–123. [Google Scholar] [CrossRef]
- Zhang, M.; Ning, T.; Sun, P.; Zhang, D.; Yan, Y.; Li, Z. Degradation mechanism of TiO2 based lambda oxygen sensor poisoned by phosphorus. Sens. Actuators B Chem. 2018, 268, 77–83. [Google Scholar] [CrossRef]
- Zhang, M.; Ning, T.; Sun, P.; Zhang, D.; Yan, Y.; Li, Z. Poisoning mechanisms of Mn-containing additives on the performance of TiO2 based lambda oxygen sensor. Sens. Actuators B Chem. 2018, 267, 565–569. [Google Scholar] [CrossRef]
- Kocemba, K. Effect of presence lead in exhaust gases on efficiency working lambda sensors. Eurosensors 1998, 2, 269–272. [Google Scholar]
- Bindra, P.; Hazra, A. Selective detection of organic vapors using TiO2 nanotubes based single sensor at room temperature. Sens. Actuators B Chem. 2019, 290, 684–690. [Google Scholar] [CrossRef]
- Li, A.; Zhao, S.; Bai, J.; Gao, S.; Wei, D.; Shen, Y.; Yuan, Z.; Meng, F. Optimal construction and gas sensing properties of SnO2@TiO2 heterostructured nanorods. Sens. Actuators B Chem. 2021, 355, 131261. [Google Scholar] [CrossRef]
- Cui, H.; Yao, C.; Cang, Y.; Liu, W.; Zhang, Z.; Miao, Y.; Xin, Y. Oxygen vacancy-regulated TiO2 nanotube photoelectrochemical sensor for highly sensitive and selective detection of tetracycline hydrochloride. Sens. Actuators B Chem. 2022, 359. [Google Scholar] [CrossRef]
- Zhang, M.L.; Song, J.P.; Yuan, Z.H.; Zheng, C. Response improvement for In2O3–TiO2 thick film gas sensors. Curr. Appl. Phys. 2012, 12, 678–683. [Google Scholar] [CrossRef]
- Jo, S.-E.; Kang, B.-G.; Heo, S.; Song, S.; Kim, Y.-J. Gas sensing properties of WO3 doped rutile TiO2 thick film at high operating temperature. Curr. Appl. Phys. 2009, 9, e235–e238. [Google Scholar] [CrossRef]
- Presicce, D.; Francioso, L.; Epifani, M.; Siciliano, P.; Ficarella, A. Temperature and doping effects on performance of titania thin film lambda probe. Sens. Actuators B Chem. 2005, 111–112, 52–57. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Pang, A.L.; Ardani, M.R.; Pung, S.-Y.; Ooi, P.C.; Hamzah, A.A.; Wee, M.M.R.; Haniff, M.A.S.M.; Dee, C.F.; Mahmoudi, E.; et al. Detection of breath acetone by semiconductor metal oxide nanostructures-based gas sensors: A review. Mater. Sci. Semicond. Process. 2022, 149, 106897. [Google Scholar] [CrossRef]
- Kang, X.; Deng, N.; Yan, Z.; Pan, Y.; Sun, W.; Zhang, Y. Resistive-type VOCs and pollution gases sensor based on SnO2: A review. Mater. Sci. Semicond. Process. 2021, 138, 106246. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, D.; Li, B.; Wang, C.; Li, J.; Crittenden, J.; Hao, J. Impacts of Pb and SO2 poisoning on CeO2-WO3/TiO2-SiO2 SCR catalyst. Environ. Sci. Technol. 2017, 51, 11943–11949. [Google Scholar] [CrossRef] [PubMed]
- Zan, G.; Wu, T.; Zhu, F.; He, P.; Cheng, Y.; Chai, S.; Wang, Y.; Huang, X.; Zhang, W.; Wan, Y.; et al. A biomimetic conductive super-foldable material. Matter 2021, 4, 3232–3247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, C.; Zhang, L.; Wu, Z.; Wang, X.; Meng, M.; Zhang, M. Study on the Deterioration Mechanism of Pb on TiO2 Oxygen Sensor. Micromachines 2023, 14, 156. https://doi.org/10.3390/mi14010156
Duan C, Zhang L, Wu Z, Wang X, Meng M, Zhang M. Study on the Deterioration Mechanism of Pb on TiO2 Oxygen Sensor. Micromachines. 2023; 14(1):156. https://doi.org/10.3390/mi14010156
Chicago/Turabian StyleDuan, Chao, Lejun Zhang, Zhaoxi Wu, Xu Wang, Meng Meng, and Maolin Zhang. 2023. "Study on the Deterioration Mechanism of Pb on TiO2 Oxygen Sensor" Micromachines 14, no. 1: 156. https://doi.org/10.3390/mi14010156



