PANI-Coated VOx Nanobelts with Core-Shell Architecture for Flexible All-Solid-State Supercapacitor
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of VOx Nanobelt
2.3. Synthesis of VOx@PANI Core-Shell Nanobelt
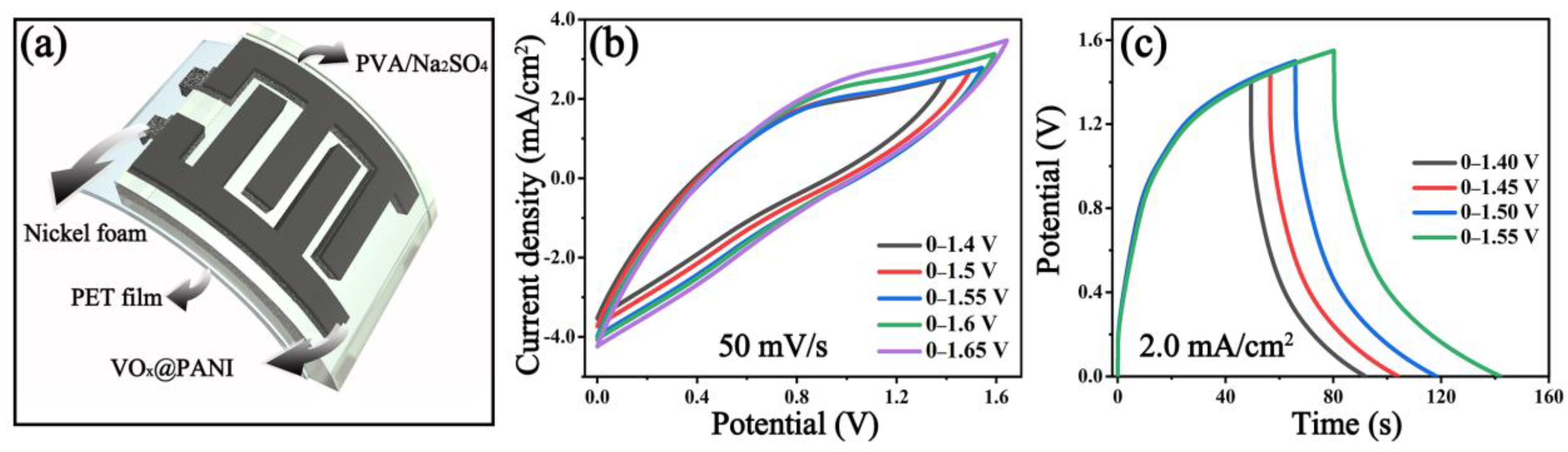
2.4. Fabrication of Symmetric VOx@PANI Supercapacitor
2.5. Electrochemical Testing
2.6. Characterization
3. Results and Discussion
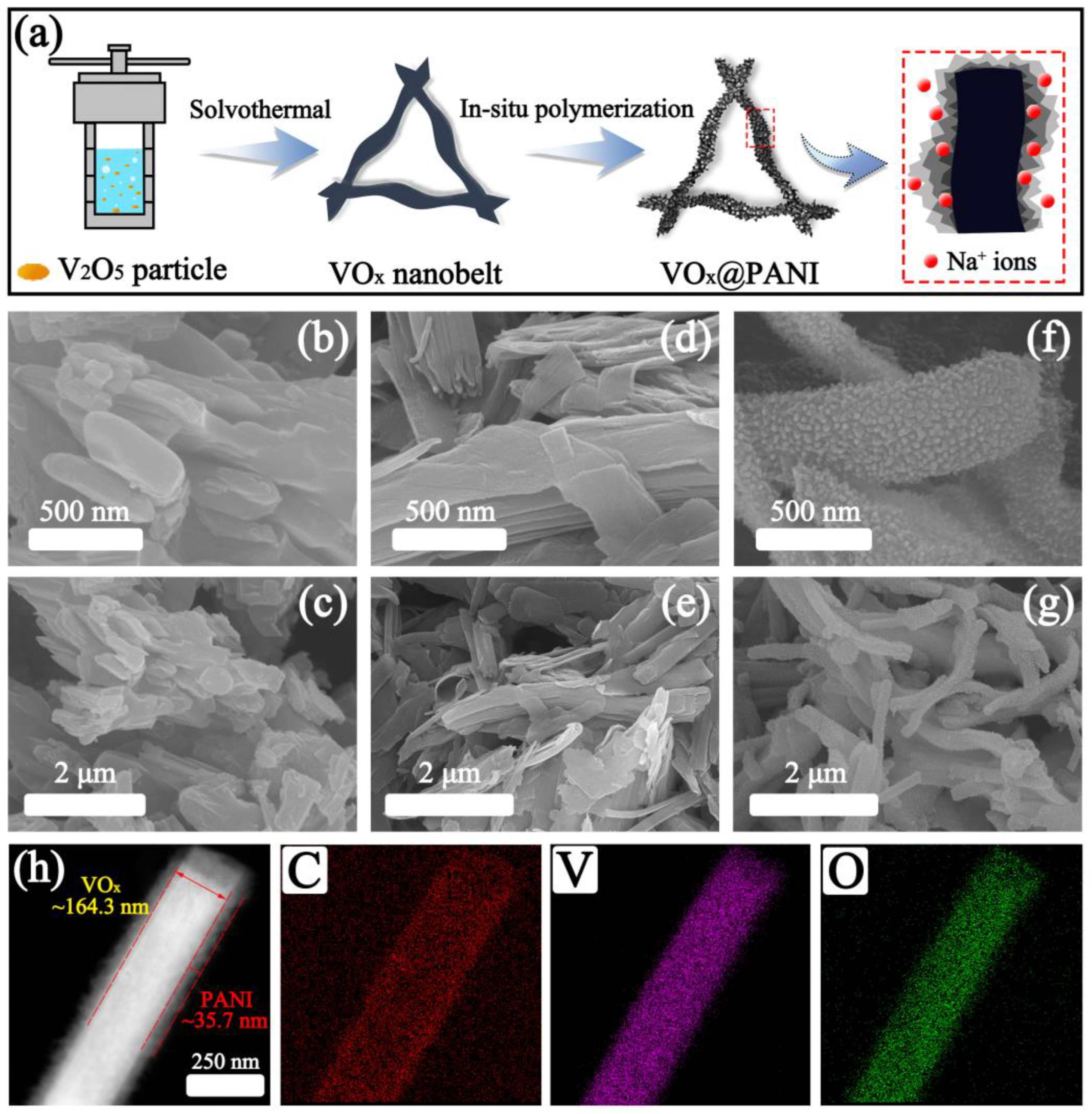
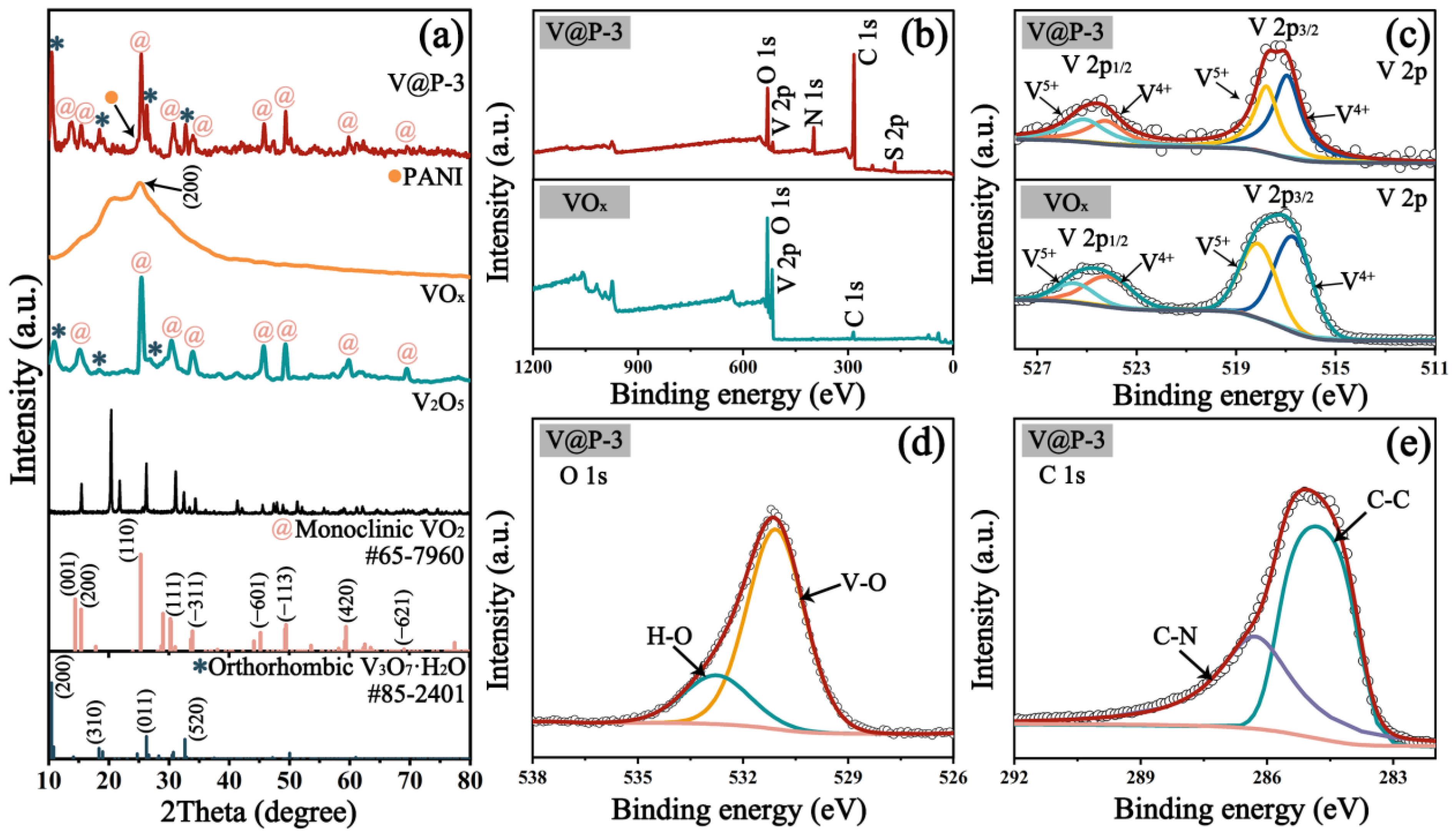
3.1. Characterization
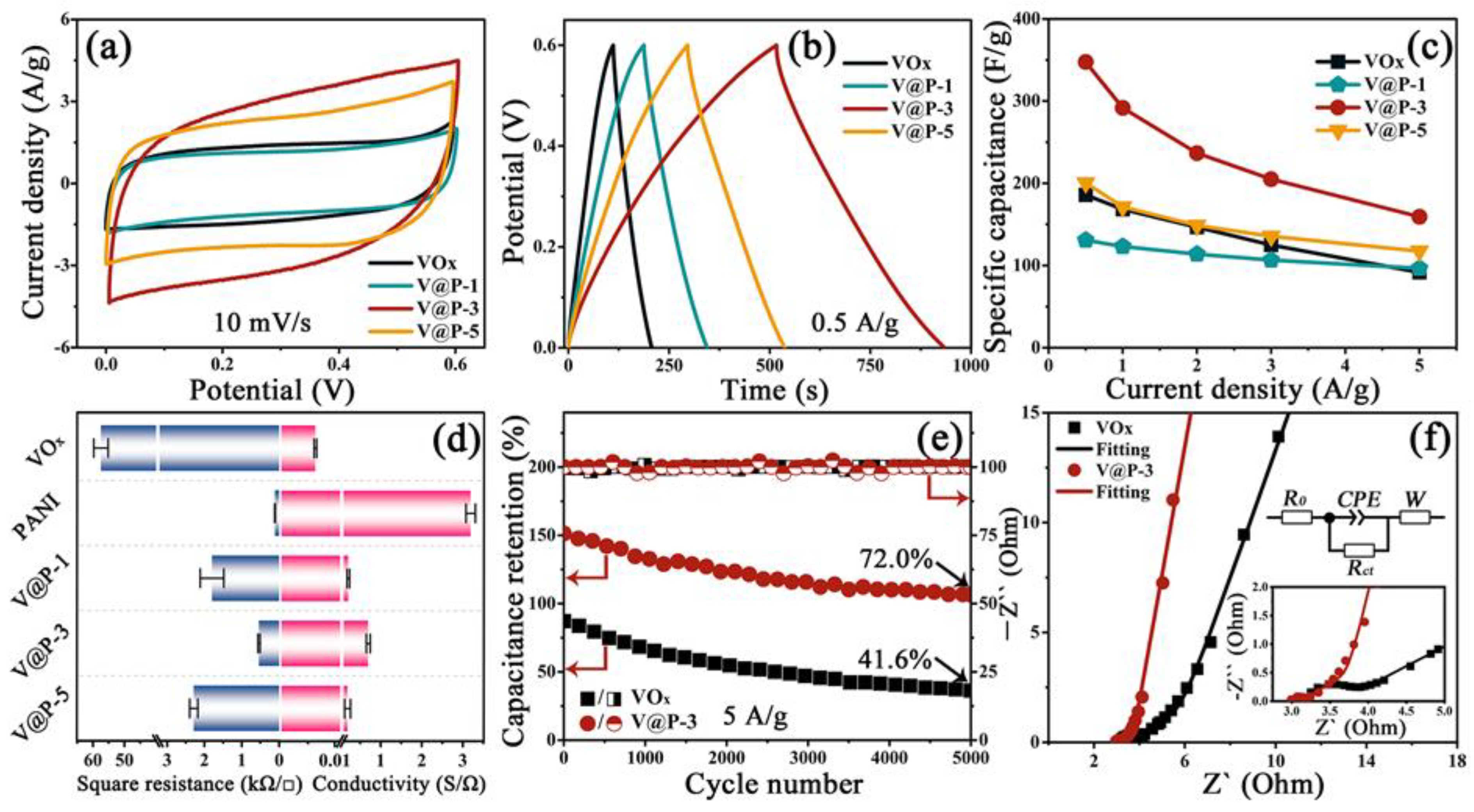
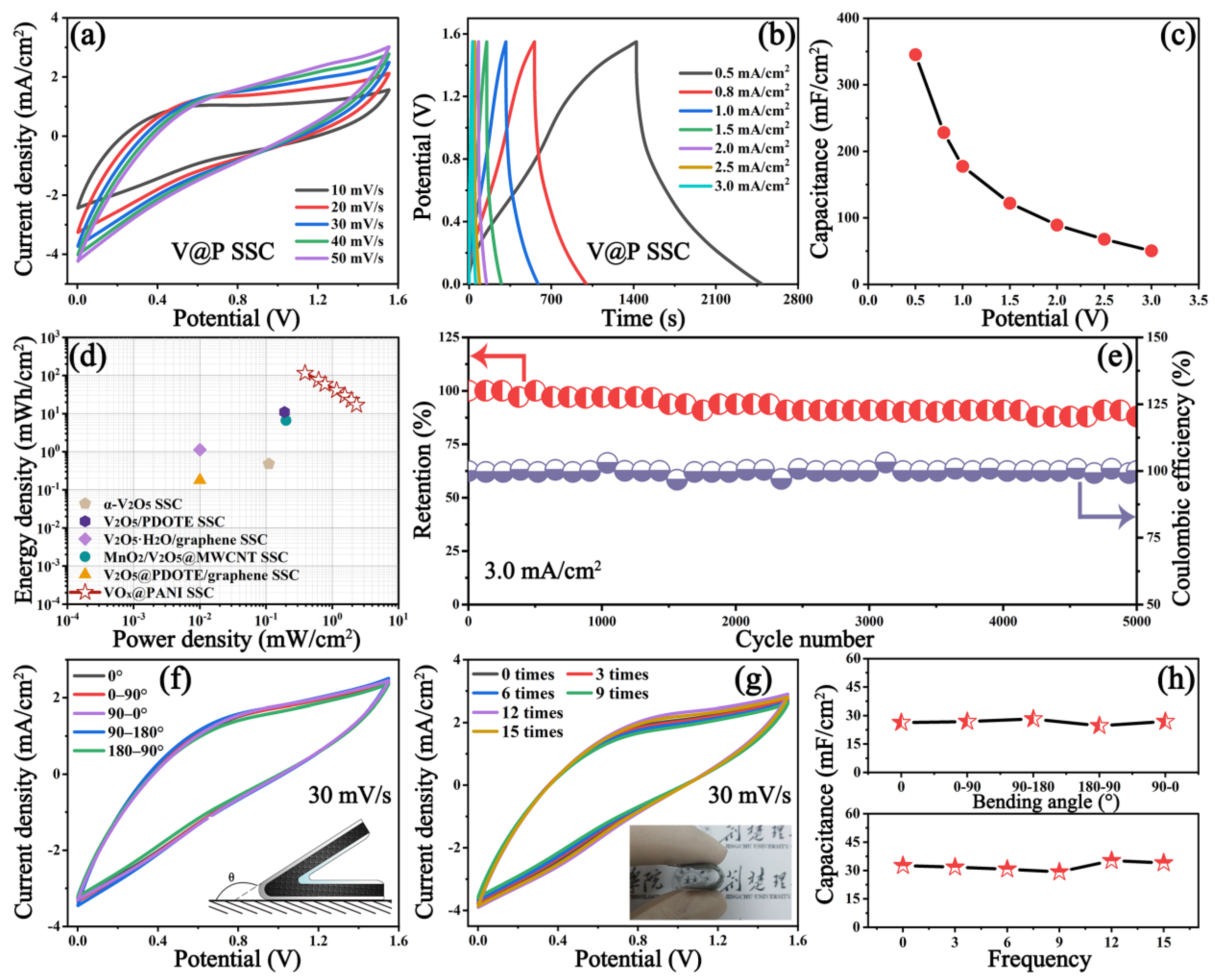
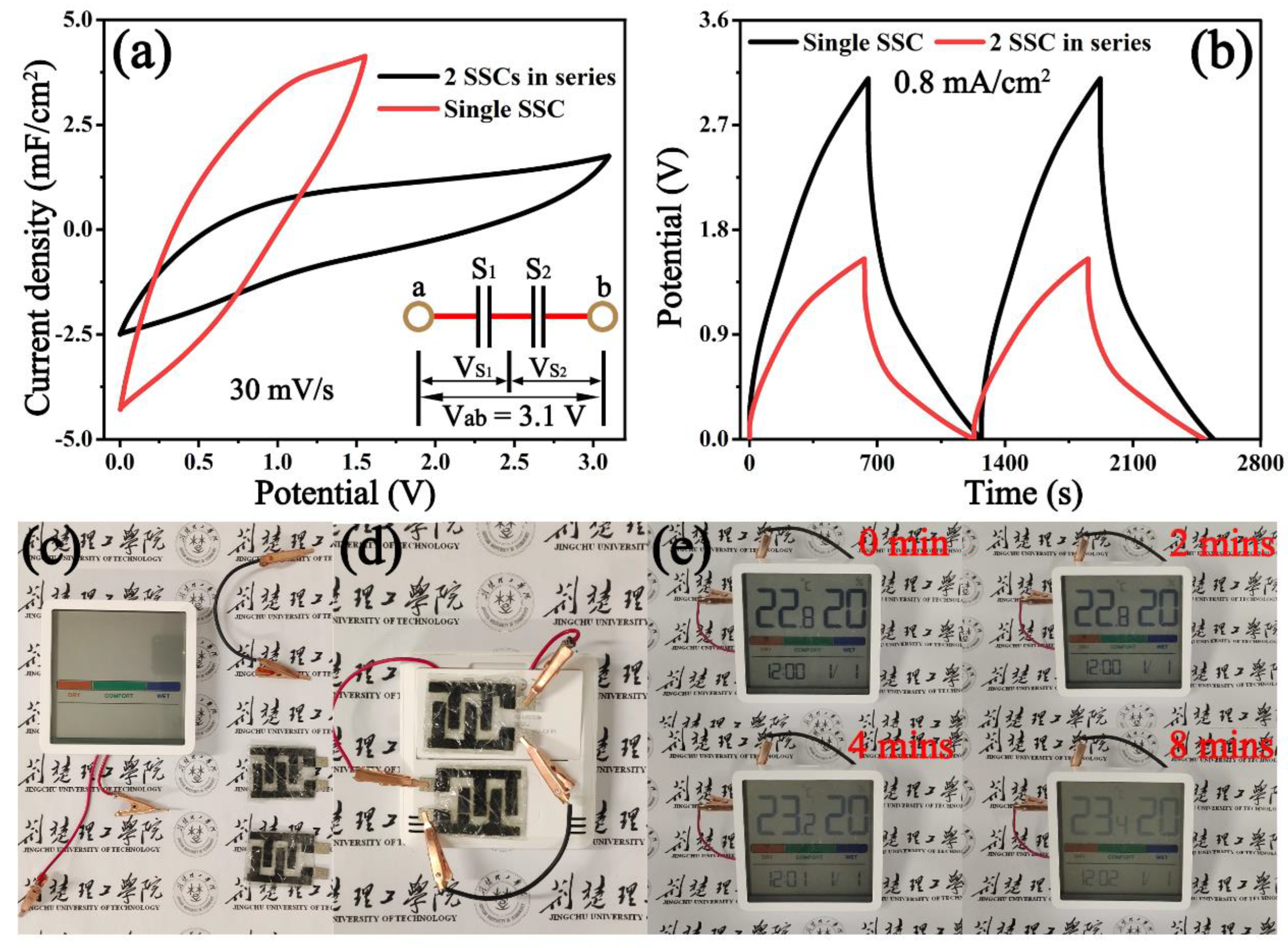
3.2. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, S.C.; Lee, Y.J.; Kang, H.; Kang, Y.C. Carbon-Coated Three-dimensional MXene/Iron Selenide Ball with Core–Shell Structure for High-Performance Potassium-ion Batteries. Nano-Micro Lett. 2021, 14, 1–17. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2020, 33, 2000619. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of Current Development in Electrical Energy Storage Technologies and the Application Potential in Power System Operation. Appl. Energy 2015, 137, 511–536. [Google Scholar]
- Liu, C.; Wang, B.; Xu, L.; Zou, K.; Deng, W.; Hou, H.; Zou, G.; Ji, X. Novel Nonstoichiometric Niobium Oxide Anode Material with Rich Oxygen Vacancies for Advanced Lithium-Ion Capacitors. ACS Appl. Mater. Interfaces 2023, 15, 5387–5398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, H.; Meng, T.; Yang, J.; Huang, B.; Gu, F.L.; Zhang, S.; Meng, C.; Tong, Y.X. Boosting Electron Transfer with Heterointerface Effect for High-Performance Lithium-Ion Storage. Energy Storage Mater. 2021, 36, 365–375. [Google Scholar] [CrossRef]
- Li, P.; Shang, T.; Dong, X.; Li, H.; Tao, Y.; Yang, Q. A Review of Compact Carbon Design for Supercapacitors with High Volumetric Performance. Small 2021, 17, 2007548. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Multidimensional Materials and Device Architectures for Future Hybrid Energy Storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, J.; Fan, Z. Carbon Materials for High Volumetric Performance Supercapacitors: Design, Progress, Challenges and Opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Patake, V.D.; Joshi, S.S.; Lokhande, C.D.; Joo, O.-S. Electrodeposited Porous and Amorphous Copper Oxide Film for Application in Supercapacitor. Mater. Chem. Phys. 2009, 114, 6–9. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Beirne, S.; Razal, J.; Chen, J. Recent Development of Fabricating Flexible Micro-Supercapacitors for Wearable Devices. Adv. Mater. Technol. 2018, 3, 1800028. [Google Scholar] [CrossRef]
- Santos, R.; Loureiro, J.; Nogueira, A.F.; Elangovan, E.; Pinto, J.V.; Veiga, J.P.; Busani, T.; Fortunato, E.; Martins, R.; Ferreira, I. Thermoelectric Properties of V2O5 Thin Films Deposited by Thermal Evaporation. Appl. Surf. Sci. 2013, 282, 590–594. [Google Scholar] [CrossRef]
- Mohd, A.; Sanger, A.; Singh, A. One-Step Sputtered Titanium Nitride Nano-Pyramid Thin Electrodes for Symmetric Super-Capacitor Device. Mater. Lett. 2019, 245, 142–146. [Google Scholar]
- Zhang, Y.; Zhu, Y.; Zheng, S.; Zhang, L.; Shi, X.; He, J.; Chou, X.; Wu, Z.-S. Ink Formulation, Scalable Applications and Challenging Perspectives of Screen Printing for Emerging Printed Microelectronics. J. Energy Chem. 2021, 63, 498–513. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Zhang, Y.J.; Ren, H.; Hu, X.; Bai, Y. Sand-Milling Fabrication of Screen-Printable Graphene Composite Inks for High-Performance Planar Micro-Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 56319–56329. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Feng, Y.; Dodabalapur, A.; Raju, V.R.; Lovinger, A.J. High-Performance Plastic Transistors Fabricated by Printing Techniques. Chem. Mater. 1997, 9, 1299–1301. [Google Scholar] [CrossRef]
- Pardo, D.A.; Jabbour, G.E.; Peyghambarian, N. Application of Screen Printing in the Fabrication of Organic Light-Emitting Devices. Adv. Mater. 2000, 12, 1249–1252. [Google Scholar] [CrossRef]
- Hyun, W.J.; Secor, E.B.; Hersam, M.C.; Frisbie, C.D.; Francis, L.F. High-Resolution Patterning of Graphene by Screen Printing with a Silicon Stencil for Highly Flexible Printed Electronics. Adv. Mater. 2014, 27, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhao, Z.; Wang, X.; Chen, Y.; Li, D.; Linghu, Y.Y.; Wang, Y.; Wang, C. A High-Performance Asymmetric Supercapacitor-Based (CuCo)Se2/GA Cathode and FeSe2/GA Anode with Enhanced Kinetics Matching. Nanoscale 2021, 13, 6489–6498. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Ding, Z.; Wei, Q.; Wang, Z.; Yang, X.; Qiu, J. Biomass-Based Hierarchical Porous Carbon for Supercapacitors: Effect of Aqueous and Organic Electrolytes on the Electrochemical Performance. ChemSusChem 2019, 12, 5099–5110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, P.; Xiong, T.; Fu, Y.; Yang, H.; Huang, Y.; Li, D.; Deng, J.; Balogun, M.-S. Sub-Thick Electrodes with Enhanced Transport Kinetics via in Situ Epitaxial Heterogeneous Interfaces for High Areal-Capacity Lithium Ion Batteries. Small 2021, 17, 2100778. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, G.; Deng, X.; Tian, Y.; Xiao, X.; Deng, W.; Hou, H.; Zou, G.; Ji, X. Strongly Coupled Interfacial Engineering Inspired by Robotic Arms Enable High-Performance Sodium-Ion Capacitors. Adv. Funct. Mater. 2022, 32, 2205453. [Google Scholar] [CrossRef]
- Hu, H.; Li, Q.; Li, L.; Teng, X.; Feng, Z.; Zhang, Y.; Wu, M.; Qiu, J. Laser Irradiation of Electrode Materials for Energy Storage and Conversion. Matter 2020, 3, 95–126. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, P.; Zhao, H.; Lei, Y. Recent Developments and Future Prospects of Transition Metal Compounds as Electrode Materials for Potassium-Ion Hybrid Capacitors. Adv. Mater. Technol. 2022, 8, 2200515. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nagaraju, D.H.; Beaujuge, P.; Alshareef, H.N. Supercapacitors Based on Two Dimensional VO2 Nanosheet Electrodes in Organic Gel Electrolyte. Electrochim. Acta 2016, 220, 601–608. [Google Scholar] [CrossRef]
- Pan, X.; Ren, G.; Hoque, N.F.; Bayne, S.; Zhu, K.; Fan, Z. Fast Supercapacitors Based on Graphene-Bridged V2O3/VOx Core-Shell Nanostructure Electrodes with a Power Density of 1 MW Kg−1. Adv. Mater. Interfaces 2014, 1, 1400398. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Wang, X. Hierarchical Branched Vanadium Oxide Nanorod@Si Nanowire Architecture for High Performance Supercapacitors. Small 2016, 13, 1603076. [Google Scholar] [CrossRef]
- Peng, L.; Li, D.; Shen, L.; Liu, Z.; Fan, W.; Qiu, H.; Yu, A.; Jiang, X. Facile One-Step Synthesis of 0D to 3D VOx Nanostructures for Energy Storage. Electrochim. Acta 2021, 392, 139021. [Google Scholar] [CrossRef]
- Bai, M.H.; Bian, L.J.; Song, Y.; Liu, X.X. Electrochemical Codeposition of Vanadium Oxide and Polypyrrole for High-Performance Supercapacitor with High Working Voltage. ACS Appl. Mater. Interfaces 2014, 6, 12656–12664. [Google Scholar] [CrossRef]
- Panigrahi, K.; Howli, P.; Chattopadhyay, K.K. Three-Dimensional VO2@PANI Micro Flower Array for Flexible Supercapacitor. Mater. Lett. 2019, 253, 90–94. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Zhang, J.; Tang, J.; Balogun, M.-S.; Wang, P.; Tong, Y.; Huang, Y. Charge Relays via Dual Carbon-Actions on Nanostructured BiVO 4 for High Performance Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2112738. [Google Scholar] [CrossRef]
- Peng, X.; Huo, K.; Fu, J.; Zhang, X.; Gao, B.; Chu, P.K. Coaxial PANI/TiN/PANI Nanotube Arrays for High-Performance Supercapacitor Electrodes. Chem. Commun. 2013, 49, 10172. [Google Scholar] [CrossRef]
- Karaca, E.; Pekmez, K.; Pekmez, N.Ö. Electrosynthesis of Polypyrrole-Vanadium Oxide Composites on Graphite Electrode in Acetonitrile in the Presence of Carboxymethyl Cellulose for Electrochemical Supercapacitors. Electrochim. Acta 2018, 273, 379–391. [Google Scholar] [CrossRef]
- Tu, Q.; Zhang, Q.; Sun, X.; Wang, J.; Lin, B.; Chen, L.; Liu, J.; Deng, Z. Construction of Three-Dimensional Nickel-Vanadium Hydrotalcite with Ball-Flower Architecture for Screen-Printed Asymmetric Supercapacitor. Appl. Surf. Sci. 2023, 615, 156347. [Google Scholar] [CrossRef]
- He, G.; Li, J.; Li, W.; Li, B.; Noor, N.; Xu, K.; Hu, J.; Parkin, I.P. One Pot Synthesis of Nickel Foam Supported Self-Assembly of NiWO4 and CoWO4 Nanostructures That Act as High Performance Electrochemical Capacitor Electrodes. J. Mater. Chem. A 2015, 3, 14272–14278. [Google Scholar] [CrossRef]
- Zhao, C.X.; Cao, J.Q.; Yang, Y.X.; Chen, W.; Li, J.S. Facile Synthesis of Hierarchical Porous VOx@Carbon Composites for Supercapacitors. J. Colloid Interface Sci. 2014, 427, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Ren, X.; Cui, G.; Asiri, A.M.; Zheng, B.; Sun, X. High-Efficiency Electrosynthesis of Ammonia with High Selectivity under Ambient Conditions Enabled by VN Nanosheet Array. ACS Sustain. Chem. Eng. 2018, 6, 9545–9549. [Google Scholar] [CrossRef]
- Mjejri, I.; Etteyeb, N.; Sediri, F. Mesoporous Vanadium Oxide Nanostructures: Hydrothermal Synthesis, Optical and Electrochemical Properties. Ceram. Int. 2014, 40, 1387–1397. [Google Scholar] [CrossRef]
- Zhao, Y.; Zong, M.Y.; Fan, C.Z.; Chen, K.; Wang, D.H.; Zhang, M.H. Effect of Doped Vanadium Dioxide on Oxidative Desulfurization Reaction. China Pet. Process. Petrochem. Technol. 2019, 21, 42–48. [Google Scholar]
- Hu, P.; Hu, P.; Vu, T.D.; Li, M.; Wang, S.; Ke, Y.; Zeng, X.; Mai, L.; Long, Y. Vanadium Oxide: Phase Diagrams, Structures, Synthesis, and Applications. Chem. Rev. 2023, 123, 4353–4415. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Verma, A. Augmented Structural, Optical and Electrical Properties of CdS Decorated PANI/RGO Nanohybrids. Opt. Mater. 2019, 96, 109310. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Q.; Chen, X.; Li, H.; Yan, F.; Dai, H.; Li, T.; Xue, R.; Chen, J.; Gong, G.; et al. The Microdefects and Enhanced Electrochemical Performances of Nano-VO2(B) Induced by Mg Doping. J. Solid State Electrochem. 2022, 27, 281–290. [Google Scholar] [CrossRef]
- Evans, G.P.; Powell, M.; Johnson, I.; Howard, D.; Bauer, D.; Darr, J.A.; Parkin, I.P. Room Temperature Vanadium Dioxide–Carbon Nanotube Gas Sensors Made via Continuous Hydrothermal Flow Synthesis. Sens. Actuators B Chem. 2018, 255, 1119–1129. [Google Scholar] [CrossRef]
- Mjejri, I.; Etteyeb, N.; Sediri, F. Hydrothermal Synthesis of Mesoporous Rod-like Nanocrystalline Vanadium Oxide Hydrate V3O7·H2O from Hydroquinone and V2O5. Mater. Res. Bull. 2013, 48, 3335–3341. [Google Scholar] [CrossRef]
- Abdulrazzaq, O.A.; Bourdo, S.E.; Saini, V.; Biris, A.S. Acid-Free Polyaniline: Graphene-Oxide Hole Transport Layer in Organic Solar Cells. J. Mater. Sci. Mater. Electron. 2020, 31, 21640–21650. [Google Scholar] [CrossRef]
- Shi, B.; Zhao, C.; Ji, Y.; Shi, J.; Yang, H. Promotion Effect of PANI on Fe-PANI/Zeolite as an Active and Recyclable Fenton-like Catalyst under Near-Neutral Condition. Appl. Surf. Sci. 2020, 508, 145298. [Google Scholar] [CrossRef]
- Liu, B.T.; Shi, X.M.; Lang, X.Y.; Gu, L.; Wen, Z.; Zhao, M.; Jiang, Q. Extraordinary Pseudocapacitive Energy Storage Triggered by Phase Transformation in Hierarchical Vanadium Oxides. Nat. Commun. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xia, Y.; Chen, Y.; Liu, Q.; Ge, T.; Xu, L.; Mai, L. Vanadium-Based Nanowires for Sodium-Ion Batteries. Adv. Energy Mater. 2019, 30, 192001. [Google Scholar] [CrossRef]
- Xiao, Y.; Yue, F.; Wen, Z.; Shen, Y.; Su, D.; Guo, H.; Rui, X.; Zhou, L.; Fang, S.; Yu, Y. Elastic Buffering Layer on CuS Enabling High-Rate and Long-Life Sodium-Ion Storage. Nano-Micro Lett. 2022, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Li, X.; Xiong, Z.; Wang, H.; Fu, J.; Chen, L. Screen-Printed Advanced All-Solid-State Symmetric Supercapacitor Using Activated Carbon on Flexible Nickel Foam. J. Energy Storage 2022, 53, 105211. [Google Scholar] [CrossRef]
- Halder, L.; Das, A.K.; Maitra, A.; Bera, A.; Paria, S.; Karan, S.K.; Si, S.K.; Ojha, S.; De, A.; Khatua, B.B. A Polypyrrole-Adorned, Self-Supported, Pseudocapacitive Zinc Vanadium Oxide Nanoflower and Nitrogen-Doped Reduced Graphene Oxide-Based Asymmetric Supercapacitor Device for Power Density Applications. New J. Chem. 2020, 44, 1063–1075. [Google Scholar] [CrossRef]
- Adewinbi, S.A.; Busari, R.A.; Animasahun, L.O.; Omotoso, E.; Taleatu, B.A. Effective pseudocapacitive performance of binder free transparent α-V2O5 thin film electrode: Electrochemical and some surface probing. Phys. B Condens. Matter 2021, 621, 413260. [Google Scholar] [CrossRef]
- QI, R.J.; Nie, J.H.; Liu, M.Y.; Lu, X.M. Stretchable V2O5/PEDOT Supercapacitors: A Modular Fabrication Process and Charging with Triboelectric Nanogenerators. Nanoscale 2018, 10, 7719–7725. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.B.; Shu, T.; Guo, S.T.; Lu, Y.; Li, M.X.; Nzabahimana, J.; Hu, X.L. Fabricating strongly coupled V2O5@PEDOT nanobelts/graphene hybrid films with high areal capacitance and facile transferability for transparent solid-state supercapacitors. Energy Storage Mater. 2020, 27, 150–158. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.D.; Bai, L.F.; Bai, W.C.; Zhou, M.; Xie, J.F.; Guan, M.L.; Zhou, J.F.; Xie, Y. All-solid-state flexible thin-film supercapacitors with high electrochemical performance based on a two-dimensional V2O5·H2O/graphene composite. J. Mater. Chem. A 2014, 2, 10876–10881. [Google Scholar] [CrossRef]
- Park, H.; Song, C.; Jin, S.W.; Lee, H.; Keum, K.; Lee, Y.H.; Lee, G.; Jeong, Y.R.; Ha, J.S. High performance flexible micro-supercapacitor for powering a vertically integrated skin-attachable strain sensor on a bio-inspired adhesive. Nano Energy 2021, 83, 105837. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, X.; Zheng, Y.; Tu, Q.; Wei, S.; Shi, H.; Tang, W.; Chen, L. PANI-Coated VOx Nanobelts with Core-Shell Architecture for Flexible All-Solid-State Supercapacitor. Micromachines 2023, 14, 1856. https://doi.org/10.3390/mi14101856
Zhang Q, Li X, Zheng Y, Tu Q, Wei S, Shi H, Tang W, Chen L. PANI-Coated VOx Nanobelts with Core-Shell Architecture for Flexible All-Solid-State Supercapacitor. Micromachines. 2023; 14(10):1856. https://doi.org/10.3390/mi14101856
Chicago/Turabian StyleZhang, Qiang, Xianran Li, Yinyin Zheng, Qian Tu, Shiwen Wei, Hong Shi, Wentao Tang, and Liangzhe Chen. 2023. "PANI-Coated VOx Nanobelts with Core-Shell Architecture for Flexible All-Solid-State Supercapacitor" Micromachines 14, no. 10: 1856. https://doi.org/10.3390/mi14101856
APA StyleZhang, Q., Li, X., Zheng, Y., Tu, Q., Wei, S., Shi, H., Tang, W., & Chen, L. (2023). PANI-Coated VOx Nanobelts with Core-Shell Architecture for Flexible All-Solid-State Supercapacitor. Micromachines, 14(10), 1856. https://doi.org/10.3390/mi14101856







