Electrolytic Characteristics of Microhole Array Manufacturing Using Polyacrylamide Electrolyte in 304 Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
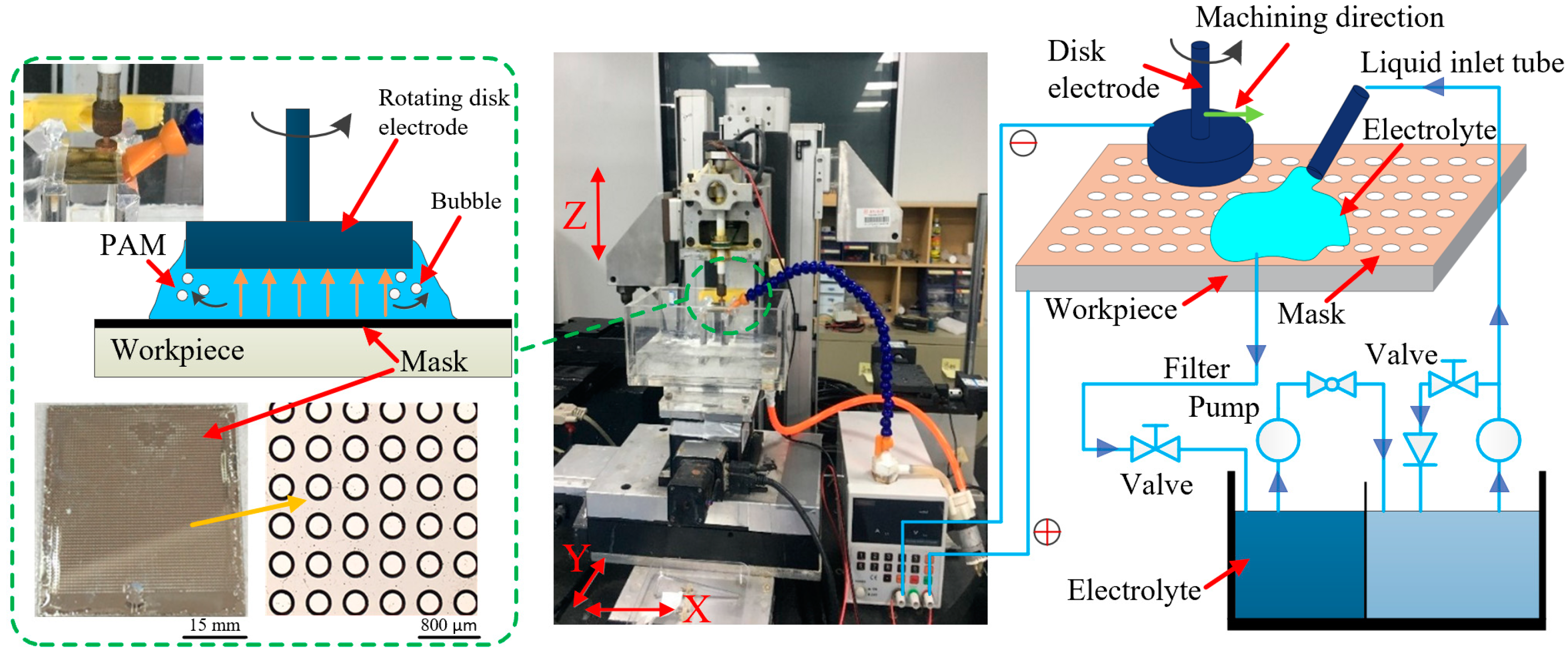
2.1. Experimental Device
2.2. Electrolyte
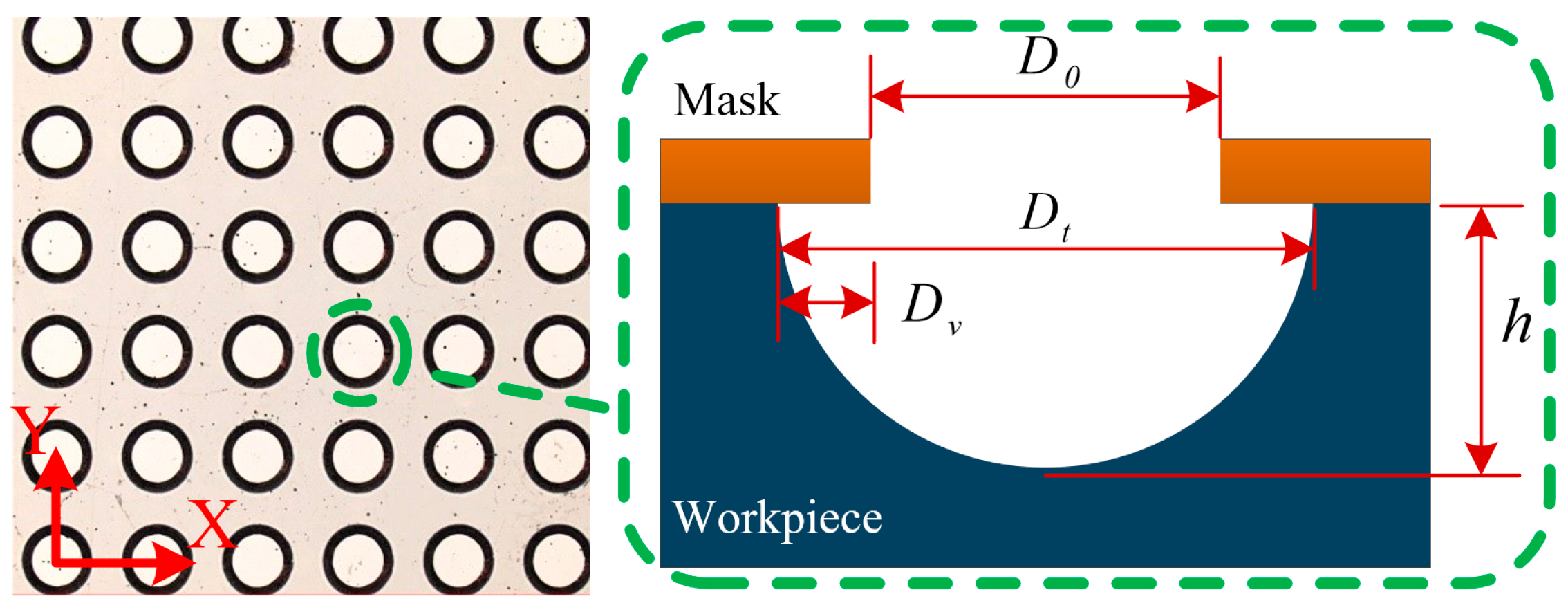
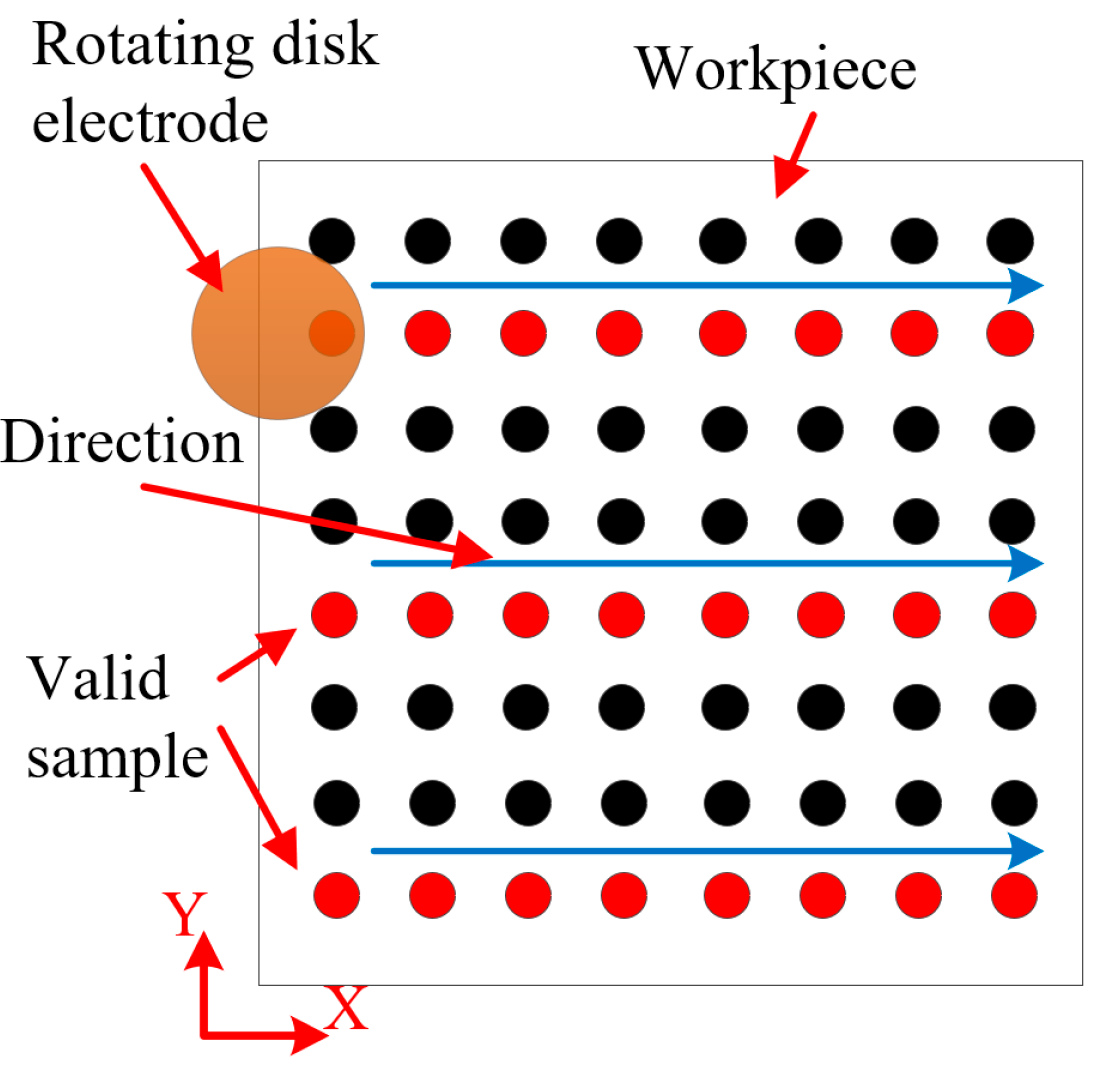
2.3. Experimental Design
3. Results and Discussion
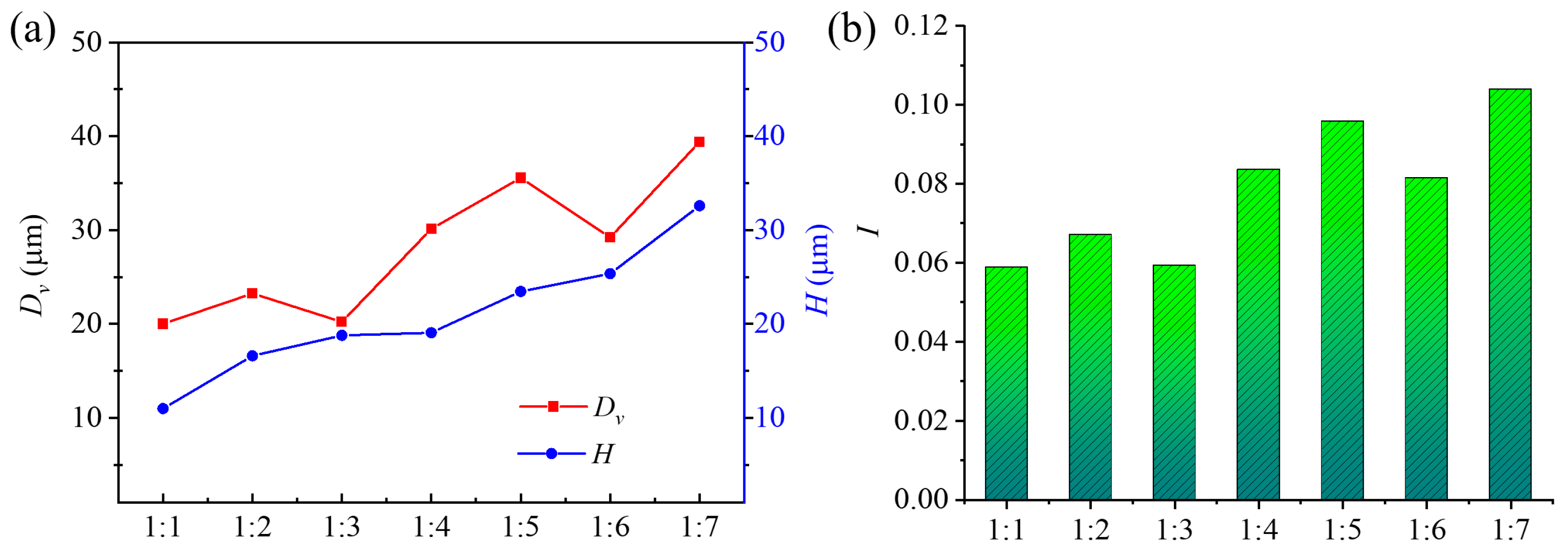
3.1. Viscosity of PAM Electrolyte
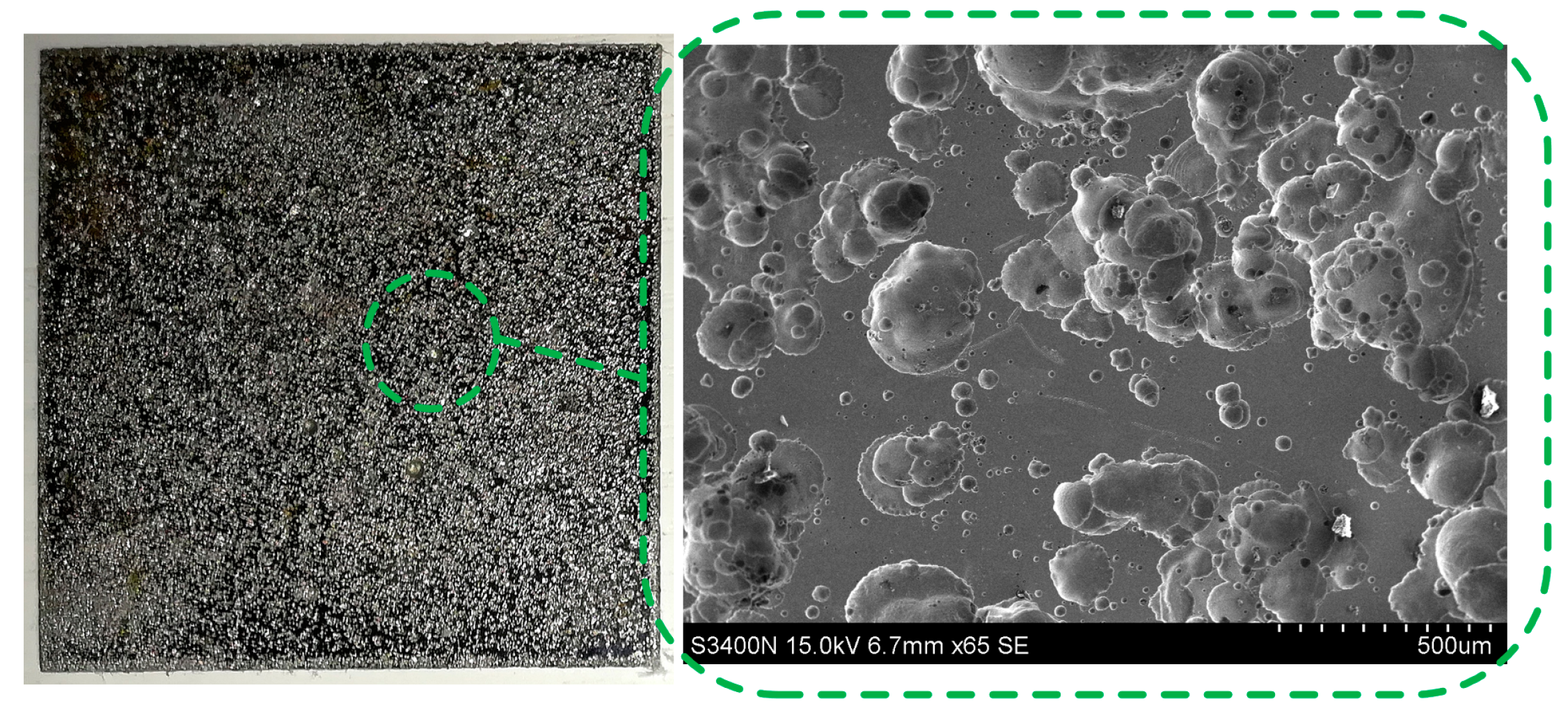
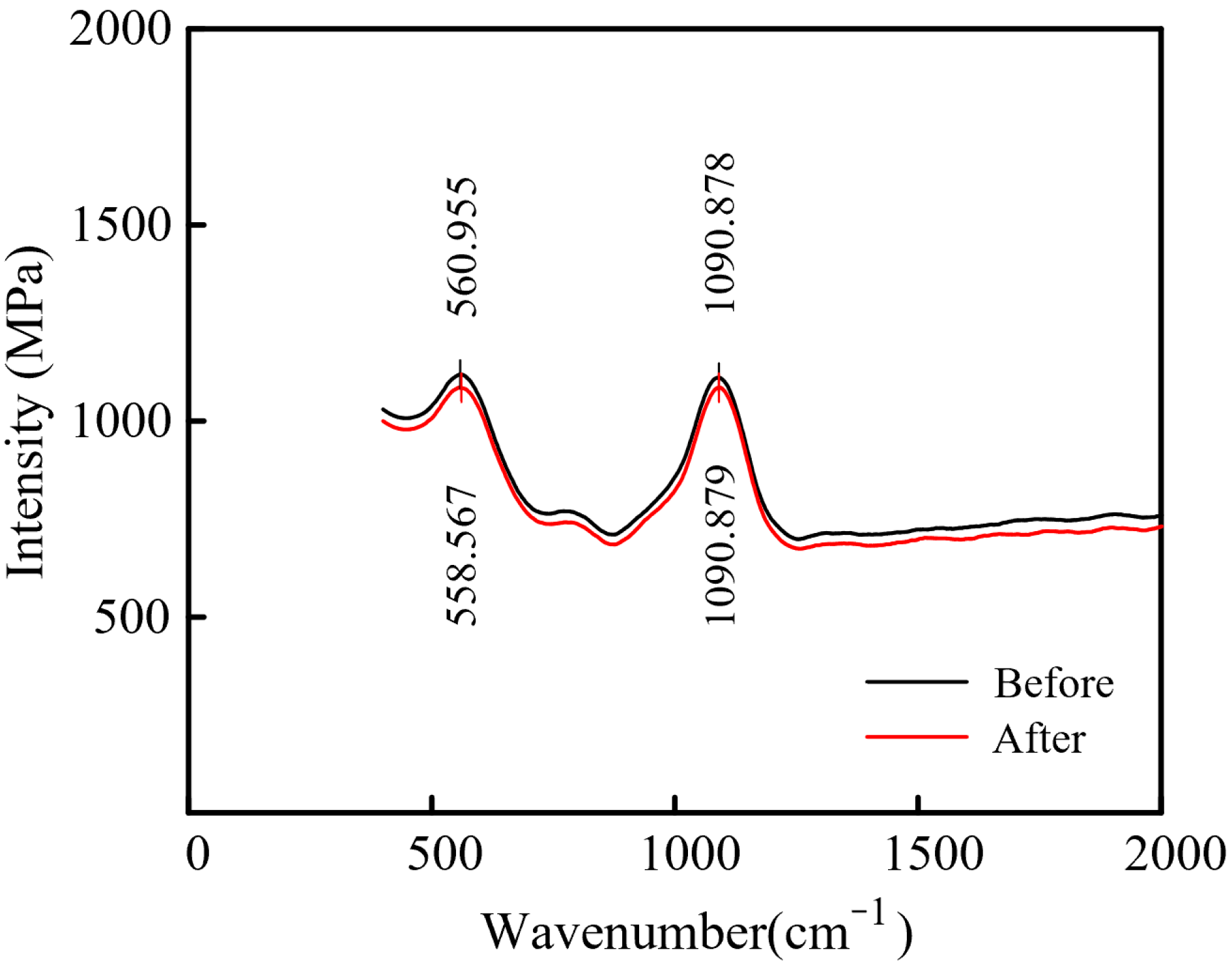
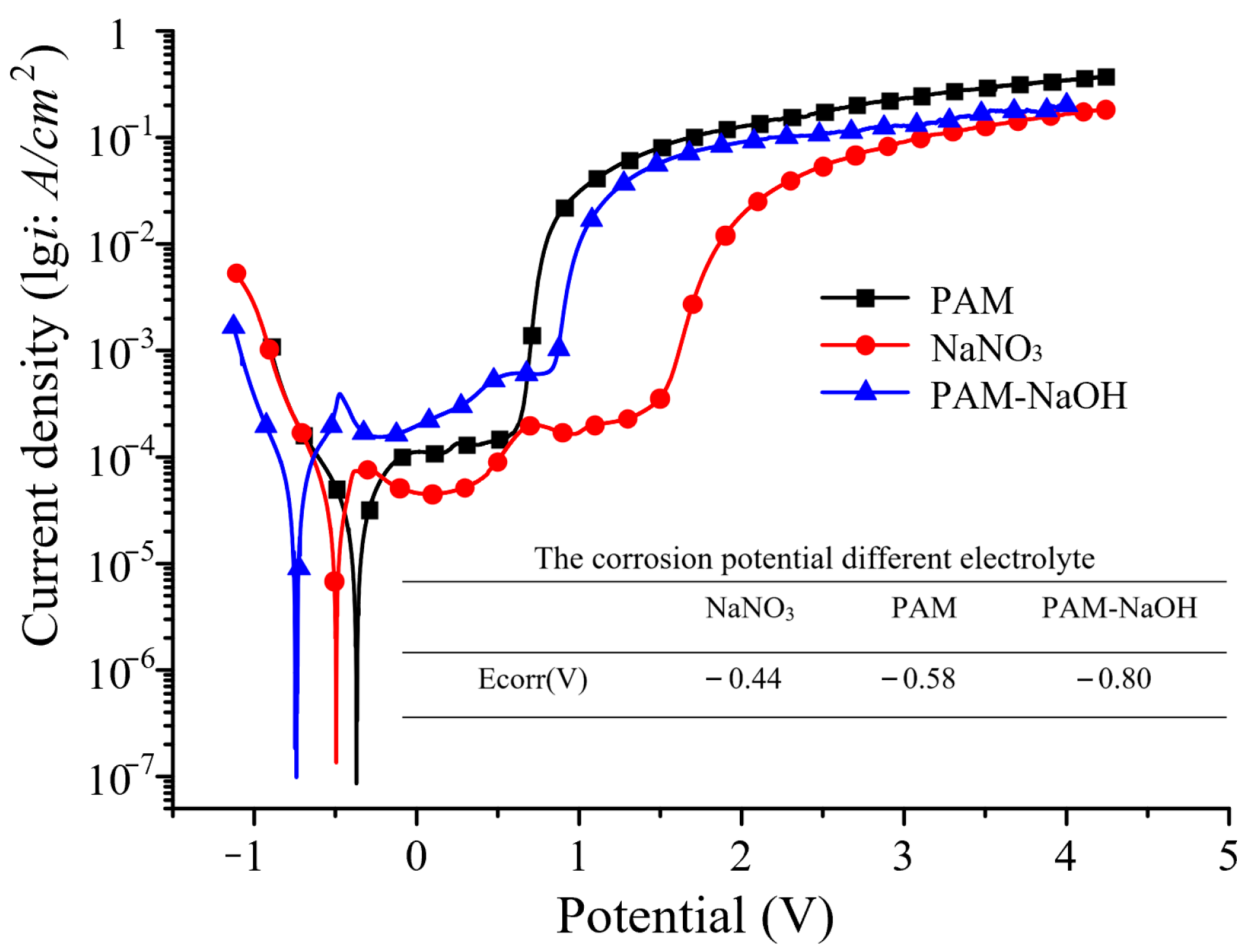
3.2. Electrochemical Dissolution Characteristics
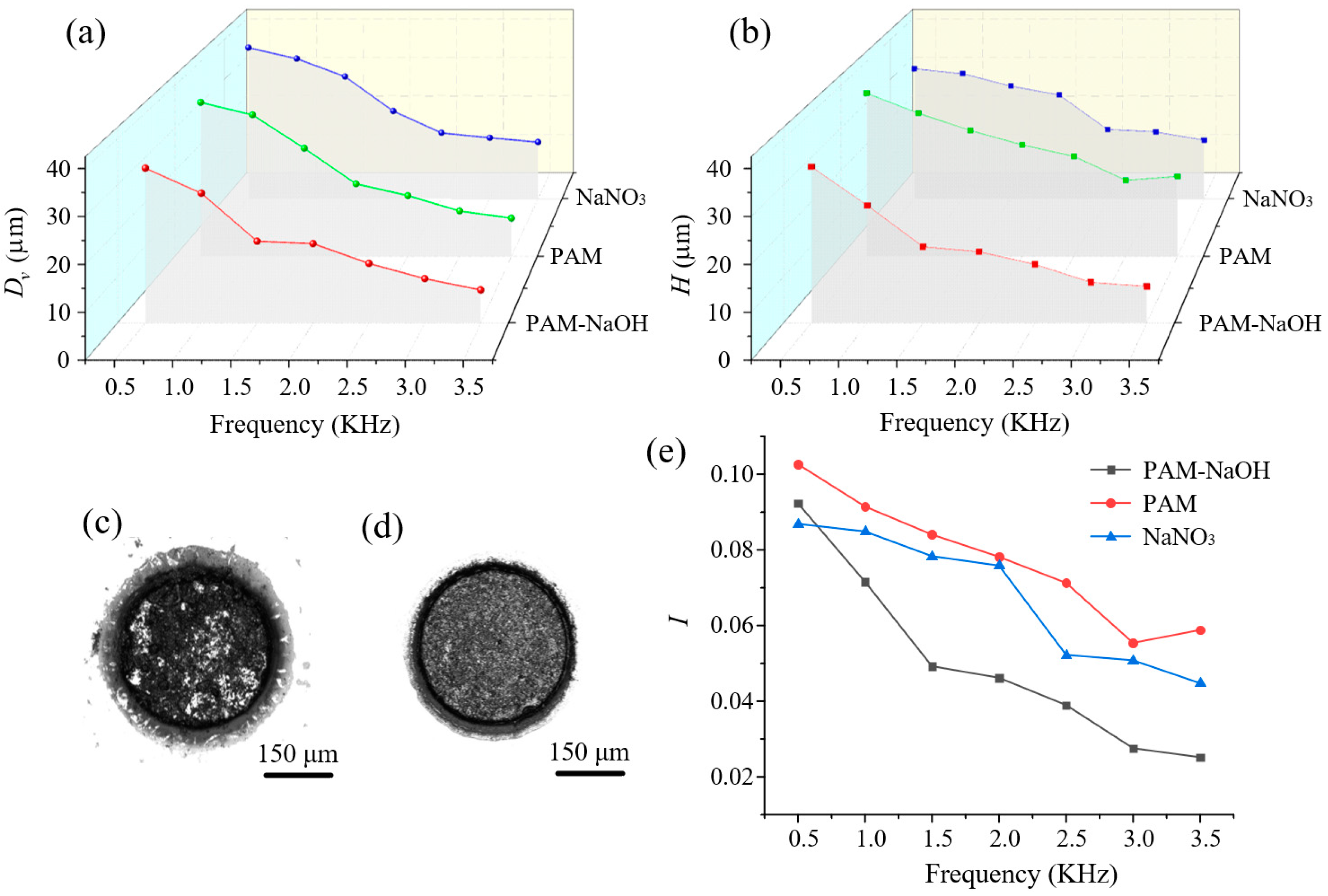
3.3. Single-Factor Experiments
3.3.1. Effects of Pulse Voltage
3.3.2. Effects of Frequency
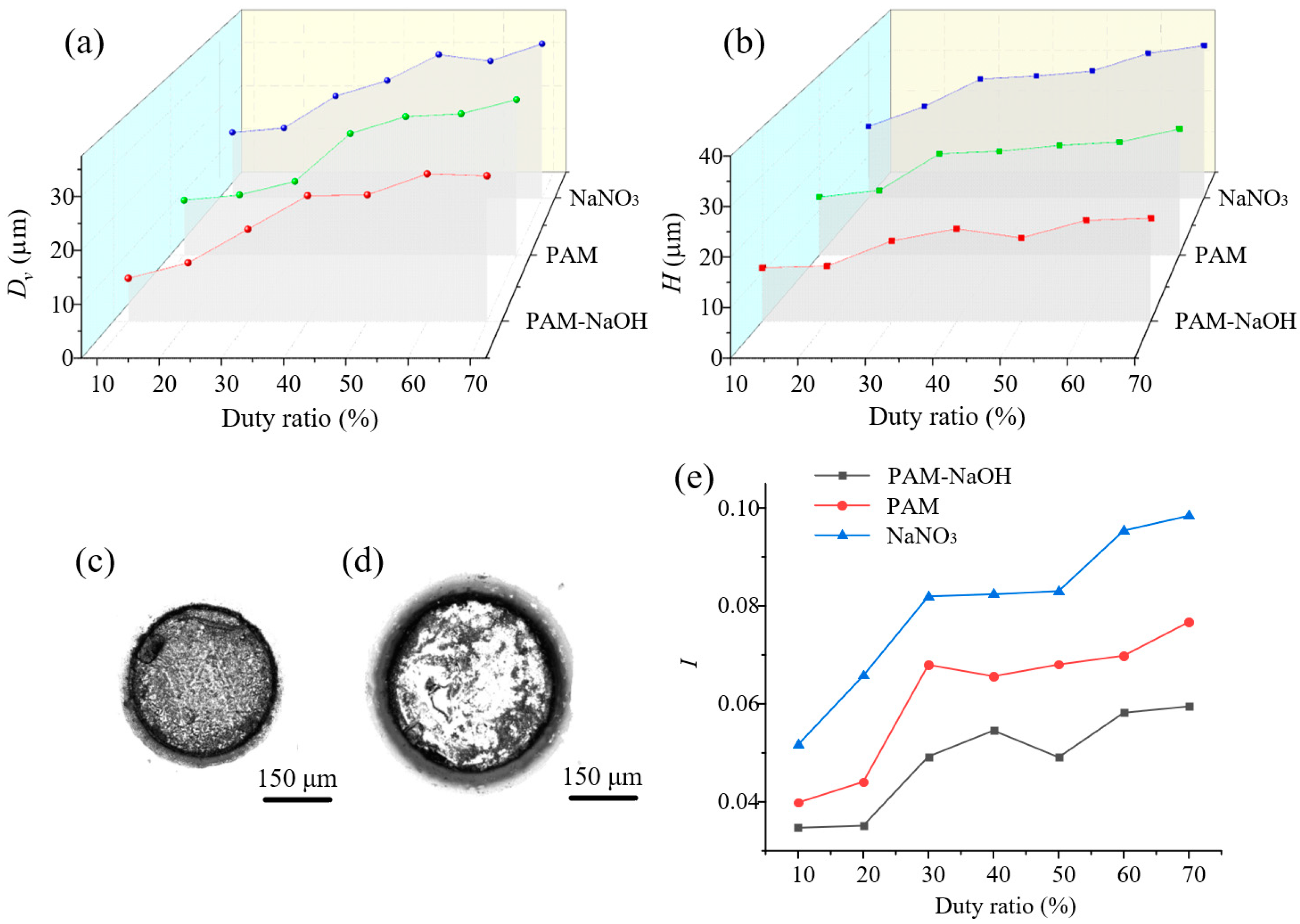
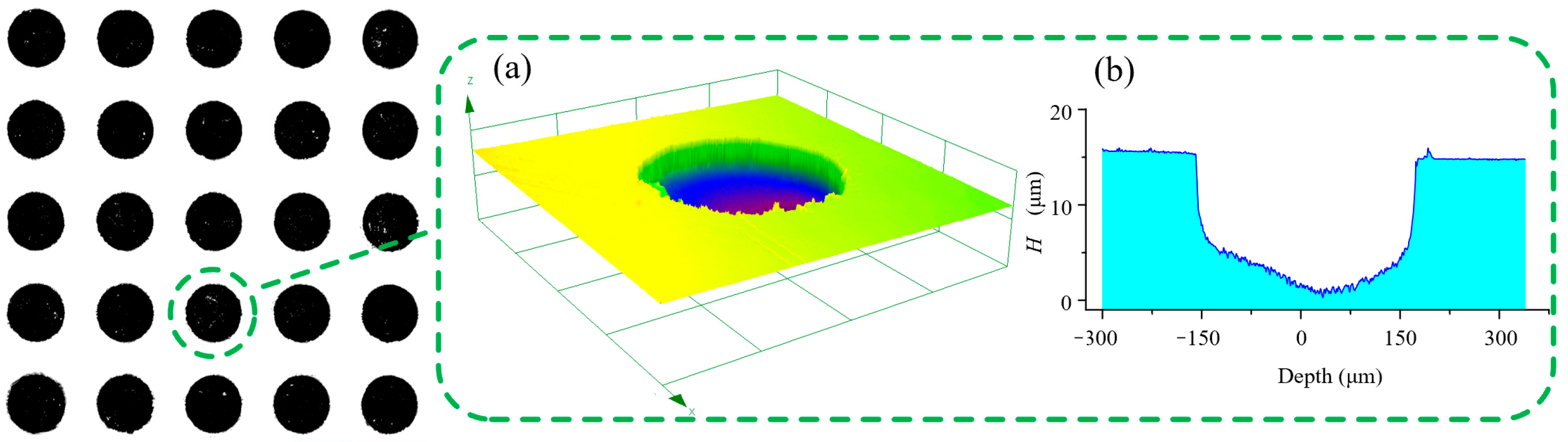
3.3.3. Effects of Duty Ratio
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, D.Y.; Zhang, J.C. Experimental research on the counter-rotating electrochemical machining of 304 stainless steel and Inconel 718 alloy. Int. J. Electrochem. Sci. 2019, 14, 9741–9754. [Google Scholar] [CrossRef]
- Gomez-Gallegos, A.; Mill, F.; Mount, A.R.; Duffield, S.; Sherlock, A. 3D multiphysics model for the simulation of electrochemical machining of stainless steel (SS316). Int. J. Adv. Manuf. Technol. 2018, 95, 2959–2972. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Ma, J.; Xu, W.; Sun, J.; Liu, X.; Song, J. Solid-Like Slippery Coating with Highly Comprehensive Performance. Adv. Funct. Mater. 2023, 33, 2302311. [Google Scholar] [CrossRef]
- Yan, D.; Chen, Y.; Liu, J.; Song, J. Super-Fast Fog Collector Based on Self-Driven Jet of Mini Fog Droplets. Small 2023, 19, 2301745. [Google Scholar] [CrossRef]
- Bian, J.; Ma, B.; Ai, H.; Qi, L. Experimental study on the influence of tool electrode material on electrochemical micromachining of 304 stainless steel. Materials 2021, 14, 2311. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.; Dong, C.; Li, X. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2017, 400, 38–48. [Google Scholar] [CrossRef]
- Qi, X.; Mao, H.; Yang, Y. Corrosion behavior of nitrogen alloyed martensitic stainless steel in chloride containing solutions. Corros. Sci. 2017, 120, 90–98. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. The electrochemical behaviour of stainless steel AISI 304 in alkaline solutions with different pH in the presence of chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Wang, J.; Natsu, W. Mechanism and characteristics of electrochemical machining using electrolyte absorbed in solid porous ball. Precis. Eng. 2022, 77, 307–319. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Liang, H.; Zhou, L.; Jian, Y.; Zhou, W. Experimental study on the evolution process of Zr702 microdimple array generated with masked jet electrochemical machining. Int. J. Adv. Manuf. Technol. 2023, 124, 973–987. [Google Scholar] [CrossRef]
- He, J.; Wang, Z.; Wang, J.; Liang, H.; Lian, H. Investigation of the microhole arrays generated by masked jet electrochemical machining with polyaluminum chloride electrolyte. Precis. Eng. 2023, 82, 370–382. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Cui, Z.; Xiao, K.; Yu, Q.; Li, X. A comparative study of primary and secondary passive films formed on AM355 stainless steel in 0.1 M NaOH. Appl. Surf. Sci. 2018, 427, 763–773. [Google Scholar] [CrossRef]
- Patel, D.S.; Sharma, V.; Jain, V.K.; Ramkumar, J. Reducing overcut in electrochemical micromachining process by altering the energy of voltage pulse using sinusoidal and triangular waveform. Int. J. Mach. Tools Manuf. 2020, 151, 103526. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, Z.; Wang, N.; Zhu, D.; Wang, H. Investigation of the electrochemical dissolution behavior of Inconel 718 and 304 stainless steel at low current density in NaNO3 solution. Electrochim. Acta 2015, 156, 301–307. [Google Scholar] [CrossRef]
- Wang, M.; Qu, N. Electrochemical dissolution behavior of S-04 high-strength stainless steel in NaNO3 aqueous solution. J. Appl. Electrochem. 2020, 50, 1149–1163. [Google Scholar] [CrossRef]
- Nie, J.; Wei, L.; Jiang, Y.; Li, Q.; Luo, H. Corrosion mechanism of additively manufactured 316 L stainless steel in 3.5 wt.% NaCl solution. Mater. Today Commun. 2021, 26, 101648. [Google Scholar] [CrossRef]
- Liu, G.; Tong, H.; Li, Y.; Tan, Q.; Zhu, Y. Passivation behavior of S136H steel in neutral electrolytes composed of NaClO3 and NaNO3 and its influence on micro electrochemical machining performance. Mater. Today Commun. 2021, 29, 102762. [Google Scholar] [CrossRef]
- Haisch, T.; Mittemeijer, E.; Schultze, J.W. Electrochemical machining of the steel 100Cr6 in aqueous NaCl and NaNO3 solutions: Microstructure of surface films formed by carbides. Electrochim. Acta 2001, 47, 235–241. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Lv, Y.; Yang, Z.; Yao, J.; He, Y.; Tian, Z. Electrochemical machining of deep narrow slits on TB6 titanium alloys. Int. J. Adv. Manuf. Technol. 2017, 92, 3063–3071. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Z.; Kunieda, M. Electrolyte jet machining of Ti1023 titanium alloy using NaCl ethylene glycol-based electrolyte. J. Mater. Process. Technol. 2020, 283, 116731. [Google Scholar] [CrossRef]
- Thanigaivelan, R.; Arunachalam, R.M.; Karthikeyan, B.; Loganathan, P. Electrochemical micromachining of stainless steel with acidified sodium nitrate electrolyte. Procedia CIRP 2013, 6, 351–355. [Google Scholar] [CrossRef]
- Lo, I.H.; Fu, Y.; Lin, C.J.; Tsai, W. Effect of electrolyte composition on the active-to-passive transition behavior of 2205 duplex stainless steel in H2SO4/HCl solutions. Corros. Sci. 2006, 48, 696–708. [Google Scholar] [CrossRef]
- Kumaravel, P.; Suresh, P.; Venkatesh Raja, K.V.; Sekar, T. Improvement of micro-electrochemical discharge machining of austenitic stainless steel 316L using NaOH electrolyte containing N2. Int. J. Electrochem. Sci. 2022, 17, 220747. [Google Scholar] [CrossRef]
- Hui, C.; YuKui, W.; ZhenLong, W.; WanSheng, Z. Effects of complexing agent on electrochemical micro machining of stainless steel. Curr. Res. Nanotechnol. 2010, 1, 7–12. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Yuk, H.; Lin, S.; Ma, C.; Takaffoli, M.; Fang, N.X.; Zhao, X. Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nat. Commun. 2017, 8, 14230. [Google Scholar] [CrossRef]




| C | Mn | P | S | Si | Cr | Ni | N | |
|---|---|---|---|---|---|---|---|---|
| SS304 (wt.%) | 0.08 | 2 | 0.035 | 0.015 | 0.75 | 18–20 | 8–10.5 | 0.1 |
| Parameter | pH Value | Surface Tension [mN/m] | Viscosity [Pa∙s] | Conductivity [mS/cm] |
|---|---|---|---|---|
| 1% | 7.3 | 45.93 | 0.021 | 6.68 |
| 2% | 7.1 | 32.3 | 0.035 | 9.54 |
| 3% | 6.9 | 31.05 | 0.58 | 12.84 |
| Parameter | Value(s) |
|---|---|
| PAM [wt.%] | 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 |
| Shear rate [rpm] | 60, 120, 180, 240, 300, 360 |
| Time [s] | 30 |
| Pressure [kPa] | 101.325 |
| Temperature [°C] | 25 |
| Parameter | Value(s) |
|---|---|
| PAM [wt.%] | 2, 3, 4, 5 |
| NaNO3 [wt.%] | 2 |
| PAM:NaOH [wt.%] | 1:1 |
| Measuring potential [V] | −2 to 4 |
| Scan rate [mV/s] | 1 |
| Temperature [°C] | 25 |
| Parameter | Value(s) |
|---|---|
| Machining gap [mm] | 1 |
| Mask aperture [μm] | 300 |
| Concentration [wt.%] | 2 |
| Feed speed [μm/s] | 50 |
| Spindle speed [rpm] | 500 |
| Pulse voltage [V] | 5, 10, 15, 20, 25, 30, 35 |
| Duty ratio [%] | 10, 20, 30, 40, 50, 60, 70 |
| Frequency [kHz] | 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Wang, Z.; Zhou, W.; Jian, Y.; Zhou, L. Electrolytic Characteristics of Microhole Array Manufacturing Using Polyacrylamide Electrolyte in 304 Stainless Steel. Micromachines 2023, 14, 1808. https://doi.org/10.3390/mi14101808
He J, Wang Z, Zhou W, Jian Y, Zhou L. Electrolytic Characteristics of Microhole Array Manufacturing Using Polyacrylamide Electrolyte in 304 Stainless Steel. Micromachines. 2023; 14(10):1808. https://doi.org/10.3390/mi14101808
Chicago/Turabian StyleHe, Junfeng, Zan Wang, Wenjie Zhou, Yue Jian, and Li Zhou. 2023. "Electrolytic Characteristics of Microhole Array Manufacturing Using Polyacrylamide Electrolyte in 304 Stainless Steel" Micromachines 14, no. 10: 1808. https://doi.org/10.3390/mi14101808
APA StyleHe, J., Wang, Z., Zhou, W., Jian, Y., & Zhou, L. (2023). Electrolytic Characteristics of Microhole Array Manufacturing Using Polyacrylamide Electrolyte in 304 Stainless Steel. Micromachines, 14(10), 1808. https://doi.org/10.3390/mi14101808





