An Amperometric Biomedical Sensor for the Determination of Homocysteine Using Gold Nanoparticles and Acetylene Black-Dihexadecyl Phosphate-Modified Glassy Carbon Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
2.2. Preparation of Different Electrodes
2.3. Electrochemical Detection of HcySH
3. Results and Discussion
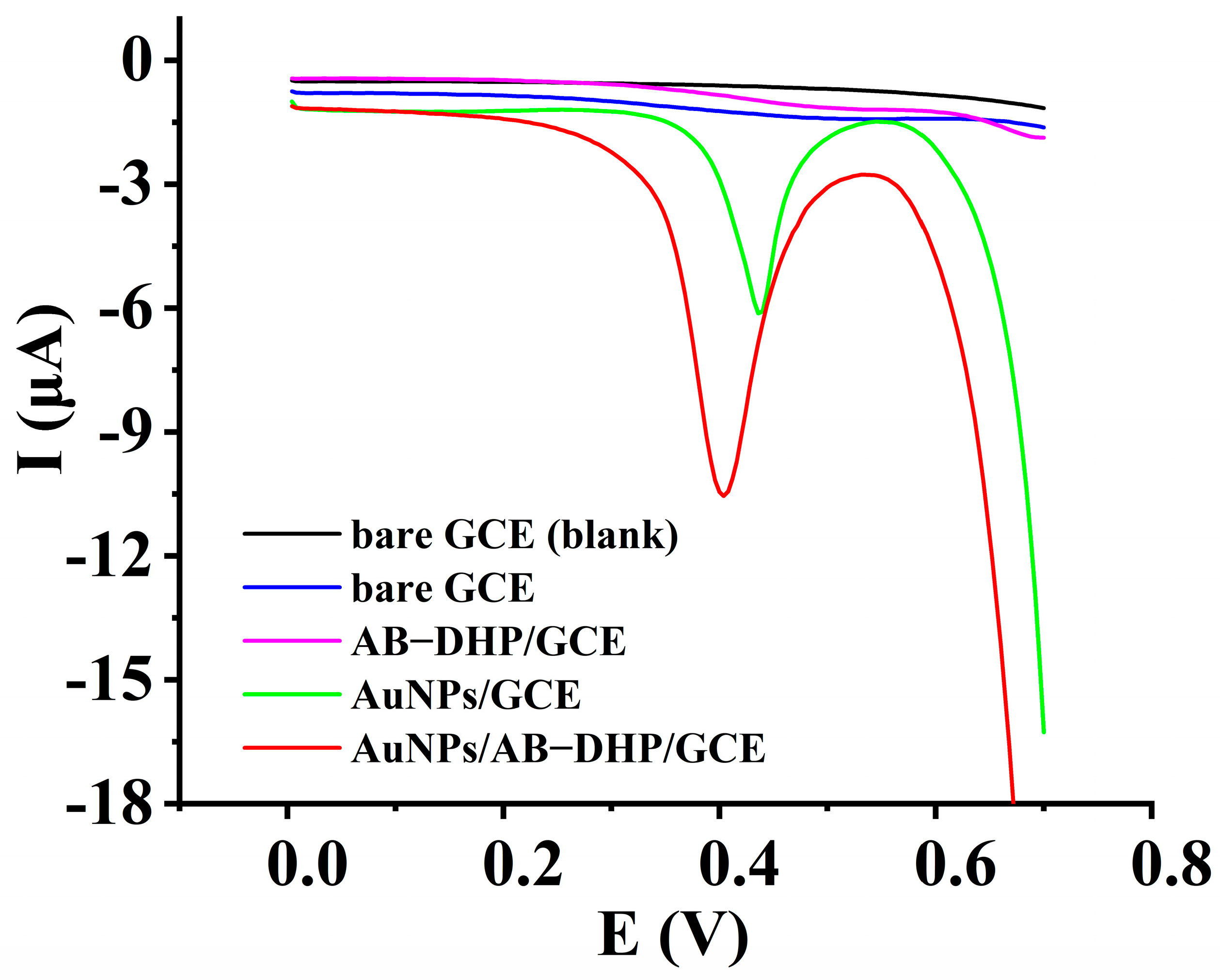
3.1. Electrochemical Response of HcySH on Different Electrodes
3.2. Characterizations of Nanocomposite Film
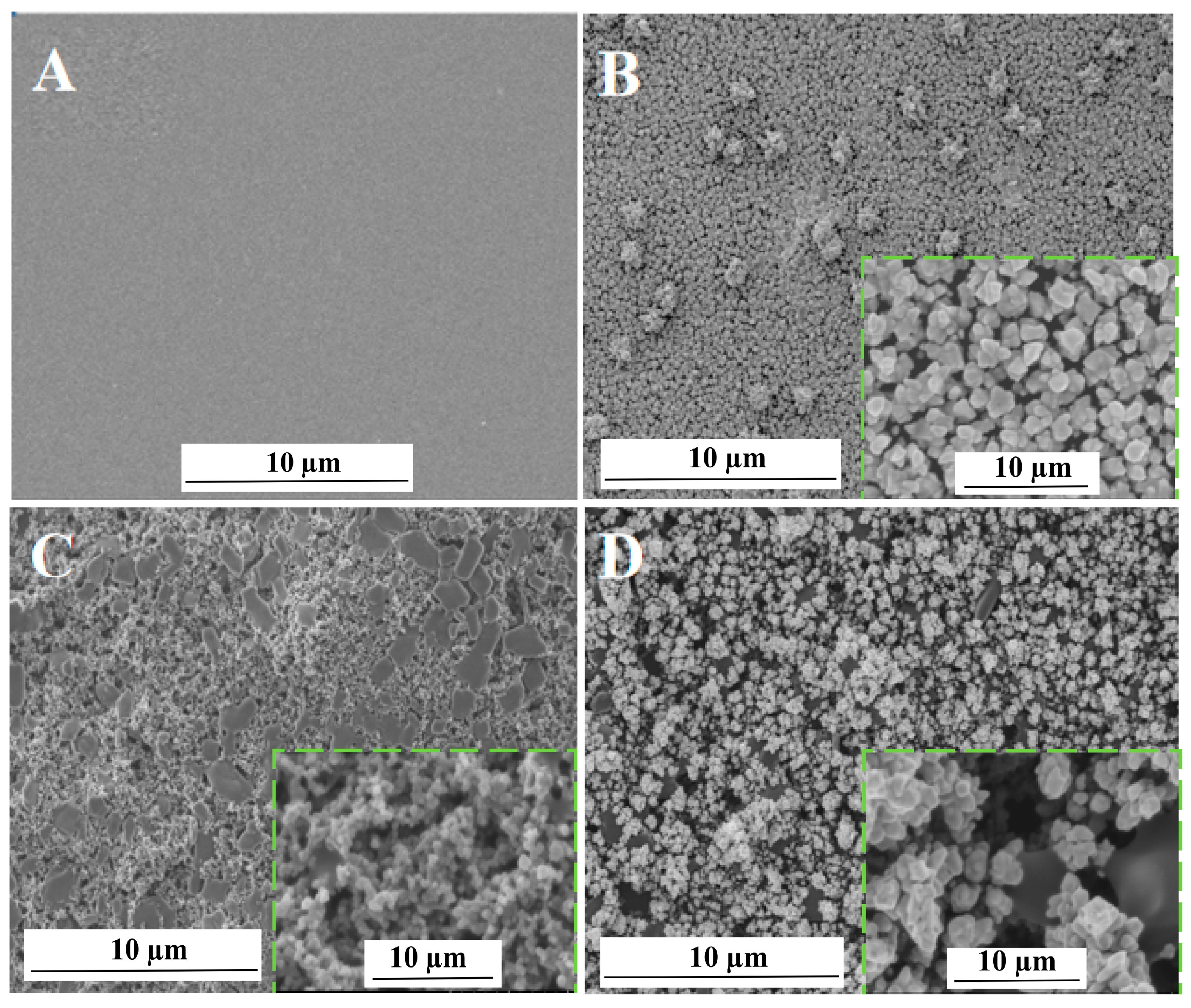
3.2.1. Characterization of Surface Topography
3.2.2. Energy Dispersive X-ray Spectrum Analysis
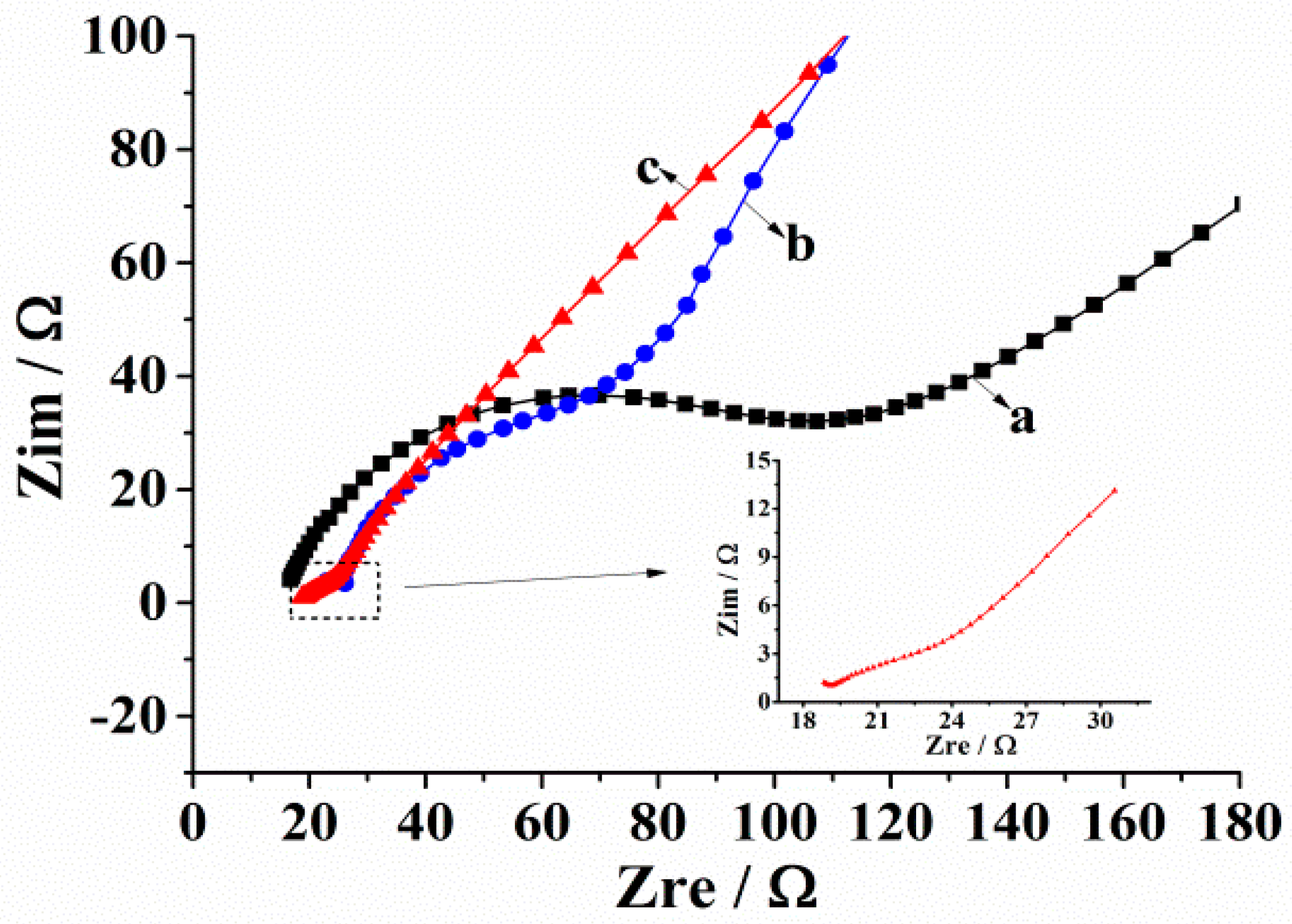
3.2.3. Characterization of Electrochemical Impedance Spectrum
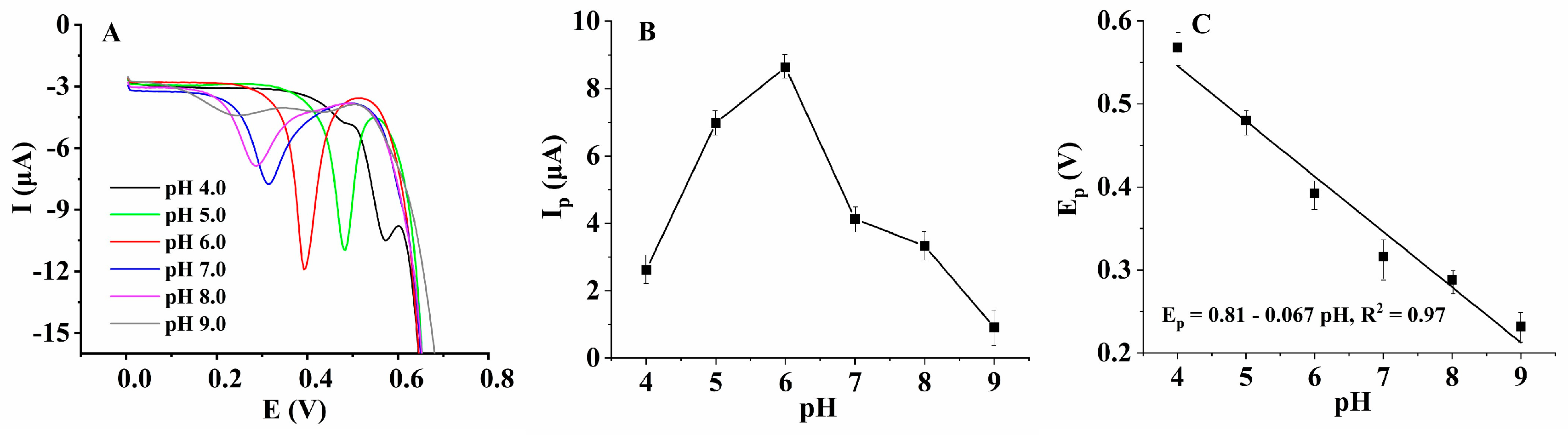
3.3. Investigation on the Electrochemical Oxidation Mechanism of HcySH
3.4. Investigation of the Electrocatalytic Mechanism of AuNPs/AB–DHP Nanocomposite Film
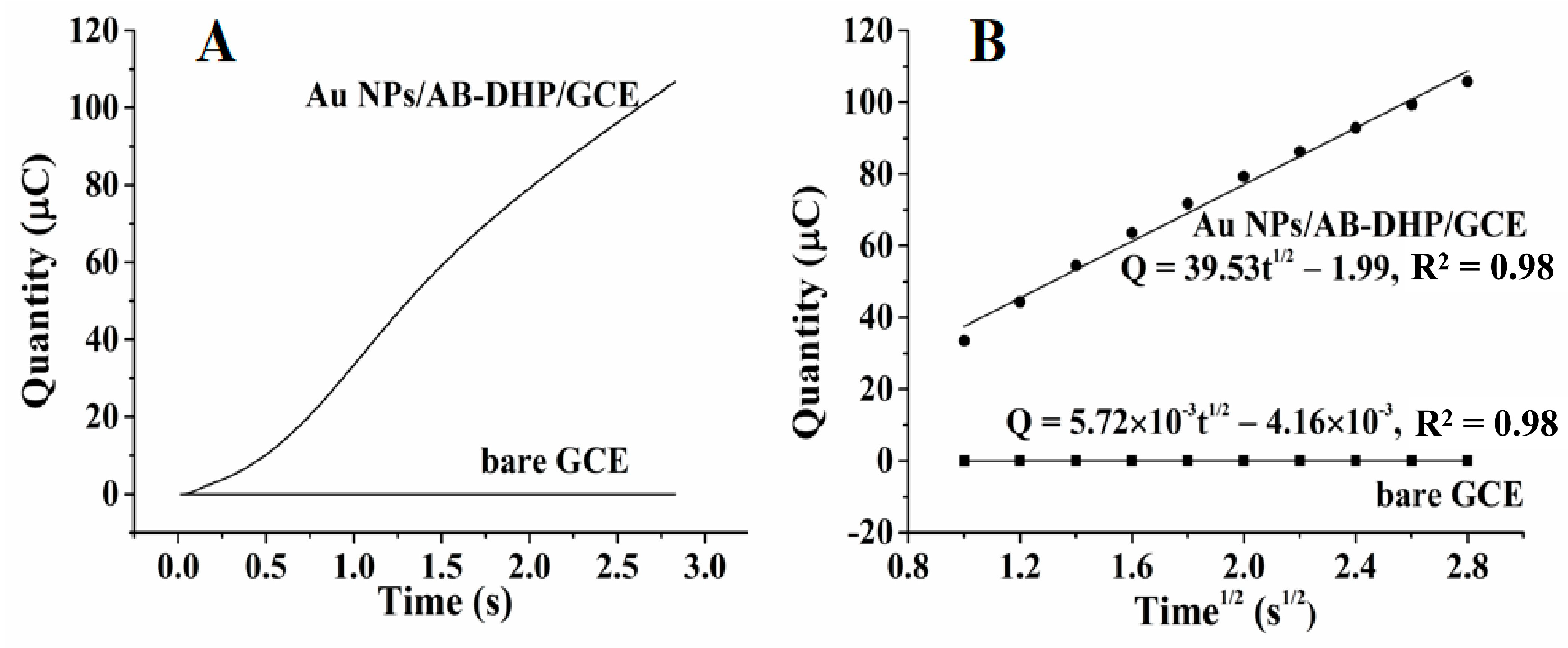
3.4.1. Specific Surface Area Increase of the Nanocomposite Film Electrode
3.4.2. Better Enrichment Ability of the Nanocomposite Film Electrode
3.5. Investigation of Sensor Performances
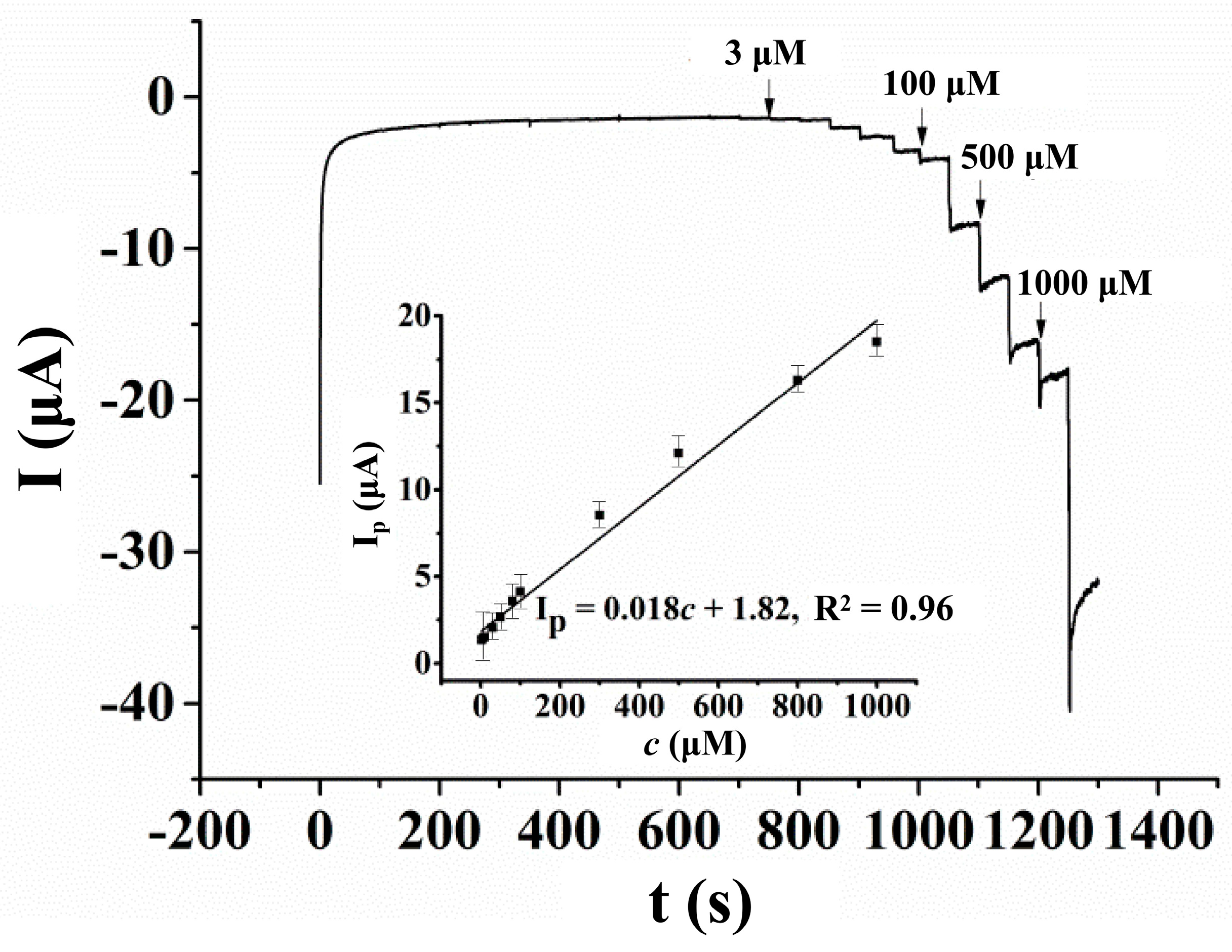
3.5.1. Linear Range, Sensitivity and Detection Limit
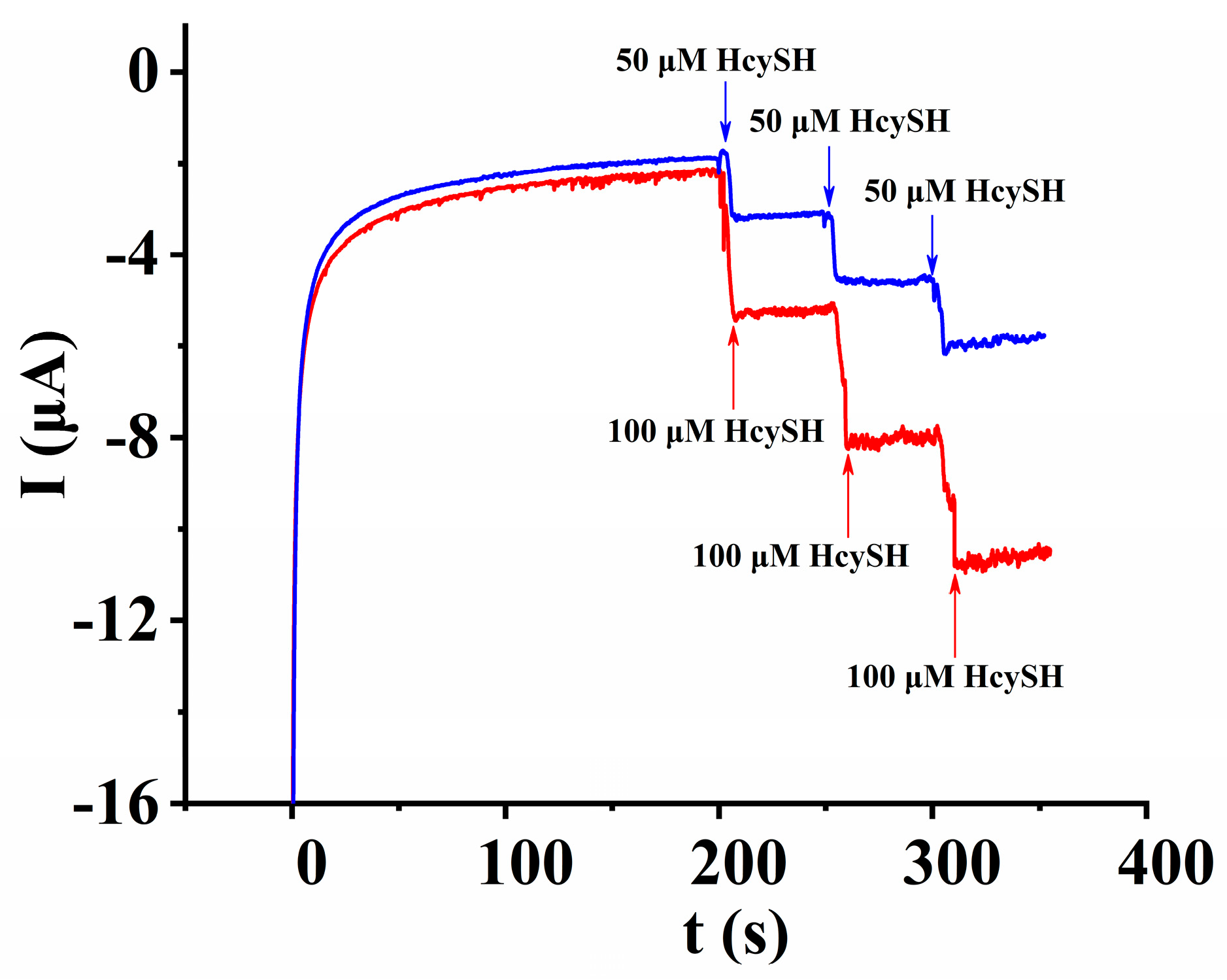
3.5.2. Anti-Interference Ability, Stability and Reproducibility
3.5.3. Determination of HcySH in Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramires, O.V.; Dos Santos, T.M.; Silveira, J.S.; Leite-Aguiar, R.; Coutinho-Silva, R.; Savio, L.E.B.; Wyse, A.T.S. Rivastigmine Reverses the Decrease in Synapsin and Memory Caused by Homocysteine: Is There Relation to Inflammation? Mol. Neurobiol. 2022, 59, 4517–4534. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.R.; Pfeiffer, C.M. Homocysteine and risk of cardiovascular disease. Acta Bioquim. Clin. Latinoam. 2010, 44, 446–453. [Google Scholar]
- Vinknes, K.J.; Refsum, H.; Turner, C.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G.; Imamura, F. Plasma sulfur amino acids and risk of cerebrovascular diseases: A nested case-control study in the EPIC-Norfolk cohort. Stroke 2021, 52, 172–180. [Google Scholar] [CrossRef]
- Xiao, W.K.; Ye, P.; Wang, F.; Cao, R.H.; Bai, Y.Y.; Wang, X.N. Plasma homocysteine is a predictive factor for accelerated renal function decline and chronic kidney disease in a community-dwelling population. Kidney Blood Press. Res. 2021, 46, 541–549. [Google Scholar] [CrossRef]
- Lee, W.J.; Peng, L.N.; Loh, C.H.; Chen, L.K. Sex-different associations between serum homocysteine, high-sensitivity C-reactive protein and sarcopenia: Results from I-Lan Longitudinal Aging Study. Exp. Gerontol. 2020, 132, 110832. [Google Scholar] [CrossRef]
- Geller, V.; Friger, M.; Sela, B.A.; Levine, J. Elevated homocysteine level in siblings of patients with schizophrenia. Psychiat. Res. 2013, 3, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Frick, B.; Schröcksnadel, K.; Neurauter, G.; Wirleitner, B.; Artner-Dworzak, E.; Fuchs, D. Rapid measurement of total plasma homocysteine by HPLC. Clin. Chim. Acta 2003, 331, 19–23. [Google Scholar] [CrossRef]
- Piechocka, J.; Wrońska, M.; Chwatko, G.; Jakubowski, H.; Glowacki, R. Quantification of homocysteine thiolactone in human saliva and urine by gas chromatography-mass spectrometry. J. Chromatogr. B 2020, 1149, 122155. [Google Scholar] [CrossRef]
- Caussé, E.; Terrier, R.; Champagne, S.; Nertz, M.; Valdiguié, P.; Salvayre, R.; Couderc, F. Quantitation of homocysteine in human plasma by capillary electrophoresis and laser-induced fluorescence detection. J. Chromatogr. A 1998, 817, 181–185. [Google Scholar] [CrossRef]
- Salehzadeh, H.; Mokhtari, B.; Nematollahia, D. Selective electrochemical determination of homocysteine in the presence of cysteine and glutathione. Electrochim. Acta 2014, 123, 353–361. [Google Scholar] [CrossRef]
- Sajid, M.; Baig, N.; Alhooshani, K. Chemically modified electrodes for electrochemical detection of dopamine: Challenges and opportunities. TrAC-Trend Anal. Chem. 2019, 118, 368–385. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC-Trend Anal. Chem. 2019, 111, 49–61. [Google Scholar] [CrossRef]
- Zhao, J.B.; Du, P.; Sun, X.Y.; Liu, H.B. Development of Electrochemical Sensor for Homocysteine Determination as a Cerebrovascular Disease Biomarker. Int. J. Electrochem. Sci. 2017, 12, 8642–8650. [Google Scholar] [CrossRef]
- Feng, J.X.; Deng, P.H.; Xiao, J.Y.; Li, J.H.; Tian, Y.L.; Wu, Y.Y.; Liu, J.; Li, G.L.; He, Q.G. New voltammetric method for determination of tyrosine in foodstuffs using an oxygen-functionalized multi-walled carbon nanotubes modified acetylene black paste electrode. J. Food Compos. Anal. 2021, 96, 103708. [Google Scholar] [CrossRef]
- Don, M.F.; Ekanayake, P.; Nakajima, H.; Mahadi, A.H.; Lim, C.M.; Atod, A. Acetylene carbon black-graphite composite as low-cost and efficient counter electrode for dye-sensitized solar cells (DSSCs). Ionics 2019, 25, 5585–5593. [Google Scholar] [CrossRef]
- Zhu, X.L.; Wang, M.B.; Xu, C.X.; Shi, S.G. Simultaneous Detection of Catechol and Hydroquinone Using Acetylene Black and Gold Nanoparticle Composite Modified Electrodes. ChemistrySelect 2022, 7, e202103384. [Google Scholar] [CrossRef]
- Aydin, E.B.; Aydin, M.; Sezginturk, M.K. A novel electrochemical immunosensor based on acetylene black/epoxy-substituted-polypyrrole polymer composite for the highly sensitive and selective detection of interleukin 6. Talanta 2021, 222, 121596. [Google Scholar] [CrossRef]
- Deng, Z.W.; Li, H.; Tian, Q.W.; Zhou, Y.S.; Yang, X.M.; Yu, Y.; Jiang, B.; Xu, Y.H.; Zhou, T.T. lectrochemical detection of methotrexate in serum sample based on the modified acetylene black sensor. Microchem. J. 2020, 157, 105058. [Google Scholar] [CrossRef]
- He, Q.G.; Tian, Y.L.; Wu, Y.Y.; Liu, J.; Li, G.L.; Deng, P.H.; Chen, D.C. Facile and Ultrasensitive Determination of 4-Nitrophenol Based on Acetylene Black Paste and Graphene Hybrid Electrode. Nanomaterials 2019, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.Z.; Cao, X.W.; Chang, K.; Xiang, H.H.; Wang, R. A novel electrochemical non-enzymatic glucose sensor based on Au nanoparticle-modified indium tin oxide electrode and boronate affinity. Electrochim. Acta 2021, 368, 137603. [Google Scholar] [CrossRef]
- Ebrahimian, J.; Khayatkashani, M.; Soltani, N.; Yousif, Q.A.; Salavati-Niasari, M. Catechin mediated green synthesis of Au nanoparticles: Experimental and theoretical approaches to the determination HOMO-LUMO energy gap and reactivity indexes for the (+)-epicatechin (2S, 3S). Arab. J. Chem. 2022, 15, 103758. [Google Scholar] [CrossRef]
- Alikhani, N.; Hekmati, M.; Karmakar, B.; Veisi, H. Green synthesis of gold nanoparticles (AuNPs) using Rosa canina fruit extractand evaluation of its catalytic activity in the degradation of organic dye pollutants of water. Inorg. Chem. Commun. 2022, 139, 109351. [Google Scholar] [CrossRef]
- Wang, R.R.; Liu, X.R.; Zhao, Y.W.; Qin, J.H.; Xu, H.; Dong, L.N.; Gao, S.M.; Zhong, L.L. Novel electrochemical non-enzymatic glucose sensor based on 3D Au@Pt core-shell nanoparticles decorated graphene oxide/multi-walled carbon nanotubes composite. Microchem. J. 2022, 174, 107061. [Google Scholar] [CrossRef]
- Fayazfar, H.; Afshar, A.; Dolati, A.; Ghalkhani, M. Modification of well-aligned carbon nanotubes with dihexadecyl hydrogen phosphate: Application to highly sensitive nanomolar detection of simvastatin. J. Apply. Electrochem. 2014, 44, 263–277. [Google Scholar] [CrossRef]
- Zheng, D.Y.; Liu, X.J.; Zhou, D.; Hu, S.S. Sensing of nitric oxide using a glassy carbon electrode modified with an electrocatalytic film composed of dihexadecyl hydrogen phosphate, platinum nanoparticles, and acetylene black. Microchim. Acta 2012, 176, 49–55. [Google Scholar] [CrossRef]
- Aquino, S.D.; Maciel, J.V.; Dias, D. Chemically modified electrode based on dihexadecyl hydrogen phosphate and carbonaceous materials: Improvement of analytical and electrochemical features applied to uranium determination. J. Solid State Electrochem. 2020, 24, 1981–1989. [Google Scholar] [CrossRef]
- Rajaram, R.; Mathiyarasu, J. An electrochemical sensor for homocysteine detection using gold nanoparticle incorporated reduced graphene oxide. Coll. Surf. B. 2018, 170, 109–114. [Google Scholar] [CrossRef]
- Park, J.; Lee, W.; Kim, I.; Kim, M.; Jo, S.; Kim, W.; Park, H.; Lee, G.; Choi, W.; Yoon, D.S. Ultrasensitive detection of fibrinogen using erythrocyte membrane-draped electrochemical impedance biosensor. Sens. Actuator B-Chem. 2019, 293, 296–303. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.T.; Lu, D.B.; Shi, X.Z.; Wang, C.M.; Duan, X.J. Sensitive determination of bisphenol A base on arginine functionalized nanocomposite graphene film. Electrochim. Acta 2012, 80, 77–83. [Google Scholar] [CrossRef]
- Zhang, J.D.; Demetriou, A.; Welinder, A.C.; Albrecht, T.; Nichols, R.J.; Ulstrup, J. Potential-induced structural transitions of DL-homocysteine monolayers on Au(111) electrode surfaces. Chem. Phys. 2005, 319, 210–221. [Google Scholar] [CrossRef]
- Shee, N.K.; Patra, S.G.; Drew, M.G.B.; Lu, L.P.; Zangrando, E.; Datta, D. Electrochemical behaviour of tris(1,10-phenanthroline)ruthenium(II) at a surface modified electrode. Electrocatalytic reduction of dioxygen. Inorg. Chim. Acta 2017, 466, 349–357. [Google Scholar] [CrossRef]
- Roffel, B.; Van de Graaf, J.J. The diffusion coefficient of ferricyanide ions in aqueous potassium chloride solutions with and without polyethylene oxide addition. J. Chem. Eng. Data 1977, 22, 300–302. [Google Scholar] [CrossRef]
- Buyuktas, D.; Ghaani, M.; Rovera, C.; Olsson, R.T.; Korel, F.; Farris, S. Development of a nano-modified glassy carbon electrode for the determination of 2,6-diaminotoluene (TDA). Food Packag. Shelf Life 2021, 29, 100714. [Google Scholar] [CrossRef]
- Wen, X.H.; Zhao, X.F.; Peng, B.F.; Yuan, K.P.; Li, X.X.; Zhu, L.Y.; Lu, H.L. Facile preparation of an electrochemical aptasensor based on Au NPs/graphene sponge for detection of homocysteine. Appl. Surf. Sci. 2021, 556, 149735. [Google Scholar] [CrossRef]
- Hosseinzadeh, L.; Khoshroo, A.; Adib, K.; Rahimi-Nasrabadi, M.; Ahmadi, F. Determination of homocysteine using a dopamine-functionalized graphene composite. Microchem. J. 2021, 165, 106124. [Google Scholar] [CrossRef]
- Beitollahi, H.; Zaimbashi, R.; Mahani, M.T.; Tajik, S. A label-free aptasensor for highly sensitive detection of homocysteine based on gold nanoparticles. Bioelectrochemistry 2020, 134, 107497. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Mi, Y.Z.; Ren, G.Y.; Chen, Y.J.; Wu, X.M. Assembly of a Nanogold-Assisted Aptamer Sensor for Highly Sensitive Detection of Homocysteine. Int. J. Electrochem. Sci. 2021, 16, 211129. [Google Scholar] [CrossRef]
- Saeed, J.; Mirzaei, M.; Torkzadeh-Mahani, M. A selective and regenerable voltammetric aptasensor for determination of homocysteine. Microchim. Acta 2016, 183, 2205–2210. [Google Scholar] [CrossRef]
- Fouladgar, M.; Mohammadzadeh, S.; Nayeri, H. Electrochemical determination of homocysteine using carbon nanotubes modified paste electrode and isoprenaline as a mediator. Russ. J. Electrochem. 2014, 50, 981–988. [Google Scholar] [CrossRef]
- Soltani, H.; Beitollahi, H.; Hatefi-Mehrjardi, A.H.; Tajik, S.; Mahani, M.T. Nanostructured base electrochemical sensor for voltammetric determination of homocysteine using a modified single-walled carbon nanotubes paste electrode. Ionics 2014, 20, 1481–1488. [Google Scholar] [CrossRef]
- Eksin, E.; Erdem, A. Electrochemical Determination of Homocysteine at Disposable Graphite Electrodes. Electroanalysis 2015, 26, 1945–1951. [Google Scholar] [CrossRef]
- Lee, P.T.; Lowinsohn, D.; Compton, R.G. The selective electrochemical detection of homocysteine in the presence of glutathione, cysteine, and ascorbic acid using carbon electrodes. Analyst 2014, 139, 3755–3762. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, S.; Fouladgar, M. Electrocatalytic oxidation and determination of homocysteine at carbon nanotubes modified paste electrode using dopamine as a mediator. J. Serb. Chem. Soc. 2013, 78, 1595–1607. [Google Scholar] [CrossRef]
- Gholamiorimi, F.; Taleshi, F.; Biparva, P.; Karimi-Maleh, H.; Beitollahi, H.; Ebrahimi, H.R.; Shamshiri, M.; Bagheri, H.; Fouladgar, M.; Taherkhani, A. Voltammetric Determination of Homocysteine Using Multiwall Carbon Nanotube Paste Electrode in the Presence of Chlorpromazine as a Mediator. J. Anal. Methods Chem. 2012, 2012, 902184. [Google Scholar] [PubMed]




| Electrode | Solution | Peak Potential (V) | Method | Linear Range (μM) | Sensitivity (nA/μM) | Detection Limit (nM) | Reference |
|---|---|---|---|---|---|---|---|
| MWCNTs/GCE | 0.2 M PB (pH 7.5) | 0.06 | LSV | 2.5−10 10−100 100−1000 | 16 8 1 | 890 | [10] |
| rGO−TiO2/GCE | 0.1 M PBS (pH 8.0) | 0.35 | I-t | 0.1−80 | 91.14 | 24 | [13] |
| AuNPs/rGO/GCE | 0.1 M PBS (pH 7.0) | 0.12 | I-t | 2000−14,000 | 14.8 | 6900 | [27] |
| Aptamer/AuNPs/GS | 0.1 M PBS (pH 7.4) | −0.14 | DPV | 1−100 | 630 | 1000 | [35] |
| DA−GO/SPE | 0.1 M PBS (pH 7.0) | 0.22 | DPV | 0.5−50 50−900 | 381 101 | 150 | [36] |
| Aptamer/nano−Au/GCE | 0.1 M PBS (pH 7.0) | 0.8 | DPV | 0.05−20.0 | 36 | 9 | [37] |
| Aptamer/G/GCE | 0.25 M PBS (pH 7.0) | 0.9 | DPV | 0.05−20.0 | / | 5 | [38] |
| Aptamer/Au electrode | 0.01 M PBS (pH 7.4) | 1.07 | DPV | 0.2−10 | / | 10 | [39] |
| ISP/MWCNTPE | Universal buffer (pH 3.5) | 0.64 | LSV | 5−800 | / | 3300 | [40] |
| BFCNPE | 0.1 M PBS (pH 7.0) | 0.67 | SWV | 0.1−10 10.0−80 | 141 54 | 50 | [41] |
| Pre-treated PGE | 0.05 M PBS (pH 7.4) | 0.564 | DPV | 2−20 | 20.03 | 1210 | [42] |
| MWCNTs/GCE | 0.15 M PBS (pH 7.0) | −0.2 | SWV | 0−10 | 200 | 660 | [43] |
| MWCNTPE | Universal buffer (pH 5.0) + 0.1 M KCl | 0.53 | DPV | 3−600 | 8 | 2080 | [44] |
| MWCNTPE | 0.04 M universal buffer (pH 4.0) + 400 μM CHP | 0.717 | SWV | 0.1−210 | 68 | 80 | [45] |
| AuNPs/AB–DHP/GCE | 0.15 M PBS (pH 6.0) | 0.4 | I-t | 3−1000 | 18 | 600 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Zhang, J.; Zhang, S.; Liu, C.; Liu, X.; Jin, J.; Zheng, D. An Amperometric Biomedical Sensor for the Determination of Homocysteine Using Gold Nanoparticles and Acetylene Black-Dihexadecyl Phosphate-Modified Glassy Carbon Electrode. Micromachines 2023, 14, 198. https://doi.org/10.3390/mi14010198
Zhu C, Zhang J, Zhang S, Liu C, Liu X, Jin J, Zheng D. An Amperometric Biomedical Sensor for the Determination of Homocysteine Using Gold Nanoparticles and Acetylene Black-Dihexadecyl Phosphate-Modified Glassy Carbon Electrode. Micromachines. 2023; 14(1):198. https://doi.org/10.3390/mi14010198
Chicago/Turabian StyleZhu, Chunnan, Jingfang Zhang, Shunrun Zhang, Chao Liu, Xiaojun Liu, Jian Jin, and Dongyun Zheng. 2023. "An Amperometric Biomedical Sensor for the Determination of Homocysteine Using Gold Nanoparticles and Acetylene Black-Dihexadecyl Phosphate-Modified Glassy Carbon Electrode" Micromachines 14, no. 1: 198. https://doi.org/10.3390/mi14010198






