Enhancing the Sensitivity of Lateral Flow Assay with Europium Nanoparticles for Accurate Human IgG Quantification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of CM-EUs Conjugation with Anti-Human IgG
2.3. Calibration
2.4. Equation to Calculate the Volume Ratio
2.5. Statistical Analysis
3. Results
3.1. Principle of the Assay Method
3.2. Optimization of Europium Chelate LFA
3.3. Effect of Detection Antibody (DAB) Concentration on the Conjugation Pad
3.4. Impact of Capture Antibody (CAB) Quantity on the Test Line
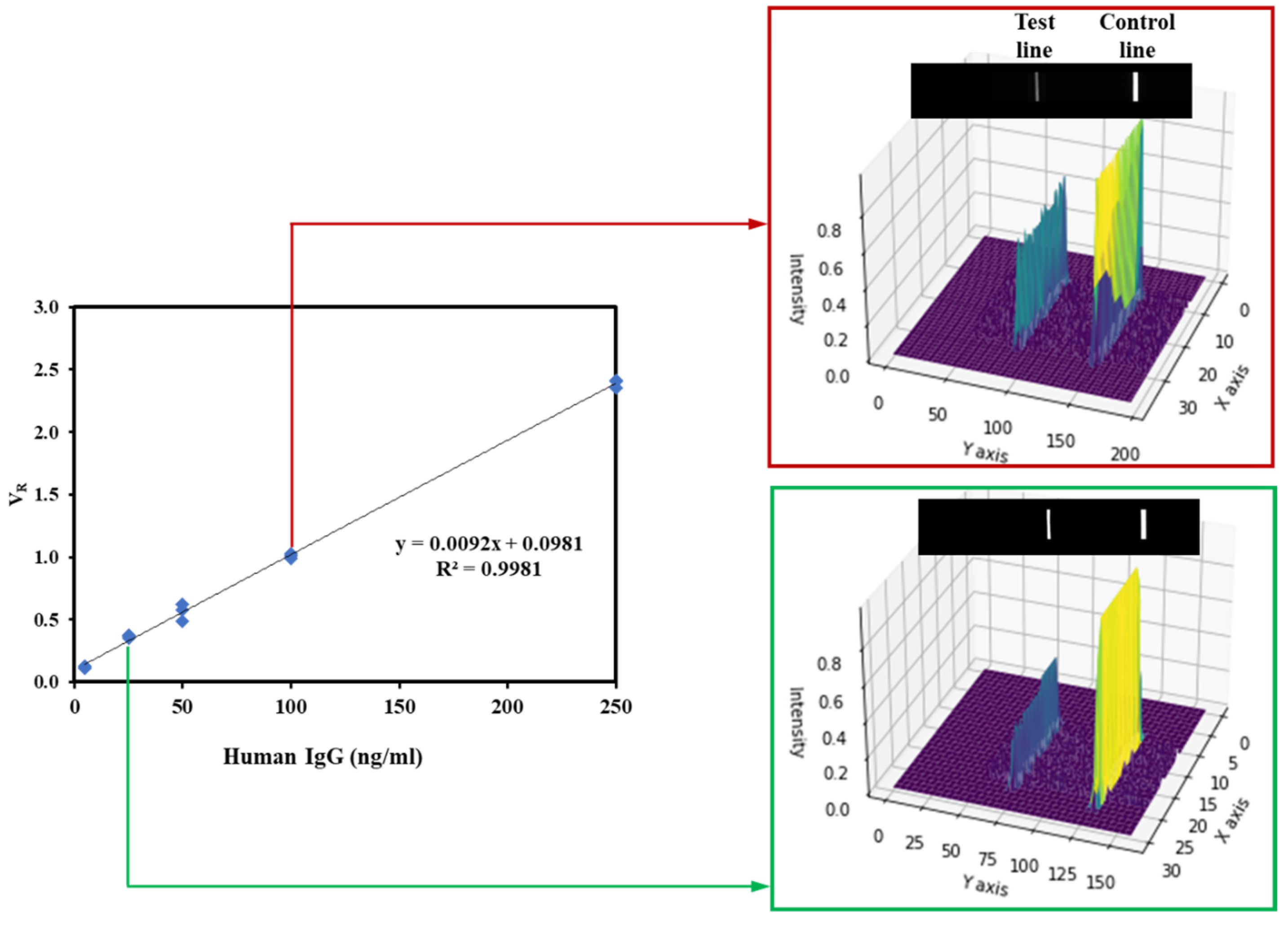
3.5. Linearity and Analytical Sensitivity
3.6. Precision
3.7. Stability of the LFIA Kit
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scott-Taylor, T.H.; Axinia, S.-C.; Amin, S.; Pettengell, R. Immunoglobulin G.; Structure and Functional Implications of Different Subclass Modifications in Initiation and Resolution of Allergy. Immun. Inflamm. Dis. 2018, 6, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Napodano, C.; Marino, M.; Stefanile, A.; Pocino, K.; Scatena, R.; Gulli, F.; Rapaccini, G.L.; Delli Noci, S.; Capozio, G.; Rigante, D. Immunological Role of IgG Subclasses. Immunol. Investig. 2021, 50, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Ballieux, R.E.; Bernier, G.M.; Tominaga, K.; Putnam, F.W. Gamma Globulin Antigenic Types Defined by Heavy Chain Determinants. Science 1964, 145, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Beeson, J.G.; Osier, F.H.; Engwerda, C.R. Recent Insights into Humoral and Cellular Immune Responses against Malaria. Trends Parasitol. 2008, 24, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Kijlstra, A.; Hoekzema, R.; Lelij, A.V.; Doekes, G.; Rothova, A. Humoral and Cellular Immune Reactions against Retinal Antigens in Clinical Disease. Curr. Eye Res. 1990, 9, 85–89. [Google Scholar] [CrossRef]
- Terry, W.D.; Fahey, J.L. Subclasses of Human Γ2-Globulin Based on Differences in the Heavy Polypeptide Chains. Science 1964, 146, 400–401. [Google Scholar] [CrossRef]
- Butler, J.E.; Hamilton, R.G. Quantitation of Specific Antibodies: Methods of Expression, Standards, Solid Phase Considerations and Specific Applications. In Immunochemistry of Solid Phase Immunoassays; CRC Press: Boca Raton, FL, USA, 1991; pp. 173–198. [Google Scholar]
- Papadea, C.; Check, I.J.; Reimer, C.B. Monoclonal Antibody-Based Solid-Phase Immunoenzymometric Assays for Quantifying Human Immunoglobulin G and Its Subclasses in Serum. Clin. Chem. 1985, 31, 1940–1945. [Google Scholar] [CrossRef]
- Djurup, R.; Weeke, B. Methods of Detecting IgG Subclass Proteins and Antibodies. Monogr. Allergy 1986, 19, 86–99. [Google Scholar]
- Hussain, R.; Poindexter, R.W.; Wistar, R.; Reimer, C.B. Use of Monoclonal Antibodies to Quantify Subclasses of Human IgG: I. Development of Two-Site Immunoenzymometric Assays for Total IgG Subclass Determinations. J. Immunol. Methods 1986, 93, 89–96. [Google Scholar] [CrossRef]
- Oxelius, V.A. IgG Subclass Pattern in Primary Immunodeficiency Disorders. Monogr. Allergy 1986, 19, 156–163. [Google Scholar]
- Peng, P.; Liu, C.; Li, Z.; Xue, Z.; Mao, P.; Hu, J.; Xu, F.; Yao, C.; You, M. Emerging ELISA Derived Technologies for in Vitro Diagnostics. TrAC Trends Anal. Chem. 2022, 152, 116605. [Google Scholar] [CrossRef]
- Pillai-Kastoori, L.; Schutz-Geschwender, A.R.; Harford, J.A. A Systematic Approach to Quantitative Western Blot Analysis. Anal. Biochem. 2020, 593, 113608. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral Flow Assays: Principles, Designs and Labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, Formats and Applications of Lateral Flow Assay: A Literature Review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Bishop, J.D.; Hsieh, H.V.; Gasperino, D.J.; Weigl, B.H. Sensitivity Enhancement in Lateral Flow Assays: A Systems Perspective. Lab Chip 2019, 19, 2486–2499. [Google Scholar] [CrossRef]
- Gong, X.; Chang, J.; Cai, J.; Zhang, B.; Zhao, Q.; Piao, J.; Peng, W.; Gao, W.; Zhou, D.; Zhao, M. A Review for Fluorescent Lateral Flow Immunochromatographic Strip. J. Mater. Chem. B 2017, 5, 5079–5091. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Nara, S. Evaluation of Gold Nanoparticle Based Lateral Flow Assays for Diagnosis of Enterobacteriaceae Members in Food and Water. Food Chem. 2015, 170, 470–483. [Google Scholar] [CrossRef]
- Ardekani, L.S.; Thulstrup, P.W. Gold Nanoparticle-Mediated Lateral Flow Assays for Detection of Host Antibodies and COVID-19 Proteins. Nanomaterials 2022, 12, 1456. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-Based Lateral Flow Biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef]
- Juntunen, E.; Myyryläinen, T.; Salminen, T.; Soukka, T.; Pettersson, K. Performance of Fluorescent Europium(III) Nanoparticles and Colloidal Gold Reporters in Lateral Flow Bioaffinity Assay. Anal. Biochem. 2012, 428, 31–38. [Google Scholar] [CrossRef]
- Karthik, S.; Shah, M.I.; Natarajan, S.; Shetty, M.J.; Joseph, J. A Motion Free Image Based TRF Reader for Quantitative Immunoassay. In Proceedings of the 2019 IEEE Healthcare Innovations and Point of Care Technologies, (HI-POCT), Bethesda, MD, USA, 20–22 November 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 163–166. [Google Scholar]
- Rijpkema, S.; Hockley, J.; Logan, A.; Rigsby, P.; Atkinson, E.; Jin, C.; Goldblatt, D.; Liang, H.; Bachtiar, N.S.; Yang, J.S.; et al. Establishment of the first International Standard for human anti-typhoid capsular Vi polysaccharide IgG. Biologicals 2018, 56, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration—FDA. Guidance for Industry; Center for Drug Evaluation and Research (CDER): Beltsville, MD, USA, 1998; p. 1000. [Google Scholar]
- Chapter, G. Validation of Compendial Methods, United States Pharmacopeia, 26th Revision, National Formulary, Rockville, MD, The United States Pharmacopeial Convention. Inc 2003, 2440, 2003–2444. [Google Scholar]
- Magnusson, B. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics; Eurachem: Gembloux, Belgium, 2014. [Google Scholar]
- Replaces, E.-P. Protocols for Determination of Limits of Detection and Limits of Quantitation; Approved Guideline; NCCLs: Wayne, PE, USA, 2004. [Google Scholar]
- Nadovich, C. Synthetic Instruments: Concepts and Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
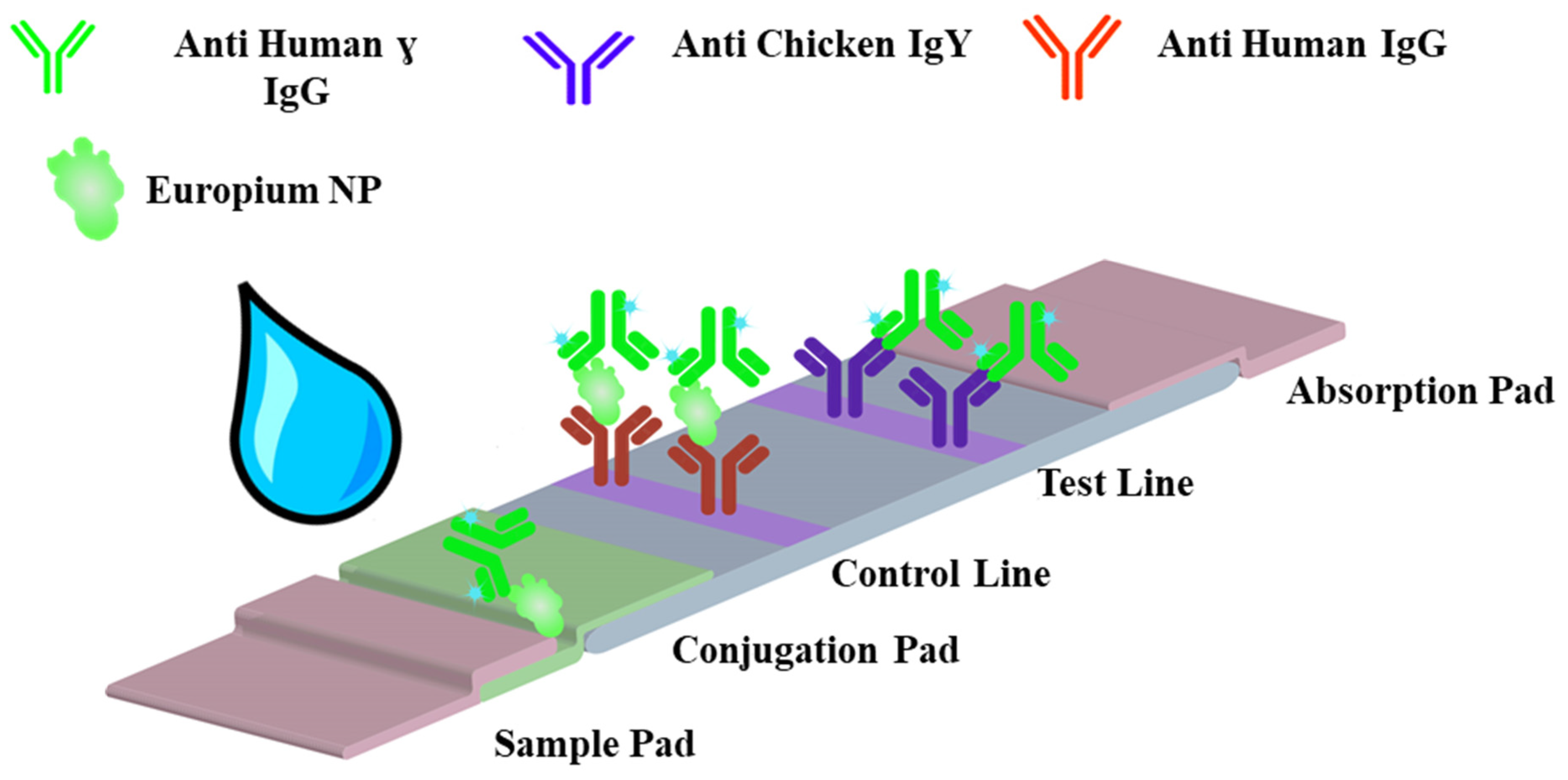

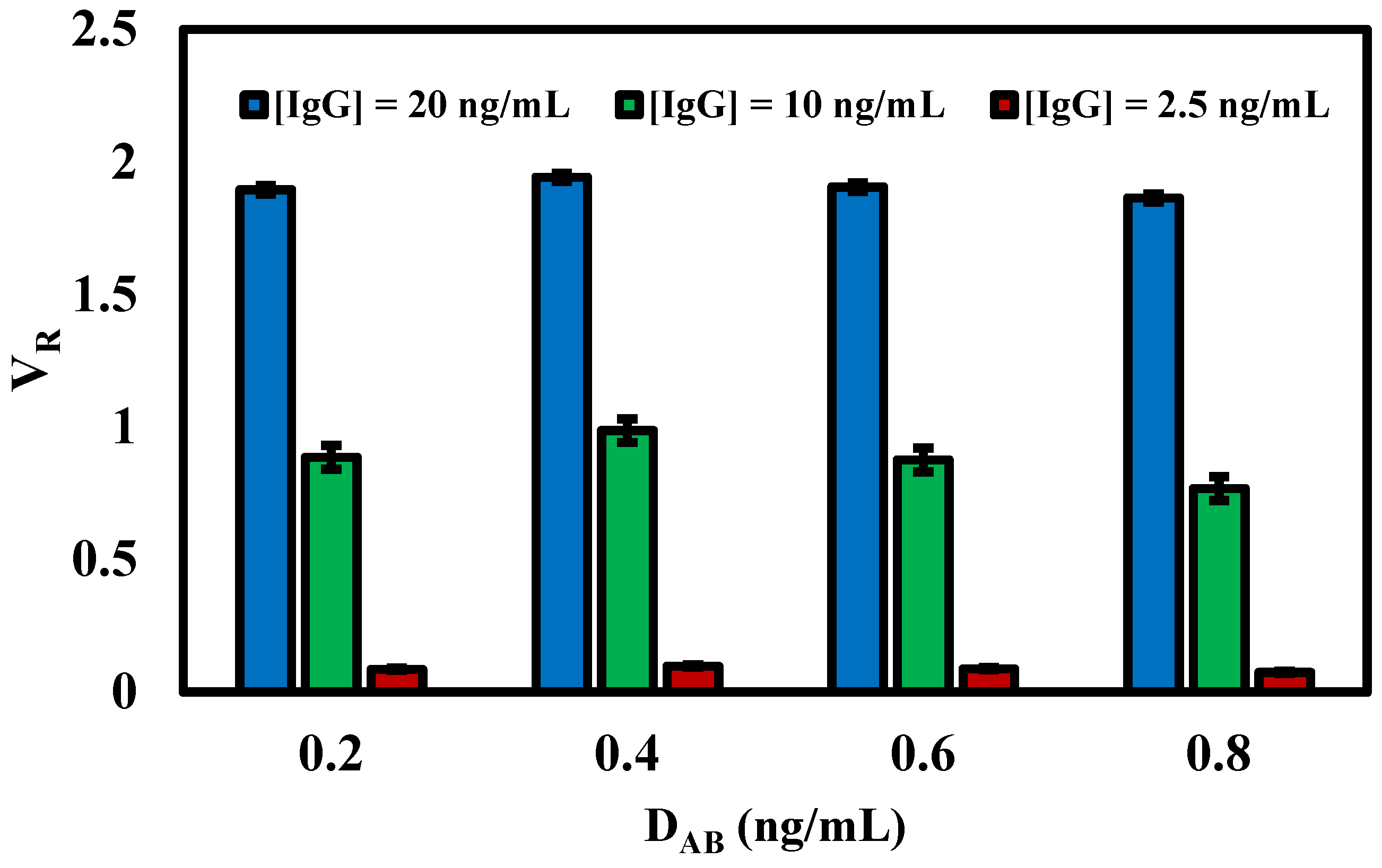


| Type of Parameters | Parameters Tested | Selected Parameter |
|---|---|---|
| Concentration of detection antibody in the conjugation pad | 0.2, 0.4, 0.6, 0.8 ng/mL | 0.4 ng/mL |
| Concentration of capture antibody in the test line | 0.5, 1.0, 1.5 mg/mL | 1 mg/mL |
| Parameter | Eu-LFA |
|---|---|
| slope | 0.0092 |
| intercept | 0.0981 |
| R | 0.9981 |
| LOD (ng/mL) | 0.004 |
| LoB (ng/mL) | 0.003 |
| Inter-Assay | |||
|---|---|---|---|
| Human IgG (ng/mL) | Mean | SD | cv |
| 0.5 | 10.8 | 6.0 | 4.5 |
| 5 | 35.9 | 1.7 | 4.9 |
| 500 | 131.8 | 0.5 | 5.1 |
| Intra-Assay | |||
| Human IgG (ng/mL) | Mean | SD | cv |
| 0.5 | 12.7 | 5.2 | 4.7 |
| 5 | 37.5 | 3.2 | 5.2 |
| 500 | 137.2 | 0.7 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natarajan, S.; Priye, A. Enhancing the Sensitivity of Lateral Flow Assay with Europium Nanoparticles for Accurate Human IgG Quantification. Micromachines 2023, 14, 1993. https://doi.org/10.3390/mi14111993
Natarajan S, Priye A. Enhancing the Sensitivity of Lateral Flow Assay with Europium Nanoparticles for Accurate Human IgG Quantification. Micromachines. 2023; 14(11):1993. https://doi.org/10.3390/mi14111993
Chicago/Turabian StyleNatarajan, Satheesh, and Aashish Priye. 2023. "Enhancing the Sensitivity of Lateral Flow Assay with Europium Nanoparticles for Accurate Human IgG Quantification" Micromachines 14, no. 11: 1993. https://doi.org/10.3390/mi14111993
APA StyleNatarajan, S., & Priye, A. (2023). Enhancing the Sensitivity of Lateral Flow Assay with Europium Nanoparticles for Accurate Human IgG Quantification. Micromachines, 14(11), 1993. https://doi.org/10.3390/mi14111993






