Tunable Visible Light and Energy Transfer Mechanism in Tm3+ and Silver Nanoclusters within Co-Doped GeO2-PbO Glasses
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuznetsov, A.S.; Tikhomirov, V.K.; Shestakov, M.V.; Moshchalkov, V.V. Ag Nanocluster Functionalized Glasses for Efficient Photonic Conversion in Light Sources, Solar Cells and Flexible Screen Monitors. Nanoscale 2013, 5, 10065–10075. [Google Scholar] [CrossRef]
- Díez, I.; Ras, R.H.A. Fluorescent Silver Nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef]
- Choi, S.; Dickson, R.M.; Yu, J. Developing Luminescent Silver Nanodots for Biological Applications. Chem. Soc. Rev. 2012, 41, 1867–1891. [Google Scholar] [CrossRef]
- Tikhomirov, V.K.; Rodríguez, V.D.; Kuznetsov, A.; Kirilenko, D.; Tendeloo, G.V.; Moshchalkov, V.V. Preparation and Luminescence of Bulk Oxyfluoride Glasses Doped with Ag Nanoclusters. Opt. Express 2010, 18, 22032–22040. [Google Scholar] [CrossRef]
- Kuznetsov, A.S.; Velázquez, J.J.; Tikhomirov, V.K.; Mendez-Ramos, J.; Moshchalkov, V.V. Quantum Yield of Luminescence of Ag Nanoclusters Dispersed within Transparent Bulk Glass vs. Glass Composition and Temperature. Appl. Phys. Lett. 2012, 101, 251106. [Google Scholar] [CrossRef]
- Tikhomirov, V.K.; Vosch, T.; Fron, E.; Rodríguez, V.D.; Velázquez, J.J.; Kirilenko, D.; Van Tendeloo, G.; Hofkens, J.; Van der Auweraer, M.; Moshchalkov, V.V. Luminescence of Oxyfluoride Glasses Co-Doped with Ag Nanoclusters and Yb3+ Ions. RSC Adv. 2012, 2, 1496–1501. [Google Scholar] [CrossRef]
- Velázquez, J.J.; Tikhomirov, V.K.; Chibotaru, L.F.; Cuong, N.T.; Kuznetsov, A.S.; Rodríguez, V.D.; Nguyen, M.T.; Moshchalkov, V.V. Energy Level Diagram and Kinetics of Luminescence of Ag Nanoclusters Dispersed in a Glass Host. Opt. Express 2012, 20, 13582–13591. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Qian, J.; Cui, S.; Qiao, X.; Wang, F.; Fan, X. Enhancing NIR Emission of Yb3+ by Silver Nanoclusters in Oxyfluoride Glass. J. Lumin. 2014, 152, 222–225. [Google Scholar] [CrossRef]
- Shestakov, M.V.; Chen, X.M.; Kaydashev, V.; Baeckelant, W.; Tikhomirov, V.K.; Vanacken, J.; Hofkens, J.; Moshchalkov, V.V. Oxyfluoride Glass (SiO2-PbF2) Co-Doped with Ag Nanoclusters and Tm3+ Ions for UV-Driven, Hg-Free, White Light Generation with a Tuneable Tint. Opt. Mater. Express 2014, 4, 1227–1235. [Google Scholar] [CrossRef]
- Wei, R.; Li, J.; Gao, J.; Guo, H. Enhancement of Eu3+ Luminescence by Ag Species (Ag NPs, ML-Ag, Ag+) in Oxyfluoride Glasses. J. Am. Ceram. Soc. 2012, 95, 3380–3382. [Google Scholar] [CrossRef]
- Ye, S.; Guo, Z.; Wang, H.; Li, S.; Liu, T.; Wang, D. Evolution of Ag Species and Molecular-like Ag Cluster Sensitized Eu3+ Emission in Oxyfluoride Glass for Tunable Light Emitting. J. Alloys Compd. 2016, 685, 891–895. [Google Scholar] [CrossRef]
- Guo, Z.; Ye, S.; Qiao, X.; Wang, D. Luminescence Properties and Tunable Emission of Ag NCs in Oxyfluoride Glass through REF3 (RE = Y, La and Gd) Doping. J. Am. Ceram. Soc. 2018, 101, 732–738. [Google Scholar] [CrossRef]
- Sandrini, M.; Muniz, R.F.; Zanuto, V.S.; Pedrochi, F.; Guyot, Y.; Bento, A.C.; Baesso, M.L.; Steimacher, A.; Neto, A.M. Enhanced and Tunable White Light Emission from Ag Nanoclusters and Eu3+-Co-Doped CaBAl Glasses. RSC Adv. 2018, 8, 35263–35270. [Google Scholar] [CrossRef]
- Fares, H.; Santos, S.N.; Santos, M.V.; Franco, D.F.; Souza, A.E.; Manzani, D.; Mendonça, C.R.; Nalin, M. Highly Luminescent Silver Nanocluster-Doped Fluorophosphate Glasses for Microfabrication of 3D Waveguides. RSC Adv. 2017, 7, 55935–55944. [Google Scholar] [CrossRef]
- Fares, H.; Castro, T.; Franco, D.F.; Fucikova, A.; da Silva, R.R.; Valenta, J.; Ribeiro, S.J.L.; Nalin, M. Tuning Multicolor Emission in AgNCs/Tm3+/Mn2+-Doped Fluorophosphate Glasses. J. Non-Cryst. Solids 2020, 535, 119968. [Google Scholar] [CrossRef]
- Fares, H.; Castro, T.; Orives, J.R.; Franco, D.F.; Nalin, M. White Light and Multicolor Emission Tuning in Ag Nanocluster Doped Fluorophosphate Glasses. RSC Adv. 2017, 7, 44356–44365. [Google Scholar] [CrossRef]
- Aseev, V.A.; Burdaev, P.A.; Kolobkova, E.V.; Nikonorov, N.V. Fluorophosphate Glasses Activated by Rare-Earth Ions and AgBr. Glass Phys. Chem. 2012, 38, 366–372. [Google Scholar] [CrossRef]
- Ennouri, M.; Gelloz, B.; Elhouichet, H. Impact of Ag Species on Luminescence and Spectroscopic Properties of Eu3+ Doped Fluoro-Phosphate Glasses. J. Non-Cryst. Solids 2021, 570, 120938. [Google Scholar] [CrossRef]
- Castro, T.; Jubera, V.; Fares, H.; Petit, Y.; Fargues, A.; Cardinal, T.; Nalin, M.; Ribeiro, S. Photoluminescence of Ag+ and Agmn+in Co-Doped Pr3+/Yb3+ Fluorophosphate Glasses: Tuning Visible Emission and Energy Transfer to Pr3+/Yb3+ Ions through Excitation in Different Silver Species. J. Mater. Sci. Mater. Electron. 2019, 30, 16878–16885. [Google Scholar] [CrossRef]
- Jiao, Q.; Wang, X.; Qiu, J.; Zhou, D. Effect of Silver Ions and Clusters on the Luminescence Properties of Eu-Doped Borate Glasses. Mater. Res. Bull. 2015, 72, 264–268. [Google Scholar] [CrossRef]
- Liao, H.; Ye, S.; Shen, R.; Li, X.; Wang, D. Effective Formation of Ag Nanoclusters and Efficient Energy Transfer to Yb3+ Ions in Borosilicate Glasses for Photovoltaic Application. Mater. Res. Bull. 2019, 111, 113–117. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, S.; Yu, J.; Liao, H.; Liu, J.; Wang, D. Simultaneous Energy Transfer from Molecular-like Silver Nanoclusters to Sm3+/Ln3+ (Ln = Eu or Tb) in Glass under UV Excitation. Opt. Express 2019, 27, 38159–38167. [Google Scholar] [CrossRef]
- Speranza, G.; Minati, L.; Chiappini, A.; Chiasera, A.; Duverger, C.A.; Ferrari, M.; Righini, G.C. Electron Confinement Effects in Silver Nanocluster Embedded in Sodalime Glasses. In Silicon Photonics and Photonic Integrated Circuits; SPIE: Bellingham, WA, USA, 2008; Volume 6996, pp. 63–69. [Google Scholar]
- Li, L.; Yang, Y.; Zhou, D.; Yang, Z.; Xu, X.; Qiu, J. Investigation of the Interaction between Different Types of Ag Species and Europium Ions in Ag+-Na+ Ion-Exchange Glass. Opt. Mater. Express 2013, 3, 806–812. [Google Scholar] [CrossRef]
- Dubrovin, V.D.; Ignatiev, A.I.; Nikonorov, N.V.; Sidorov, A.I.; Shakhverdov, T.A.; Agafonova, D.S. Luminescence of Silver Molecular Clusters in Photo-Thermo-Refractive Glasses. Opt. Mater. 2014, 36, 753–759. [Google Scholar] [CrossRef]
- Hamzaoui, H.E.; Capoen, B.; Razdobreev, I.; Bouazaoui, M. In Situ Growth of Luminescent Silver Nanoclusters inside Bulk Sol-Gel Silica Glasses. Mater. Res. Express 2017, 4, 076201. [Google Scholar] [CrossRef]
- Ghafoor, M.; Sgibnev, Y.; Marasanov, D.; Nikonorov, N. Energy Transfer between Silver Clusters and Europium Eu3+ Ions in Photo-Thermo-Refractive Glasses. In Third International Conference on Applications of Optics and Photonics; SPIE: Bellingham, WA, USA, 2017; Volume 10453, pp. 138–143. [Google Scholar]
- Nishimura, M.V.M.; Bordon, C.D.S.; da Silva, D.M.; Kassab, L.R.P. Tunable visible emission and white light generation by Ag nanoclusters in Tm3+/Yb3+ doped GeO2-PbO glasses. In Proceedings of the 2021 35th Symposium on Microelectronics Technology and Devices (SBMicro), Campinas, Brazil, 23–27 August 2021. [Google Scholar]
- Chattopadhyay, R.; Haldar, A.; Paul, M.C.; Das, S.; Bhadra, S.K. Fluorescence Enhancement in Tm-Yb-Ag Codoped Fiber by Super-Radiance. In Proceedings of the 12th International Conference on Fiber Optics and Photonics (2014), paper T4A.5, Kharagpur, India, 13–16 December 2014; p. T4A.5. [Google Scholar]
- Halder, A.; Chattopadhyay, R.; Majumder, S.; Bysakh, S.; Paul, M.C.; Das, S.; Bhadra, S.K.; Unnikrishnan, M. Highly Fluorescent Silver Nanoclusters in Alumina-Silica Composite Optical Fiber. Appl. Phys. Lett. 2015, 106, 011101. [Google Scholar] [CrossRef]
- Nishimura, M.V.M.; Bordon, C.D.S.; Miretzcky, L.M.; Kassab, L.R.P. Broadband visible light emission by GeO2-PbO glasses doped with Ag nanoclusters. In Proceedings of the 2021 SBMO/IEEE MTT-S International Microwave and Optoelectronics Conference (IMOC), Fortaleza, Brazil, 24–27 October 2021. [Google Scholar]
- Amaro, A.A.; Mattos, G.R.d.S.; Nishimura, M.V.d.M.; Dipold, J.; Wetter, N.U.; Kassab, L.R.P. Silver Nanoclusters Tunable Visible Emission and Energy Transfer to Yb3+ Ions in Co-Doped GeO2-PbO Glasses for Photonic Applications. Nanomaterials 2023, 13, 1177. [Google Scholar] [CrossRef] [PubMed]
- Halperin, W.P. Quantum Size Effects in Metal Particles. Rev. Mod. Phys. 1986, 58, 533–606. [Google Scholar] [CrossRef]
- Dousti, M.R.; Amjad, R.J. Plasmon Assisted Luminescence in Rare Earth Doped Glasses. In Reviews in Plasmonics 2015; Geddes, C.D., Ed.; Reviews in Plasmonics; Springer International Publishing: Cham, Switzerland, 2016; pp. 339–386. [Google Scholar]
- Camilo, M.E.; Assumpção, T.A.A.; da Silva, D.M.; da Silva, D.S.; Kassab, L.R.P.; de Araújo, C.B. Influence of Silver Nanoparticles on the Infrared-to-Visible Frequency Upconversion in Tm3+/Er3+/Yb3+ Doped GeO2-PbO Glass. J. Appl. Phys. 2013, 113, 153507. [Google Scholar] [CrossRef]
- Kassab, L.R.P.; Bomfim, F.A.; Martinelli, J.R.; Wetter, N.U.; Neto, J.J.; de Araújo, C.B. Energy Transfer and Frequency Upconversion in Yb3+–Er3+-Doped PbO-GeO2 Glass Containing Silver Nanoparticles. Appl. Phys. B 2009, 94, 239–242. [Google Scholar] [CrossRef]
- Pontuschka, W.M.; Giehl, J.M.; Miranda, A.R.; Da Costa, Z.M.; Alencar, A.M. Effect of the Al2O3 Addition on the Formation of Silver Nanoparticles in Heat Treated Soda-Lime Silicate Glasses. J. Non-Cryst. Solids 2016, 453, 74–83. [Google Scholar] [CrossRef]
- McCamy, C.S. Correlated Color Temperature as an Explicit Function of Chromaticity Coordinates. Color Res. Appl. 1992, 17, 142–144. [Google Scholar] [CrossRef]
- Nishimura, M.V.M.; Bordon, C.D.S.; Miretzcky, L.M.; Kassab, L.R.P. A review of recent results of white light generation and tunable visible light emission by Ag nanoclusters: Undoped and doped Tm3+/Yb3+ doped GeO2-BbO glasses. In Smart Systems: Theory and Advances; Amplla Editora: Campina Grande, Brazil, 2022; pp. 57–73. [Google Scholar] [CrossRef]
- Lim, K.-S.; Babu, P.; Jayasankar, C.K.; Lee, S.; Pham, V.-T.; Seo, H.-J. Optical Spectroscopy of Thulium-Doped Oxyfluoroborate Glass. J. Alloys Compd. 2004, 385, 12–18. [Google Scholar] [CrossRef]
- Rajesh, D.; Amjad, R.J.; Reza Dousti, M.; de Camargo, A.S.S. Enhanced VIS and NIR Emissions of Pr3+ Ions in TZYN Glasses Containing Silver Ions and Nanoparticles. J. Alloys Compd. 2017, 695, 607–612. [Google Scholar] [CrossRef]
- Jiao, Q.; Qiu, J.; Zhou, D.; Xu, X. Contribution of Eu Ions on the Precipitation of Silver Nanoparticles in Ag-Eu Co-Doped Borate Glasses. Mater. Res. Bull. 2014, 51, 315–319. [Google Scholar] [CrossRef]
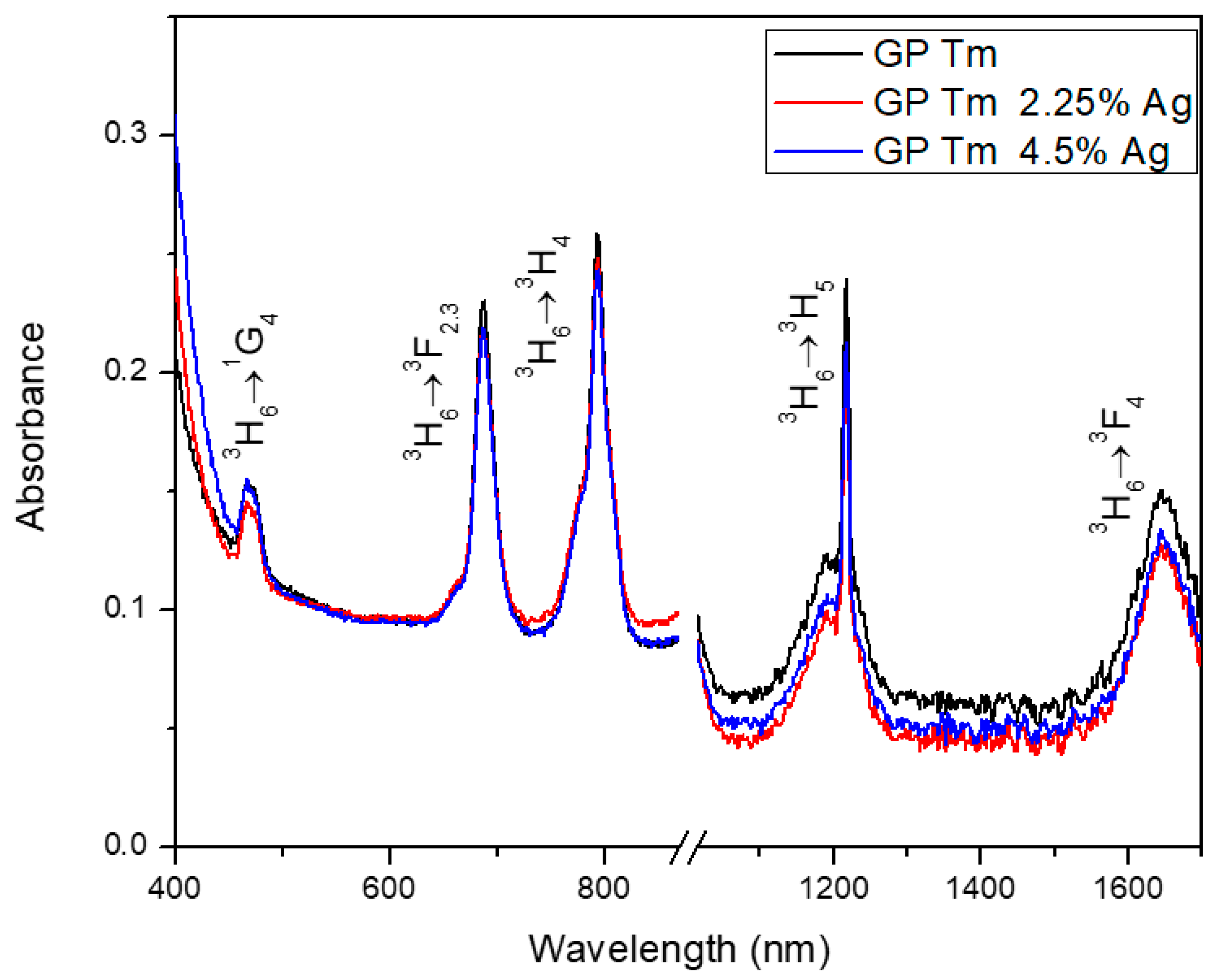
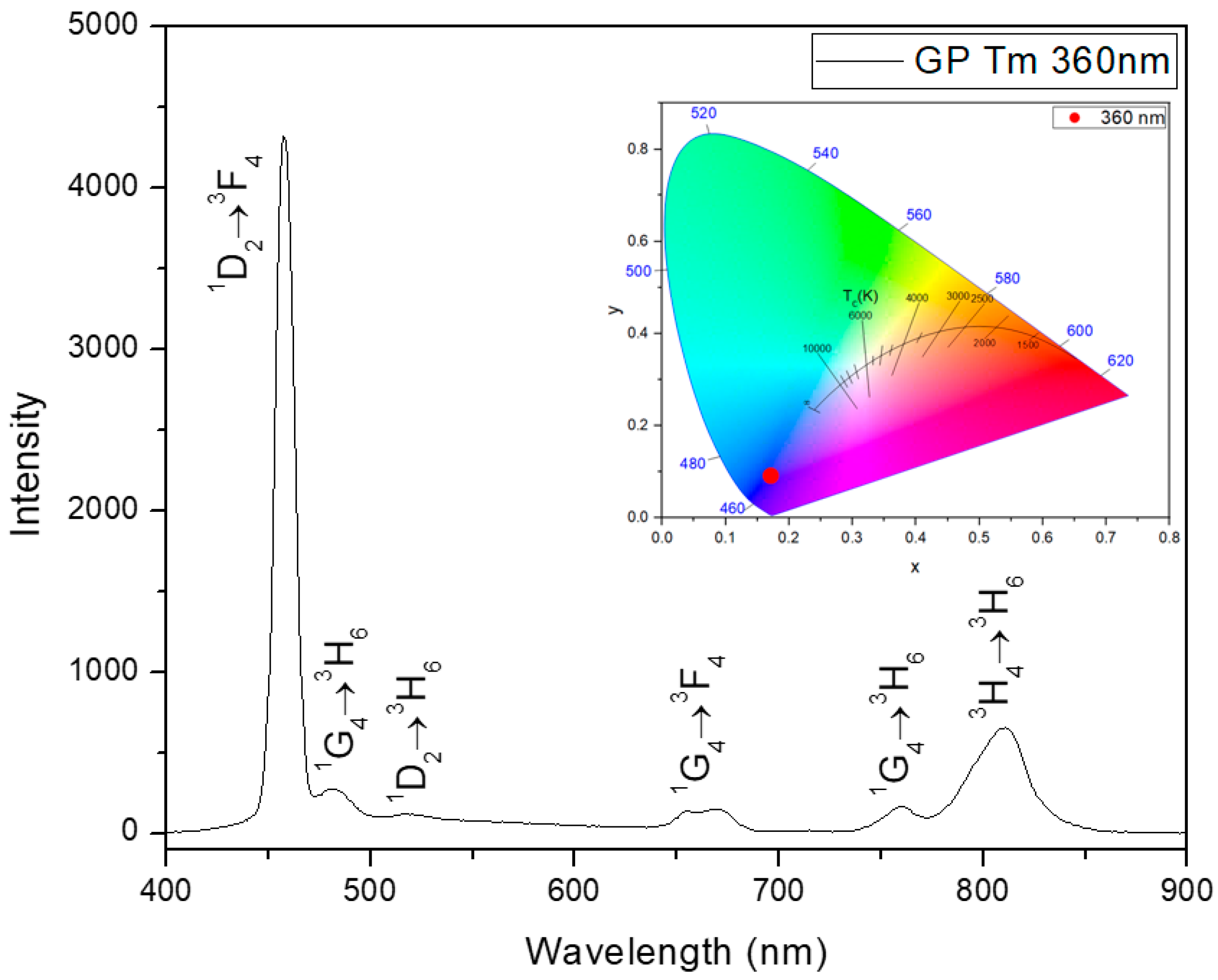
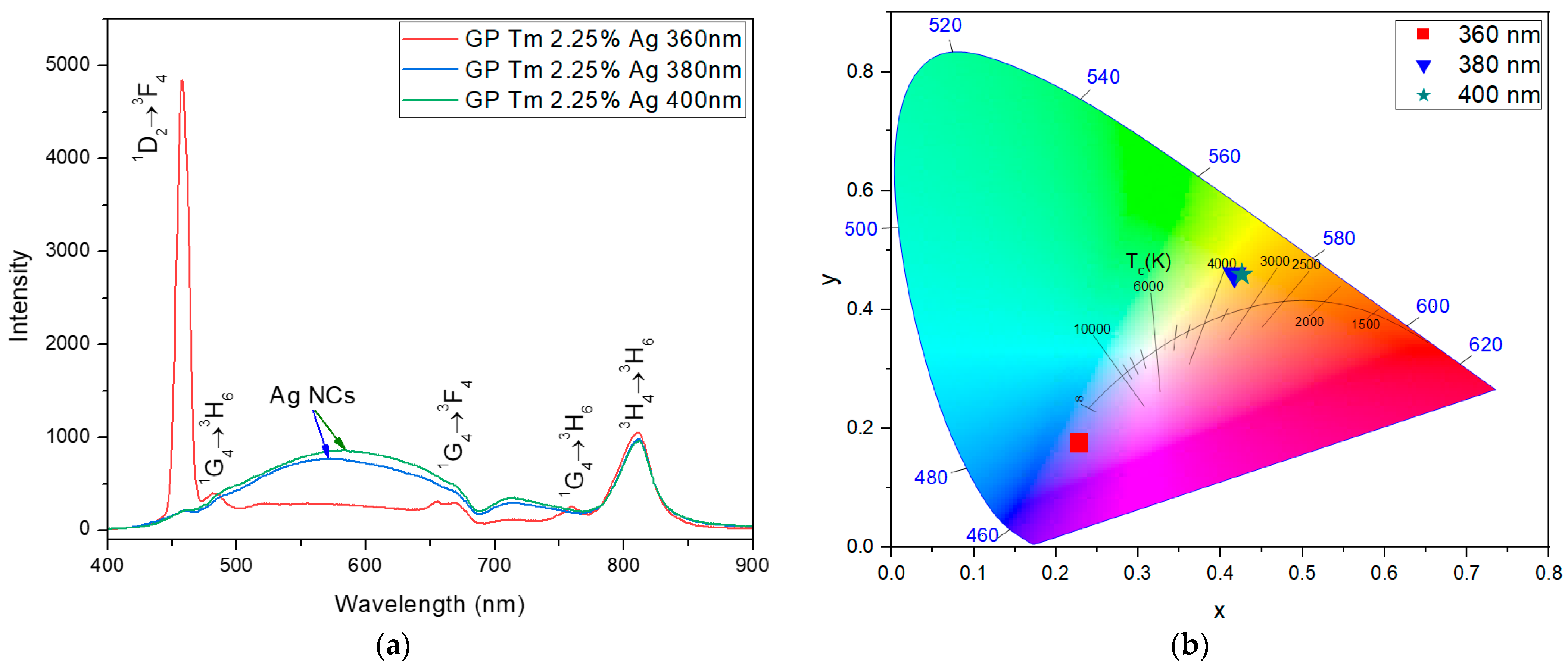


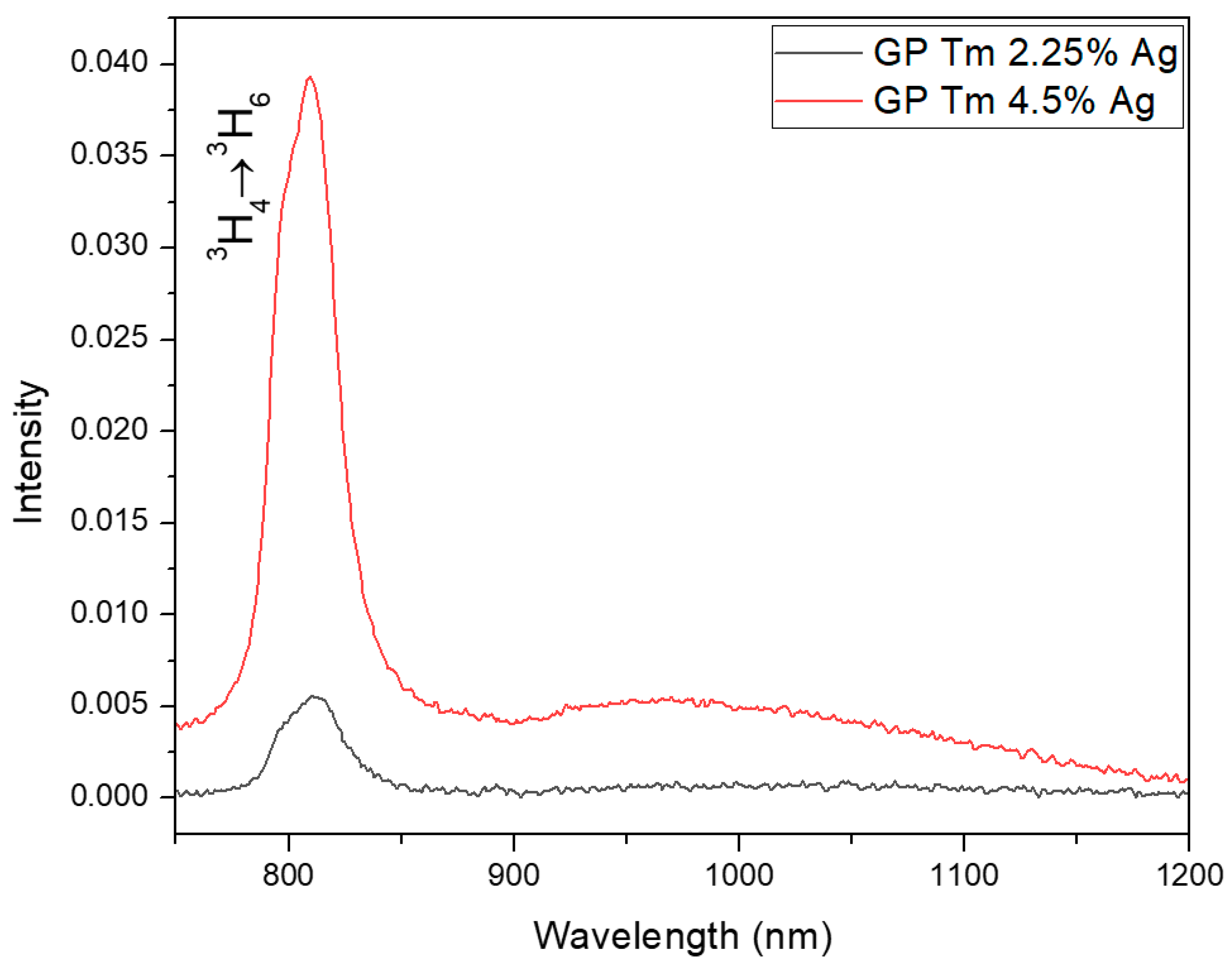
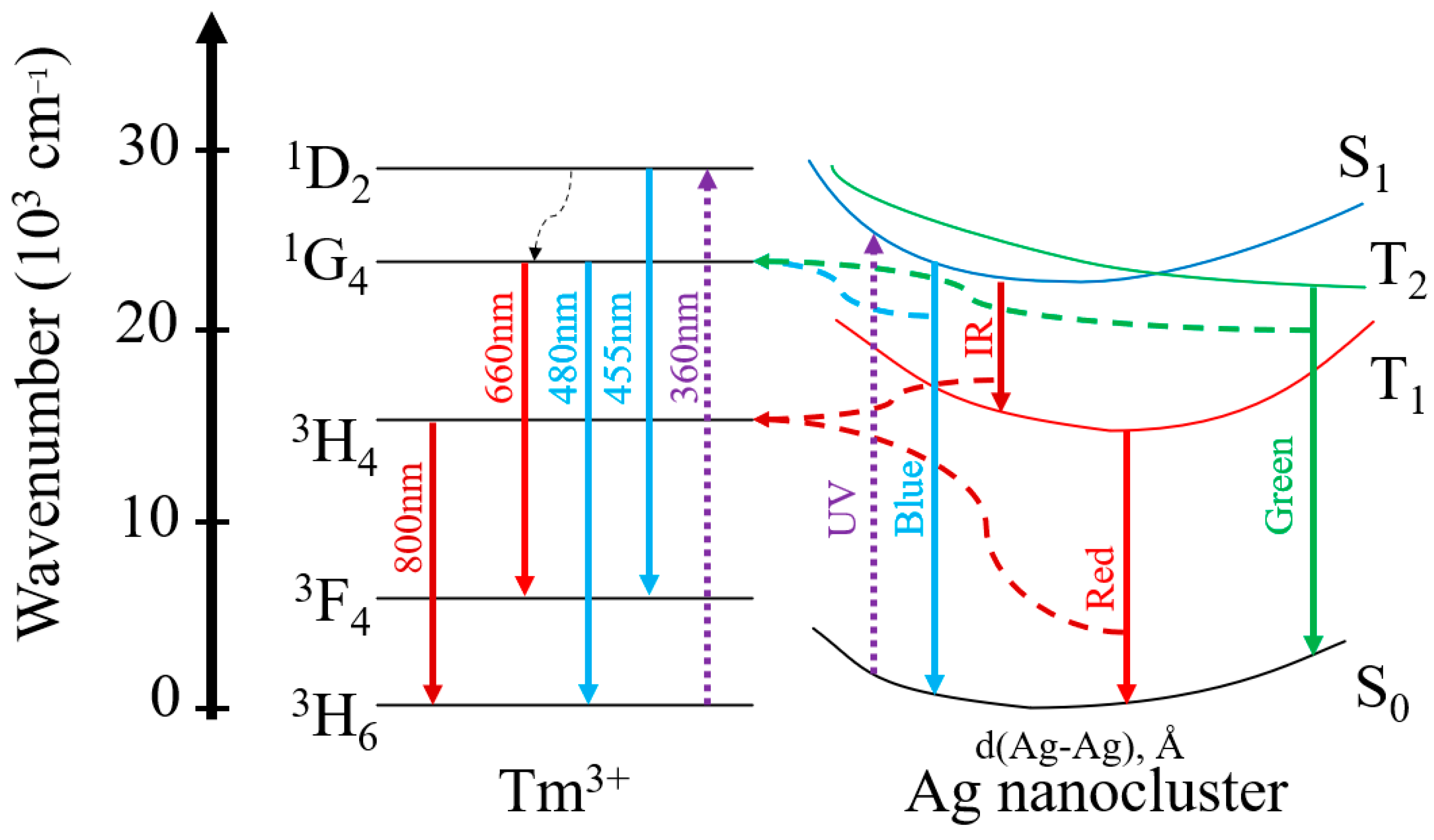

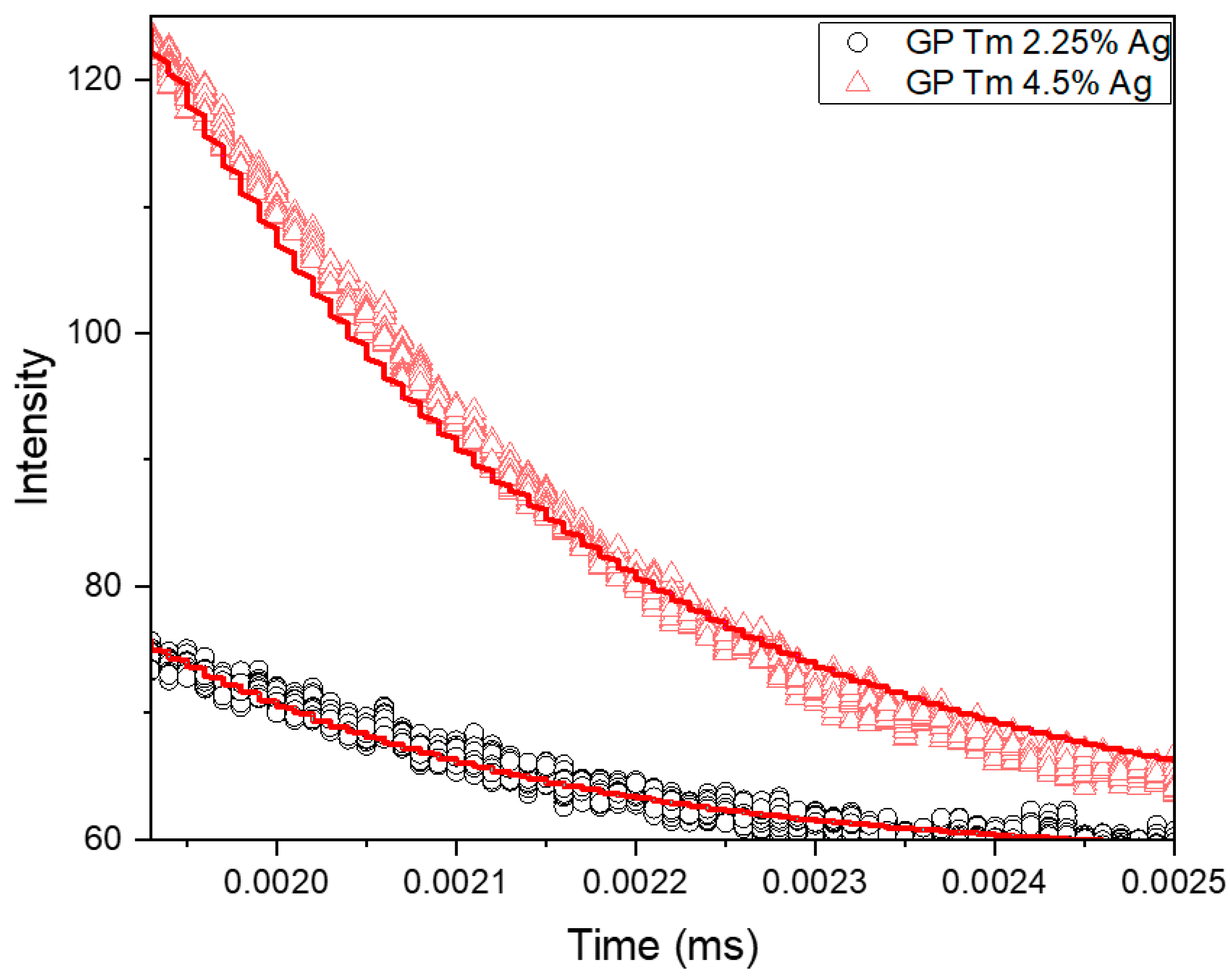
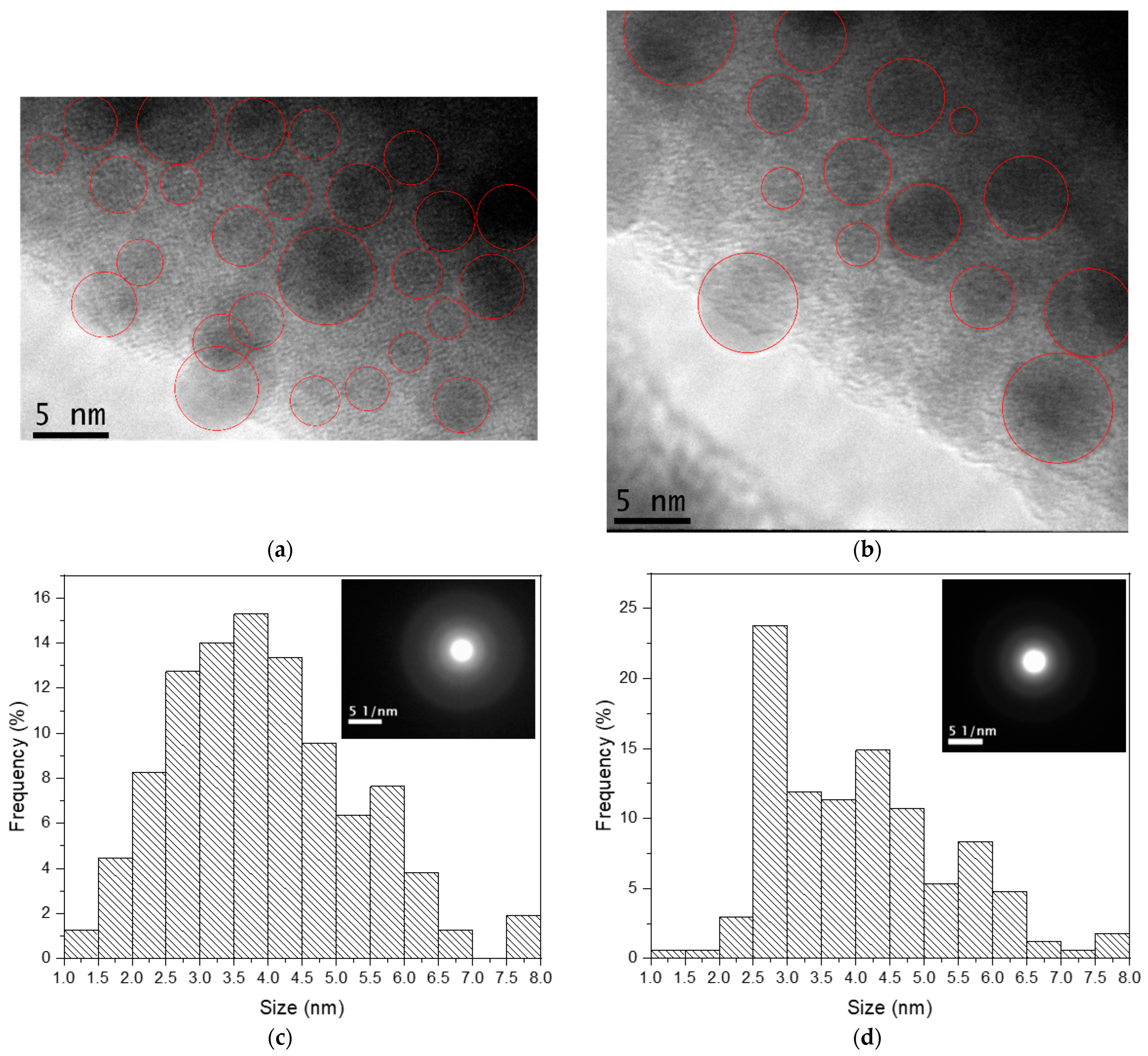
| Sample | Excitation Wavelength (nm) | τfast (μs) | τslow (μs) |
|---|---|---|---|
| GP 4.5% Ag | 380 | 11.7 ± 0.3 | 85 ± 2 |
| 400 | 3.7 ± 0.4 | 72 ± 8 | |
| GP Tm 4.5% Ag | 380 | 3.3 ± 0.1 | 26.2 ± 0.3 |
| 400 | 5.8 ± 0.2 | 38 ± 2 |
| Sample | Τime (μs) |
|---|---|
| GP Tm 2.25% Ag | 220 ± 4 |
| GP Tm 4.5% Ag | 239 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, M.V.d.M.; Amaro, A.A.; Bordon, C.D.d.S.; Dipold, J.; Wetter, N.U.; Kassab, L.R.P. Tunable Visible Light and Energy Transfer Mechanism in Tm3+ and Silver Nanoclusters within Co-Doped GeO2-PbO Glasses. Micromachines 2023, 14, 2078. https://doi.org/10.3390/mi14112078
Nishimura MVdM, Amaro AA, Bordon CDdS, Dipold J, Wetter NU, Kassab LRP. Tunable Visible Light and Energy Transfer Mechanism in Tm3+ and Silver Nanoclusters within Co-Doped GeO2-PbO Glasses. Micromachines. 2023; 14(11):2078. https://doi.org/10.3390/mi14112078
Chicago/Turabian StyleNishimura, Marcos Vinicius de Morais, Augusto Anselmo Amaro, Camila Dias da Silva Bordon, Jessica Dipold, Niklaus Ursus Wetter, and Luciana Reyes Pires Kassab. 2023. "Tunable Visible Light and Energy Transfer Mechanism in Tm3+ and Silver Nanoclusters within Co-Doped GeO2-PbO Glasses" Micromachines 14, no. 11: 2078. https://doi.org/10.3390/mi14112078
APA StyleNishimura, M. V. d. M., Amaro, A. A., Bordon, C. D. d. S., Dipold, J., Wetter, N. U., & Kassab, L. R. P. (2023). Tunable Visible Light and Energy Transfer Mechanism in Tm3+ and Silver Nanoclusters within Co-Doped GeO2-PbO Glasses. Micromachines, 14(11), 2078. https://doi.org/10.3390/mi14112078








