Fabrication of a Highly NO2-Sensitive Gas Sensor Based on a Defective ZnO Nanofilm and Using Electron Beam Lithography
Abstract
:1. Introduction
2. Materials and Methods
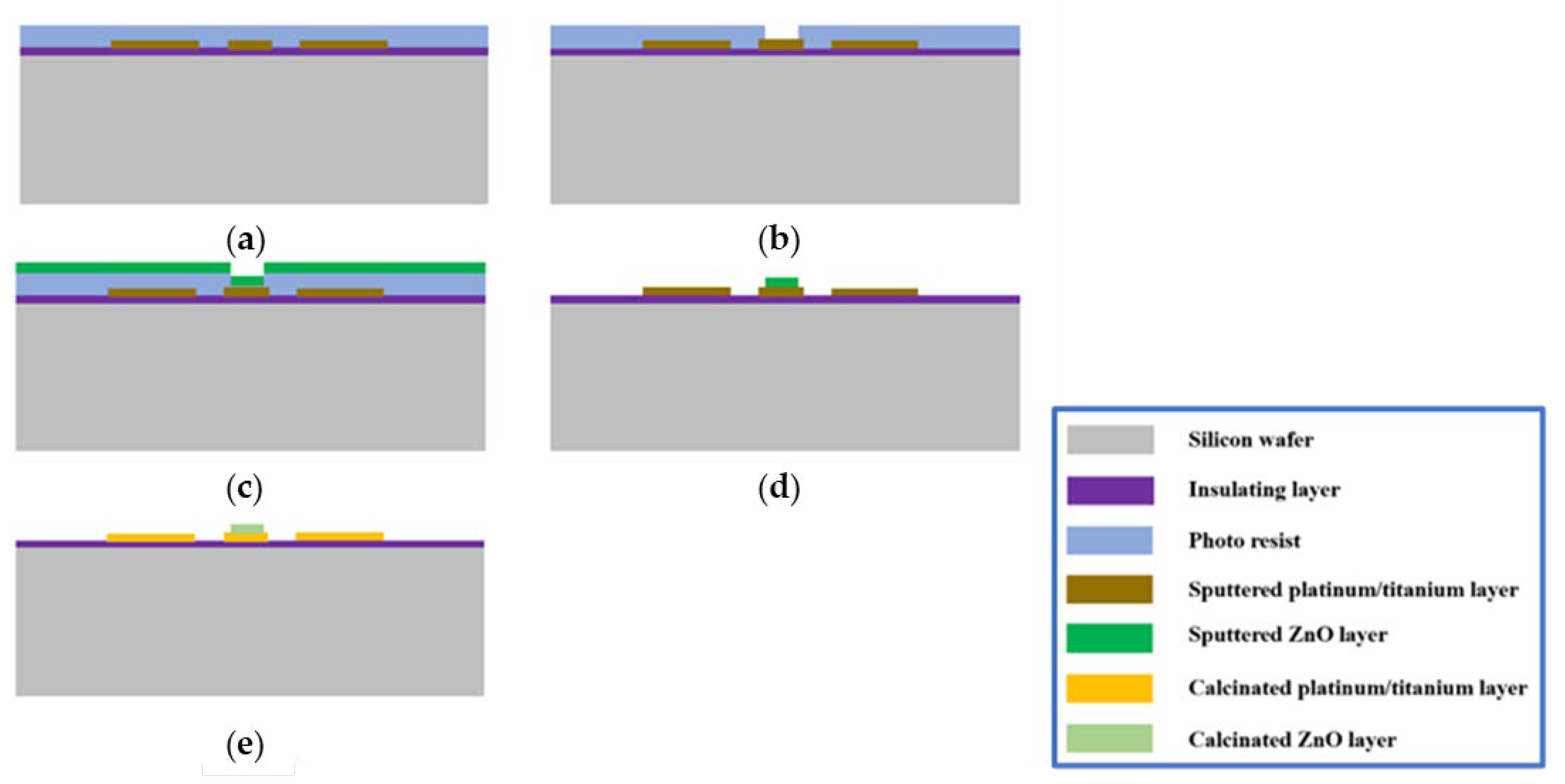
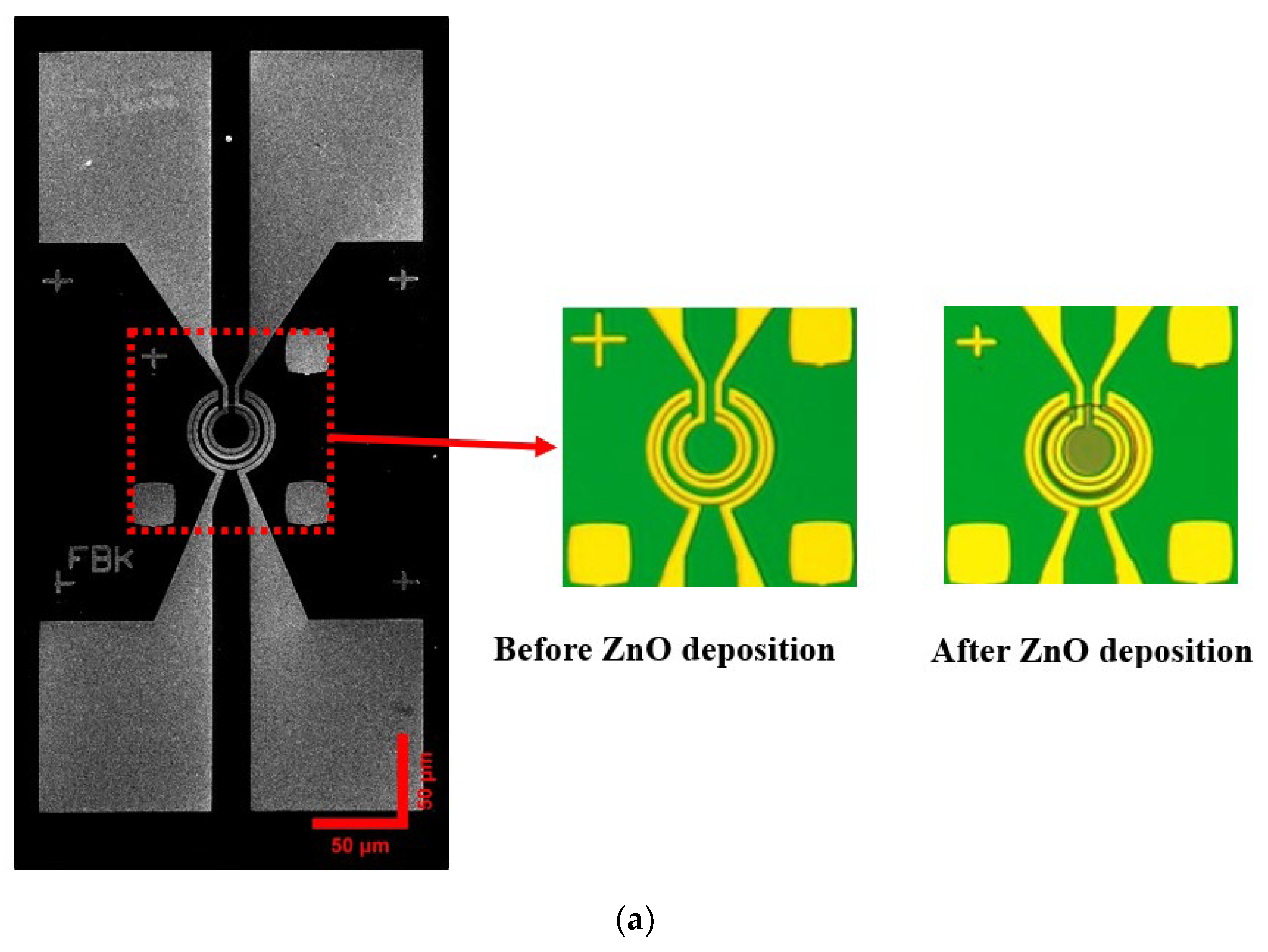
2.1. Fabrication Process
2.2. Characterization Tools
2.3. Gas Sensing Measurement
3. Results
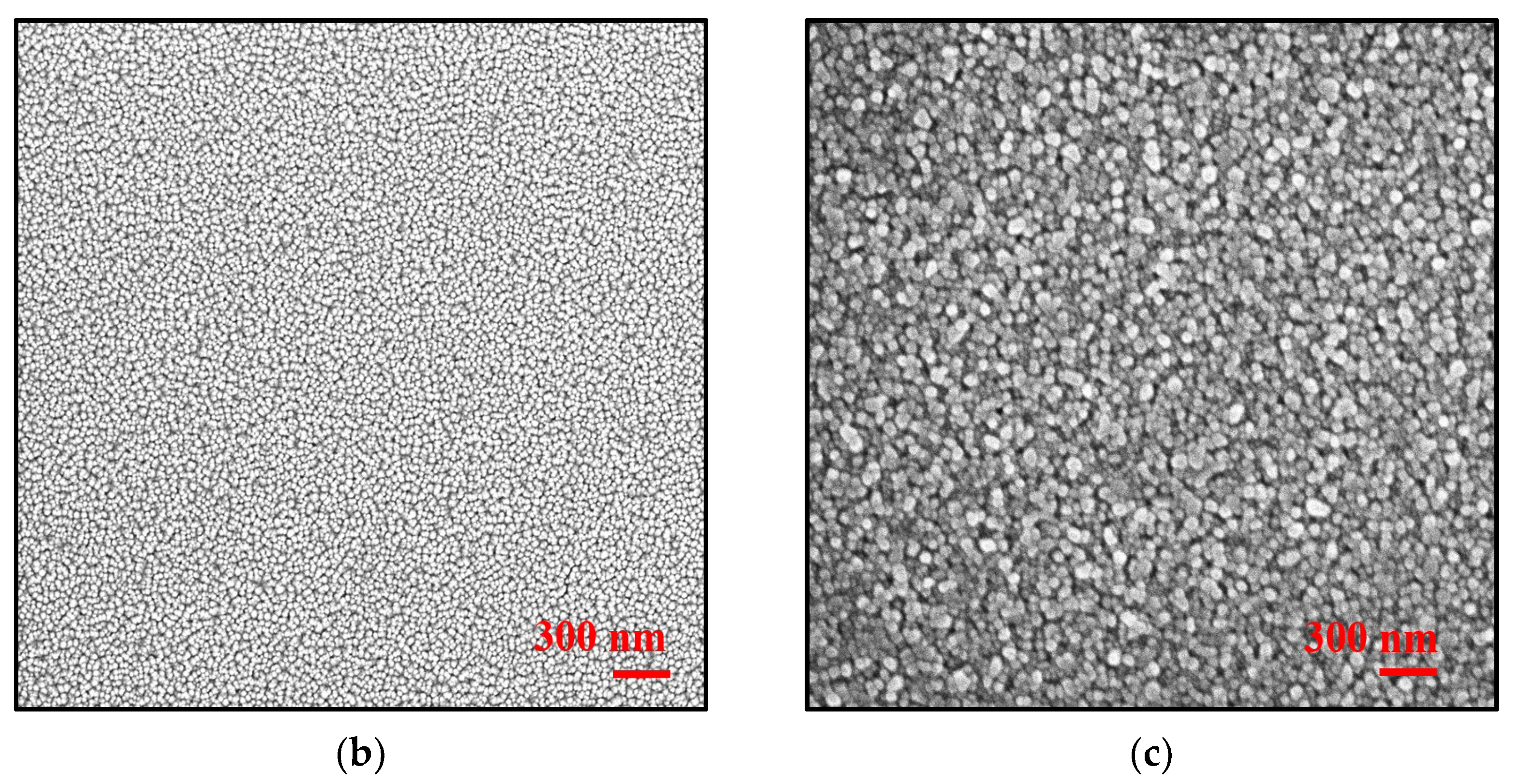
Structural and Morphological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naz, S.; Javid, I.; Konwar, S.; Singh, P.K.; Sahni, M.B. Solid state gas sensor. Mater. Today: Proc. 2022, 49, 3245–3249. [Google Scholar] [CrossRef]
- Dhall, S.; Mehta, B.R.; Tyagi, A.K.; Sood, K. A review on environmental gas sensors: Materials and technologies. Sens. Int. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, W.; Li, Y. Metal oxide gas sensors for detecting NO2 in industrial exhaust gas: Recent developments. Sens. Actuators B Chem. 2022, 359, 131579. [Google Scholar] [CrossRef]
- Eom, T.H.; Cho, S.H.; Suh, J.M.; Kim, T.; Lee, T.H.; Jun, S.E.; Yang, J.W.; Lee, J.; Hong, S.-H.; Jang, H.W. Substantially improved room temperature NO2 sensing in 2-dimensional SnS2 nanoflowers enabled by visible light illumination. J. Mater. Chem. A 2021, 9, 11168–11178. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Luo, N.; Chen, J.; Cheng, J.; Ren, W.; Xu, J. Enhanced H2S sensing performance of BiFeO3 based MEMS gas sensor with corona poling. Sens. Actuators B Chem. 2022, 358, 131477. [Google Scholar] [CrossRef]
- Yaqoob, U.; Younis, M.I. Chemical Gas Sensors: Recent Developments, Challenges, and the Potential of Machine Learning—A Review. Sensors 2021, 21, 2877. [Google Scholar] [CrossRef]
- Landini, N.; Anania, G.; Astolfi, M.; Fabbri, B.; Guidi, V.; Rispoli, G.; Valt, M.; Zonta, G.; Malagu, C. Nanostructured Chemoresistive Sensors for Oncological Screening and Tumor Markers Tracking: Single Sensor Approach Applications on Human Blood and Cell Samples. Sensors 2020, 20, 1411. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Current state of knowledge on the metal oxide based gas sensing mechanism. Sens. Actuators B Chem. 2022, 358, 131531. [Google Scholar] [CrossRef]
- Spagnoli, E.; Gaiardo, A.; Fabbri, B.; Valt, M.; Krik, S.; Ardit, M.; Cruciani, G.; Della Ciana, M.; Vanzetti, L.; Vola, G.; et al. Design of a Metal-Oxide Solid Solution for Sub-ppm H2 Detection. ACS Sens. 2022, 7, 573–583. [Google Scholar] [CrossRef]
- Feng, Z.; Gaiardo, A.; Valt, M.; Fabbri, B.; Casotti, D.; Krik, S.; Vanzetti, L.; Ciana, M.D.; Fioravanti, S.; Caramori, S.; et al. Investigation on Sensing Performance of Highly Doped Sb/SnO2. Sensors 2022, 22, 1233. [Google Scholar] [CrossRef]
- Raju, P.; Li, Q. Review—Semiconductor Materials and Devices for Gas Sensors. J. Electrochem. Soc. 2022, 169, 057518. [Google Scholar] [CrossRef]
- Kwon, J.; Hong, S.; Kim, G.; Suh, Y.D.; Lee, H.; Choo, S.-Y.; Lee, D.; Kong, H.; Yeo, J.; Ko, S.H. Digitally patterned resistive micro heater as a platform for zinc oxide nanowire based micro sensor. Appl. Surf. Sci. 2018, 447, 1–7. [Google Scholar] [CrossRef]
- Gaiardo, A.; Fabbri, B.; Giberti, A.; Guidi, V.; Bellutti, P.; Malagù, C.; Valt, M.; Pepponi, G.; Gherardi, S.; Zonta, G.; et al. ZnO and Au/ZnO thin films: Room-temperature chemoresistive properties for gas sensing applications. Sens. Actuators B Chem. 2016, 237, 1085–1094. [Google Scholar] [CrossRef]
- Miao, X.; Zhao, X.; Qin, H.; Jin, Q.; Chen, Y.; Cao, Z.; Yang, W.; Wang, Q.; Pan, Q. Synergistic effect of cubic C3N4_ZnO_C hybrid composite for selective detection of sulfur dioxide. Rare Met. 2022, 41, 3662–3670. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, Y.; Yang, L.; Xiong, Z.; Lv, Z.; Xing, X.; Zhou, Y.; Zhang, B.; Su, C.; Liao, Q.; et al. MXene-ZnO Memristors: MXene-ZnO Memristor for Multimodal In-Sensor Computing. Adv. Funct. Mater 2021, 31, 2170152. [Google Scholar] [CrossRef]
- Khudiar, S.S.; Nayef, U.M.; Mutlak, F.A.H.; Abdulridha, S.K. Characterization of NO2 gas sensing for ZnO nanostructure grown hydrothermally on porous silicon. Optik 2022, 249, 168300. [Google Scholar] [CrossRef]
- Bai, M.; Chen, M.; Li, X.; Wang, Q. One-step CVD growth of ZnO nanorod/SnO2 film heterojunction for NO2 gas sensor. Sens. Actuators B Chem. 2022, 373, 132738. [Google Scholar] [CrossRef]
- Yang, D.; Ramu, A.G.; Lee, Y.; Kim, S.; Jeon, H.; Sathishkumar, V.E.; Al-Mohaimeed, A.M.; Al-onazi, W.A.; Algarni, T.S.; Choi, D. Fabrication of ZnO nanorods based gas sensor pattern by photolithography and lift off techniques. J. King Saud Univ. Sci. 2021, 33, 101397. [Google Scholar] [CrossRef]
- Raj, I.L.P.; Gobalakrishnan, S.; Praseetha, P.K.; Chidhambaram, N.; Saravanakumar, S.; Ganesh, V.; AlFaify, S.; Algarni, H.; Yahia, I.S. Improved ammonia vapor sensing properties of Al-doped ZnO nanoparticles prepared by sol-gel process. Phys. Scr. 2021, 96, 085802. [Google Scholar]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuators B Chem. 2019, 301, 126845. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Meng, L.; Sun, Z.; Chen, Z.; Huang, X.; Qin, Y. Adjustment of oxygen vacancy states in ZnO and its application in ppb-level NO2 gas sensor. Sci. Bull. 2020, 65, 1650–1658. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Tao, T.; Leng, B.; Xu, W.; Mao, L. Structural inheritance and change from ZnSn(OH)6 to ZnSnO3 compounds used for ethanol sensors: Effects of oxygen vacancies, temperature and UV on gas-sensing properties. J. Alloys Compd. 2020, 829, 154445. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Sahdan, M.Z.; Nafarizal, N.; Saim, H.; Embong, Z.; Rohaida, C.H.C.; Adriyanto, F. Influence of substrate annealing on inducing Ti3+ and oxygen vacancy in TiO2 thin films deposited via RF magnetron sputtering. Appl. Surf. Sci. 2018, 462, 575–582. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, H.; Wang, X.; Xue, G.; Tian, Z.; Zhang, J.; Yuan, S.; Yu, T.; Zou, Z. The role of oxygen vacancy-Ti3+ states on TiO2 nanotubes’ surface in dye-sensitized solar cells. Mater. Lett. 2013, 100, 51–53. [Google Scholar] [CrossRef]
- Yang, G.; Wang, T.; Yang, B.; Yan, Z.; Ding, S.; Xiao, T. Enhanced visible-light activity of F-N co-doped TiO2 nanocrystals via nonmetal impurity, Ti3+ ions and oxygen vacancies. Appl. Surf. Sci. 2013, 287, 135–142. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, W.; Ma, X.; Pan, C.; Zong, R.; Zhu, Y. The surface oxygen vacancy induced visible activity and enhanced UV activity of a ZnO1−x photocatalyst. Catal. Sci. Technol. 2013, 3, 3136–3146. [Google Scholar] [CrossRef]
- HShwetha, R.; Sharath, S.M.; Guruprasad, B.; Rudraswamy, S.B. MEMS based metal oxide semiconductor carbon dioxide gas sensor. Micro Nano Eng. 2022, 16, 100156. [Google Scholar] [CrossRef]
- Nedelcu, S.; Thodkar, K.; Hierold, C. A customizable, low-power, wireless, embedded sensing platform for resistive nanoscale sensors. Microsyst Nanoeng 2022, 8, 10. [Google Scholar] [CrossRef]
- Luo, N.; Wang, C.; Zhang, D.; Guo, M.; Wang, X.; Cheng, Z.; Xu, J. Ultralow detection limit MEMS hydrogen sensor based on SnO2 with oxygen vacancies. Sens. Actuators B: Chem. 2022, 354, 130982. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Gohain, M.; Bezuidenhoudt, B.C.; van Vuuren, A.J.; Lee, M.; Ntwaeaborwa, O.M. The role of neutral and ionized oxygen defects in the emission of tin oxide nanocrystals for near white light application. Nanotechnology 2015, 26, 295703. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Y.; Zheng, F.; Li, C.; Wu, J.; Wang, T.; Fu, Y.; Zhang, X. Introducing oxygen vacancies on sodium titanate via NaBH4 treatment for conductometric hydrogen gas sensors. Sens. Actuators B Chem. 2023, 375, 132916. [Google Scholar] [CrossRef]
- Jeroish, Z.E.; Bhuvaneshwari, K.S.; Samsuri, F.; Narayanamurthy, V. Microheater: Material, design, fabrication, temperature control, and applications-a role in COVID-19. Biomed Microdevices 2021, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, C.; Wang, Z.; Song, Z.; Zhou, X.; Han, N.; Chen, Y. Sputtered SnO2:NiO thin films on self-assembled Au nanoparticle arrays for MEMS compatible NO2 gas sensors. Sens. Actuators B: Chem. 2019, 278, 28–38. [Google Scholar] [CrossRef]
- Schoendorfer, C.; Hetzel, M.; Pongratz, P.; Lugstein, A.; Bertagnolli, E. Focused ion beam induced synthesis of antimony nanowires for gas sensor applications. Nanotechnology 2012, 23, 435502. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, H.; Zhao, H.; Tao, M.; Liu, J. Fast and accurate proximity effect correction algorithm based on pattern edge shape adjustment for electron beam lithography. Microelectron. J. 2023, 133, 105718. [Google Scholar] [CrossRef]
- Matsumoto, H.; Okabe, T.; Taniguchi, J. Microchannel fabrication via ultraviolet-nanoimprint lithography and electron-beam lithography using an ultraviolet-curable positive-tone electron-beam resist. Microelectron. Eng. 2020, 226, 111278. [Google Scholar] [CrossRef]
- EEriksen, H.; Nazir, A.; Balling, P.; Vester-Petersen, J.; Christiansen, R.E.; Sigmund, O.; Madsen, S.P. Dose regularization via filtering and projection: An open-source code for optimization-based proximity-effect-correction for nanoscale lithography. Microelectron. Eng. 2018, 199, 52–57. [Google Scholar] [CrossRef]
- Herr, D.J.C.; Liu, C.-H.; Tien, P.-L.; Ng, P.C.W.; Shen, Y.-T.; Tsai, K.-Y. Model-based proximity effect correction for electron-beam direct-write lithography. In Alternative Lithographic Technologies II; SPIE: Bellingham, WA, USA, 2010. [Google Scholar]
- Bolten, J.; Wahlbrink, T.; Schmidt, M.; Gottlob, H.D.B.; Kurz, H. Implementation of electron beam grey scale lithography and proximity effect correction for silicon nanowire device fabrication. Microelectron. Eng. 2011, 88, 1910–1912. [Google Scholar] [CrossRef]
- Bagolini, A.; Gaiardo, A.; Crivellari, M.; Demenev, E.; Bartali, R.; Picciotto, A.; Valt, M.; Ficorella, F.; Guidi, V.; Bellutti, P. Development of MEMS MOS gas sensors with CMOS compatible PECVD inter-metal passivation. Sens. Actuators B Chem. 2019, 292, 225–232. [Google Scholar] [CrossRef]
- Speranza, G.; Canteri, R. RxpsG a New Open Project for Photoelectron and Electron Spectroscopy Data Processing. SoftwareX 2019, 10, 100282. [Google Scholar] [CrossRef]
- Algamili, A.S.; Khir, M.H.; Ahmed, A.Y.; Rabih, A.A.; Ba-Hashwan, S.S.; Alabsi, S.S.; Al-Mahdi, O.L.; Isyaku, U.B.; Ahmed, M.G.; Junaid, M. Fabrication and Characterization of the Micro-Heater and Temperature Sensor for PolyMUMPs-Based MEMS Gas Sensor. Micromachines 2022, 13, 525. [Google Scholar] [CrossRef] [PubMed]
- Gaiardo, A.; Novel, D.; Scattolo, E.; Crivellari, M.; Picciotto, A.; Ficorella, F.; Iacob, E.; Bucciarelli, A.; Petti, L.; Lugli, P.; et al. Optimization of a Low-Power Chemoresistive Gas Sensor: Predictive Thermal Modelling and Mechanical Failure Analysis. Sensors 2021, 21, 783. [Google Scholar] [CrossRef]
- Au, B.W.-C.; Chan, K.-Y. Sodium and potassium doped P-type ZnO films by sol-gel spin-coating technique. Appl. Phys. A 2017, 123, 485. [Google Scholar] [CrossRef]
- Cui, S.; Feng, W.; Hu, H.; Feng, Z.; Wang, Y. Structural and electronic properties of ZnO under high pressure. J. Alloys Compd. 2009, 476, 306–310. [Google Scholar] [CrossRef]
- Ruso, J.M.; Gravina, A.N.; D’Elía, N.L.; Messina, P.V. Highly efficient photoluminescence of SiO2 and Ce-SiO2 microfibres and microspheres. Dalton Trans. 2013, 42, 7991–8000. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Boehme, I.; Weimar, U.; Barsan, N. Unraveling the surface chemistry of CO sensing with In2O3 based gas sensors. Sens. Actuator B Chem. 2021, 326, 129004. [Google Scholar] [CrossRef]
- Saito, N.; Haneda, H.; Watanabe, K.; Shimanoe, K.; Sakaguchi, I. Highly sensitive isoprene gas sensor using Au-loaded pyramid-shaped ZnO particles. Sens. Actuators B Chem. 2021, 326, 128999. [Google Scholar] [CrossRef]
- Staerz, A.; Kobald, A.; Russ, T.; Weimar, U.; Hémeryck, A.; Barsan, N. Thermal Water Splitting on the WO3 Surface: Experimental Proof. ACS Appl. Electron. Mater. 2020, 2, 3254–3262. [Google Scholar] [CrossRef]
- Eadi, S.B.; Shin, H.; Nguyen, K.T.; Song, K.; Choi, H.; Kim, S.; Lee, H. Indium-gallium–zinc oxide (IGZO) thin-film gas sensors prepared via post-deposition high-pressure annealing for NO2 detection. Sens. Actuators: B Chem. 2022, 353, 131082. [Google Scholar] [CrossRef]
- Yuan, H.; Aljneibi, S.A.A.A.; Yuan, J.; Wang, Y.; Liu, H.; Fang, J.; Tang, C.; Yan, X.; Cai, H.; Gu, Y.; et al. ZnO Nanosheets Abundant in Oxygen Vacancies Derived from Metal-Organic Frameworks for ppb-Level Gas Sensing. Adv. Mater. 2019, 31, 1807161. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Kim, M.Y.; Mirzaei, A.; Kim, H.-S.; Kim, S.-I.; Baek, S.-H.; Chun, D.W.; Jin, C.; Lee, K.H. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 568, 150910. [Google Scholar] [CrossRef]
- Kasirajan, K.; Chandrasekar, L.B.; Maheswari, S.; Karunakaran, M.; Sundaram, P.S. A comparative study of different rare-earth (Gd, Nd, and Sm) metals doped ZnO thin films and its room temperature ammonia gas sensor activity: Synthesis, characterization, and investigation on the impact of dopant. Opt. Mater. 2021, 121, 111554. [Google Scholar] [CrossRef]
- Duoc, V.T.; Hung, C.M.; Nguyen, H.; Duy, N.V.; Hieu, N.V.; Hoa, N.D. Room temperature highly toxic NO2 gas sensors based on rootstock/scion nanowires of SnO2/ZnO, ZnO/SnO2, SnO2/SnO2 and, ZnO/ZnO. Sens. Actuators B Chem. 2021, 348, 130652. [Google Scholar] [CrossRef]
- Kim, H.W.; Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S. Synthesis of zinc oxide semiconductors-graphene nanocomposites by microwave irradiation for application to gas sensors. Sens. Actuators B Chem. 2017, 249, 590–601. [Google Scholar] [CrossRef]
- Öztürk, S.; Kılınç, N.; Öztürk, Z.Z. Fabrication of ZnO nanorods for NO2 sensor applications Effect of dimensions and electrode position. J. Alloys Compd. 2013, 581, 196–201. [Google Scholar] [CrossRef]
- Şahin, Y.; Öztürk, S.; Kılınç, N.; Kösemen, A.; Erkovan, M.; Öztürk, Z.Z. Electrical conduction and NO2 gas sensing properties of ZnO nanorods. Appl. Surf. Sci. 2014, 303, 90–96. [Google Scholar] [CrossRef]
- Oh, E.; Choi, H.-Y.; Jung, S.-H.; Cho, S.; Kim, J.C.; Lee, K.-H.; Kang, S.-W.; Kim, J.; Yun, J.-Y.; Jeong, S.-H. High-performance NO2 gas sensor based on ZnO nanorod grown by ultrasonic irradiation. Sens. Actuators B Chem. 2009, 141, 239–243. [Google Scholar] [CrossRef]
- Yan, D.; Hu, M.; Li, S.; Liang, J.; Wu, Y.; Ma, S. Electrochemical deposition of ZnO nanostructures onto porous silicon and their enhanced gas sensing to NO2 at room temperature. Electrochim. Acta 2014, 115, 297–305. [Google Scholar] [CrossRef]


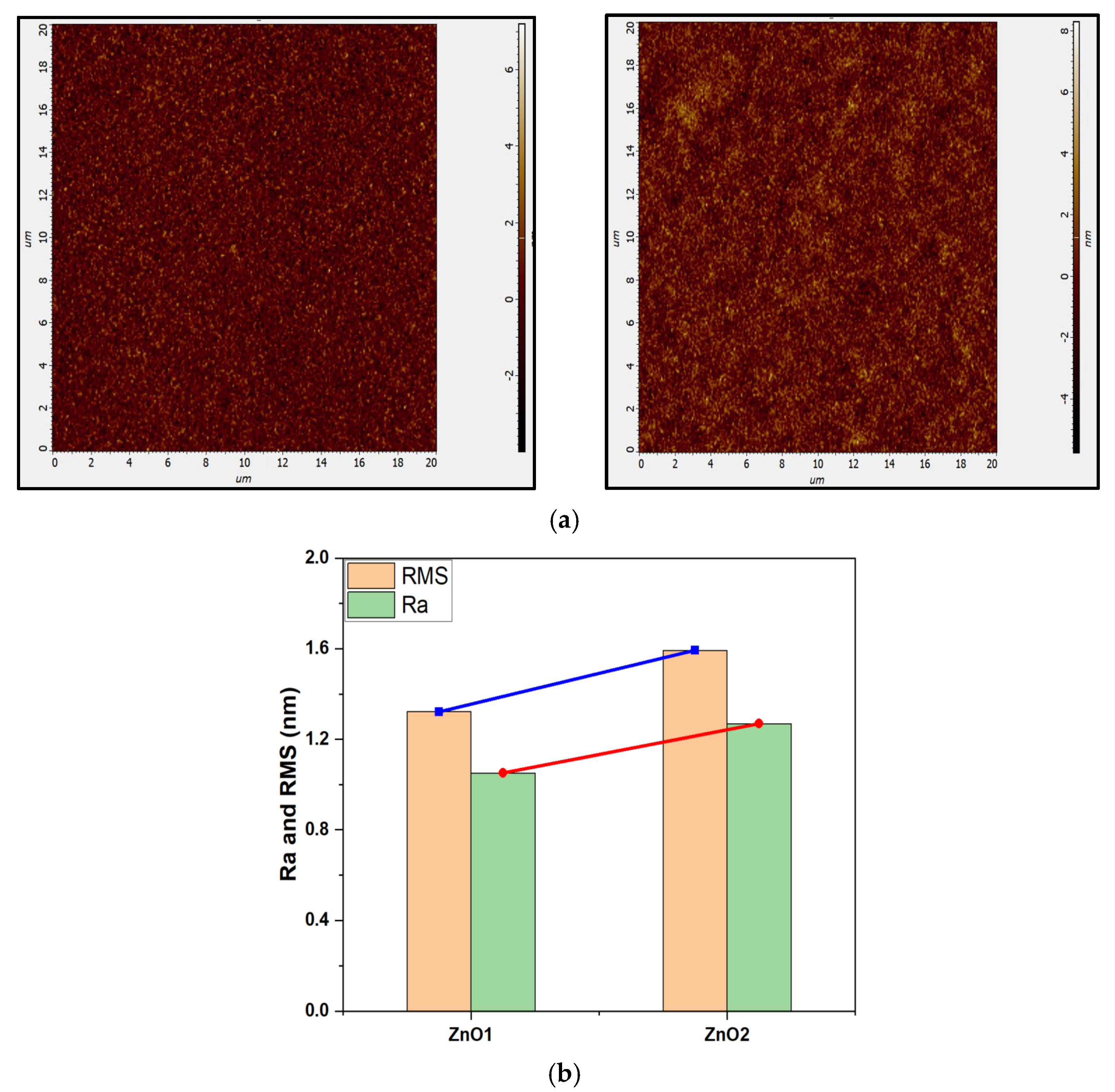
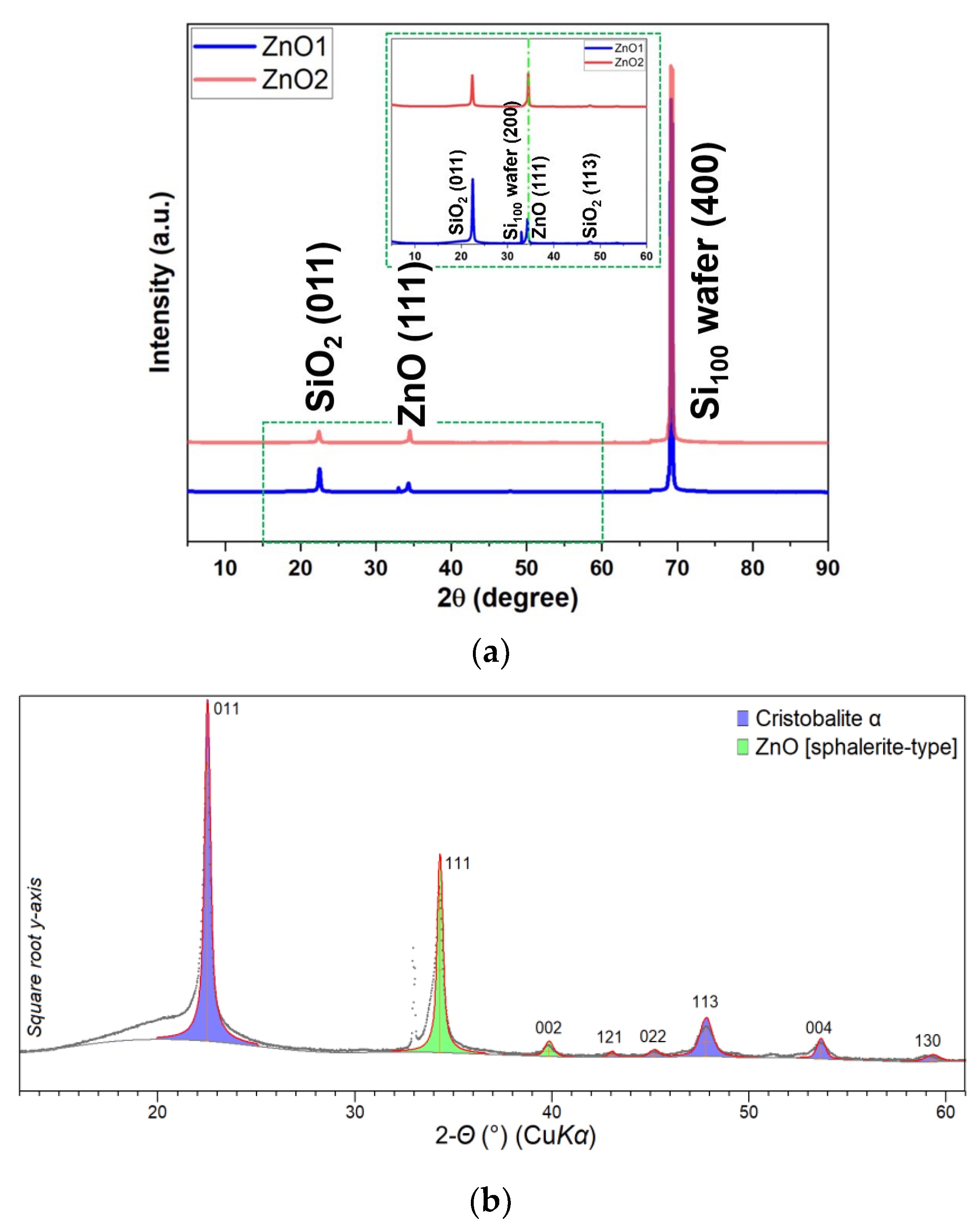
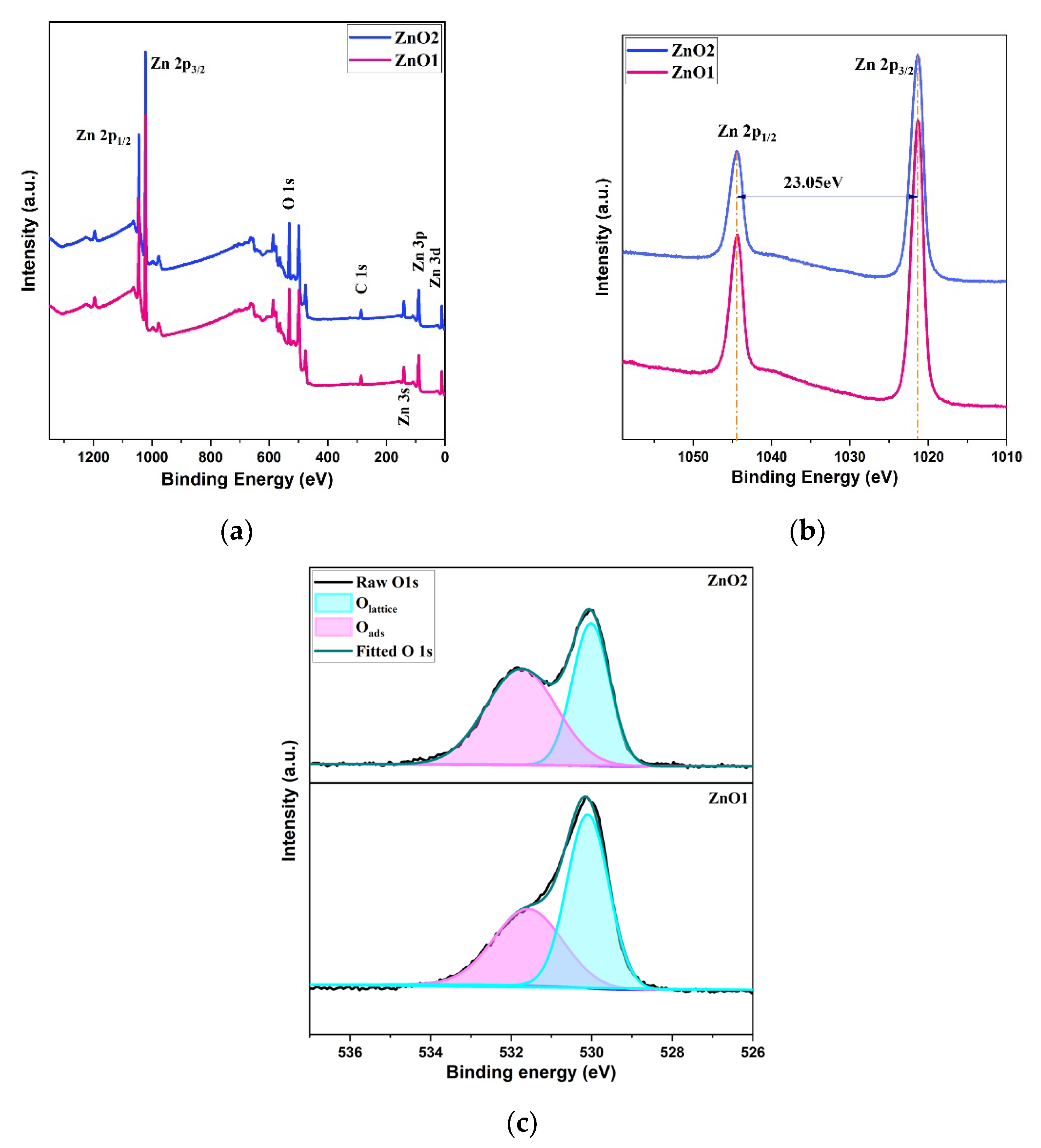
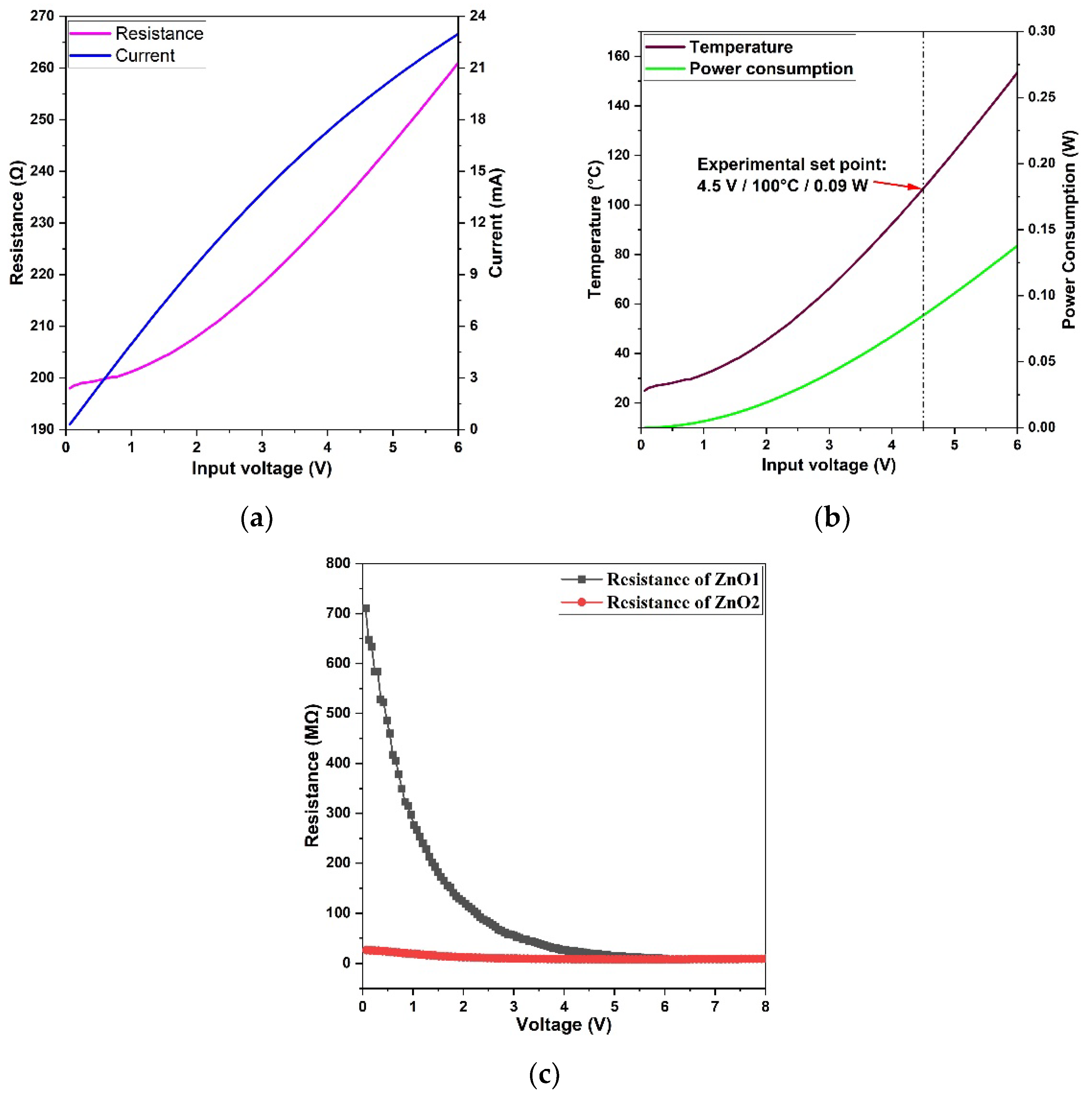

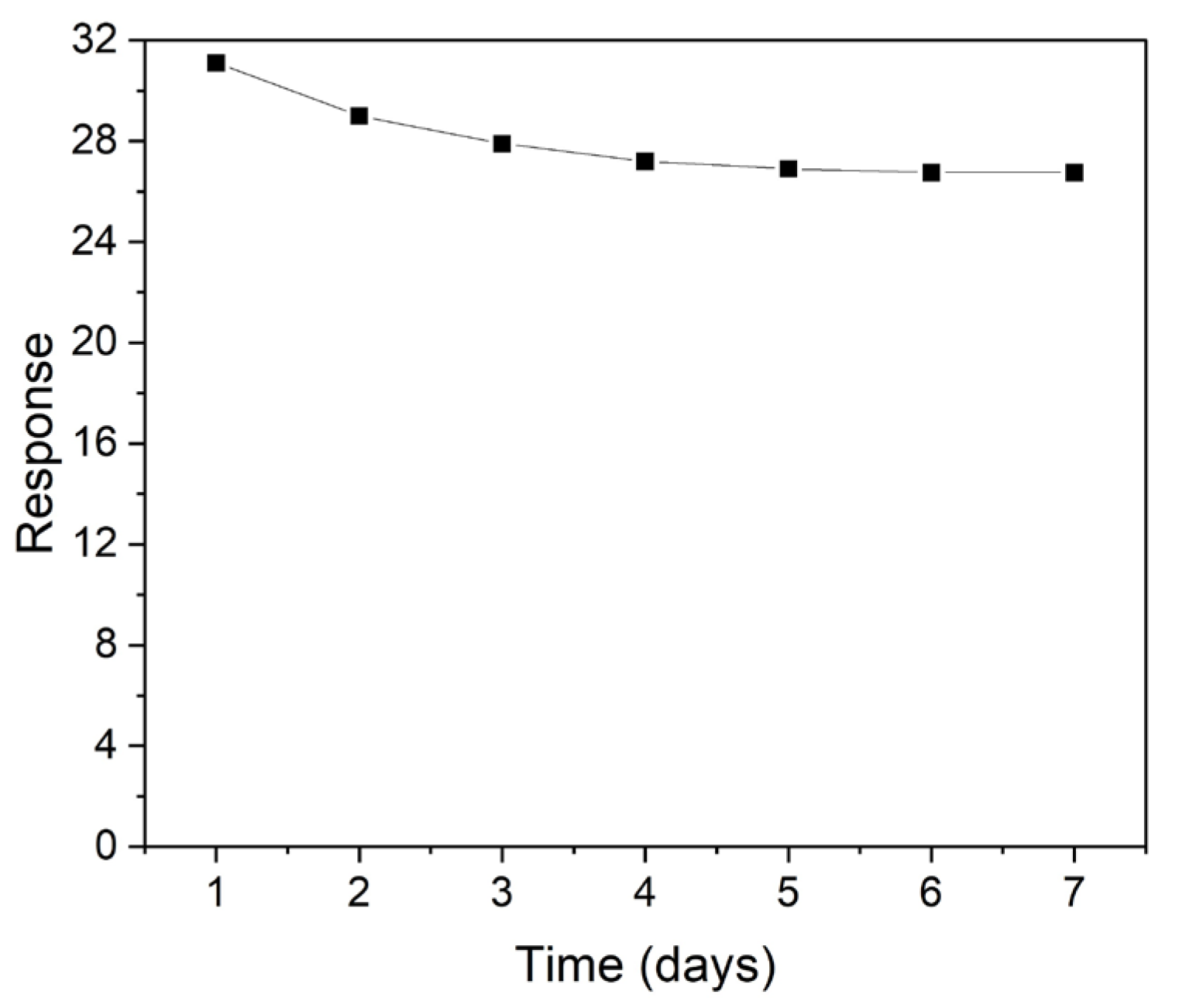

| Samples | Zn (at%) | O (at%) | C (at%) | O/Zn (%) |
|---|---|---|---|---|
| ZnO1 | 41.29 | 43.96 | 14.74 | 1.065 |
| ZnO2 | 32.45 | 33.71 | 33.83 | 1.039 |
| Material | Dimensions | Size (nm) | Operational Temperature (°C) | NO2 Concentration (ppm) | Sensor Response (Rgas/Rair) | Reference |
|---|---|---|---|---|---|---|
| ZnO nanosheets | 2D | 80 | 100 | 2 | 2.6 | [53] |
| ZnO | Nanosheets | 790 | RT | 25 | 23 | [54] |
| ZnO | Nanowire | 30–50 | RT | 1 | Around 10 | [55] |
| ZnO | Nanofilm | RT | 0.125 | 5.5 | [21] | |
| ZnO | Nanoparticles | 100 | 300 | 10 | 6.8 | [56] |
| ZnO | Nanorods | 60 | 100 | 1 | 13.4 | [57] |
| ZnO | Nanorods | 100 | 200 | 1 | 2.3 | [58] |
| ZnO | Nanorods | 50–500 | 250 | 10 | 13 | [59] |
| ZnO | Nanosheets | 200 | 100 | 1 | 2.5 | [60] |
| ZnO | Nanoparticles | 25–31 | 100 | 3% | 1.2 | [16] |
| ZnO/SnO2 | Heterojunction | 2000 | 300 | 1 | 1.3 | [17] |
| ZnO | nanofilm | 60–90 | 100 | 5 | 31.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Giubertoni, D.; Cian, A.; Valt, M.; Ardit, M.; Pedrielli, A.; Vanzetti, L.; Fabbri, B.; Guidi, V.; Gaiardo, A. Fabrication of a Highly NO2-Sensitive Gas Sensor Based on a Defective ZnO Nanofilm and Using Electron Beam Lithography. Micromachines 2023, 14, 1908. https://doi.org/10.3390/mi14101908
Feng Z, Giubertoni D, Cian A, Valt M, Ardit M, Pedrielli A, Vanzetti L, Fabbri B, Guidi V, Gaiardo A. Fabrication of a Highly NO2-Sensitive Gas Sensor Based on a Defective ZnO Nanofilm and Using Electron Beam Lithography. Micromachines. 2023; 14(10):1908. https://doi.org/10.3390/mi14101908
Chicago/Turabian StyleFeng, Zhifu, Damiano Giubertoni, Alessandro Cian, Matteo Valt, Matteo Ardit, Andrea Pedrielli, Lia Vanzetti, Barbara Fabbri, Vincenzo Guidi, and Andrea Gaiardo. 2023. "Fabrication of a Highly NO2-Sensitive Gas Sensor Based on a Defective ZnO Nanofilm and Using Electron Beam Lithography" Micromachines 14, no. 10: 1908. https://doi.org/10.3390/mi14101908
APA StyleFeng, Z., Giubertoni, D., Cian, A., Valt, M., Ardit, M., Pedrielli, A., Vanzetti, L., Fabbri, B., Guidi, V., & Gaiardo, A. (2023). Fabrication of a Highly NO2-Sensitive Gas Sensor Based on a Defective ZnO Nanofilm and Using Electron Beam Lithography. Micromachines, 14(10), 1908. https://doi.org/10.3390/mi14101908







