Effects of Block Copolymer Terminal Groups on Toughening Epoxy-Based Composites: Microstructures and Toughening Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
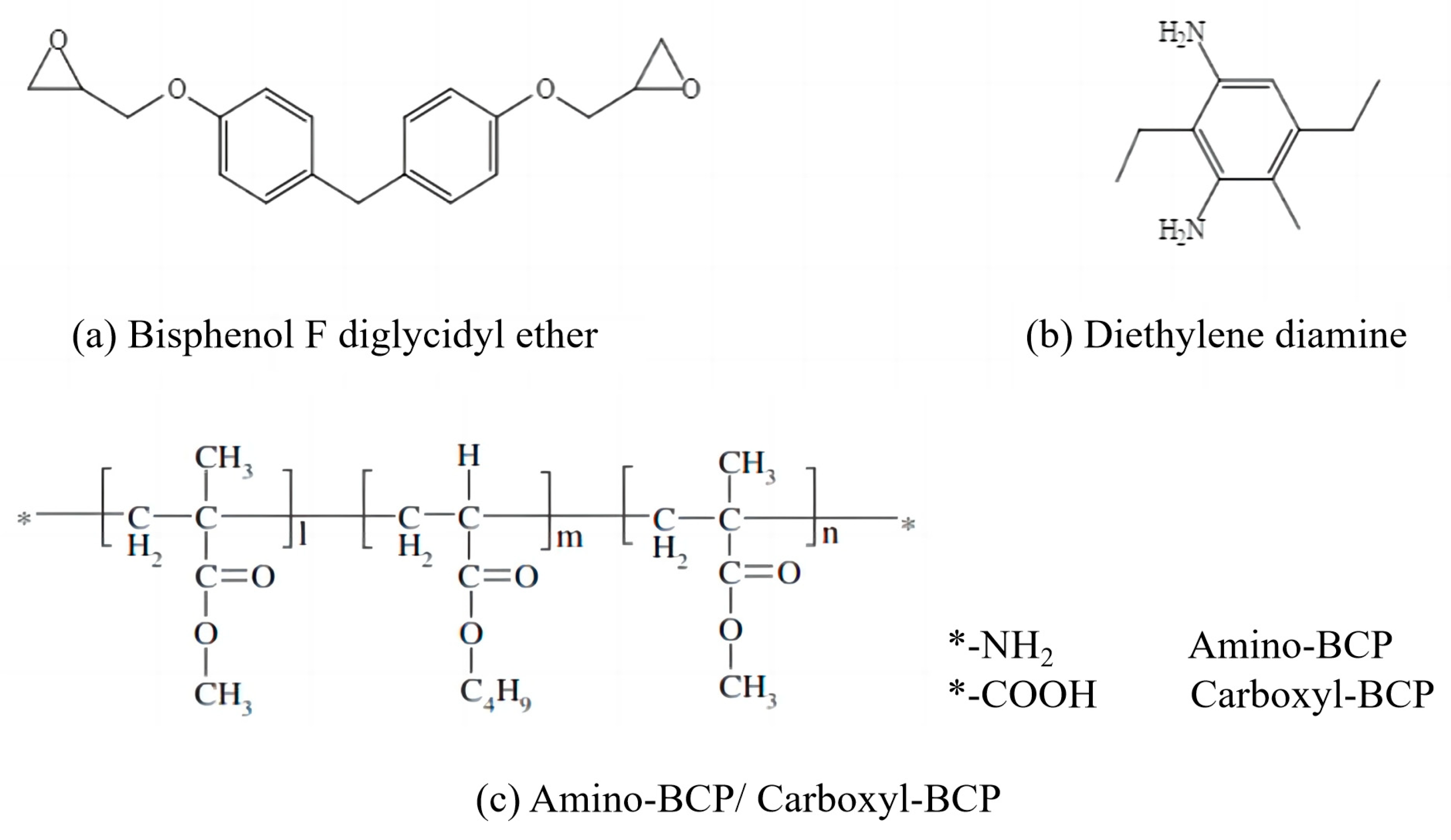
2.1. Material
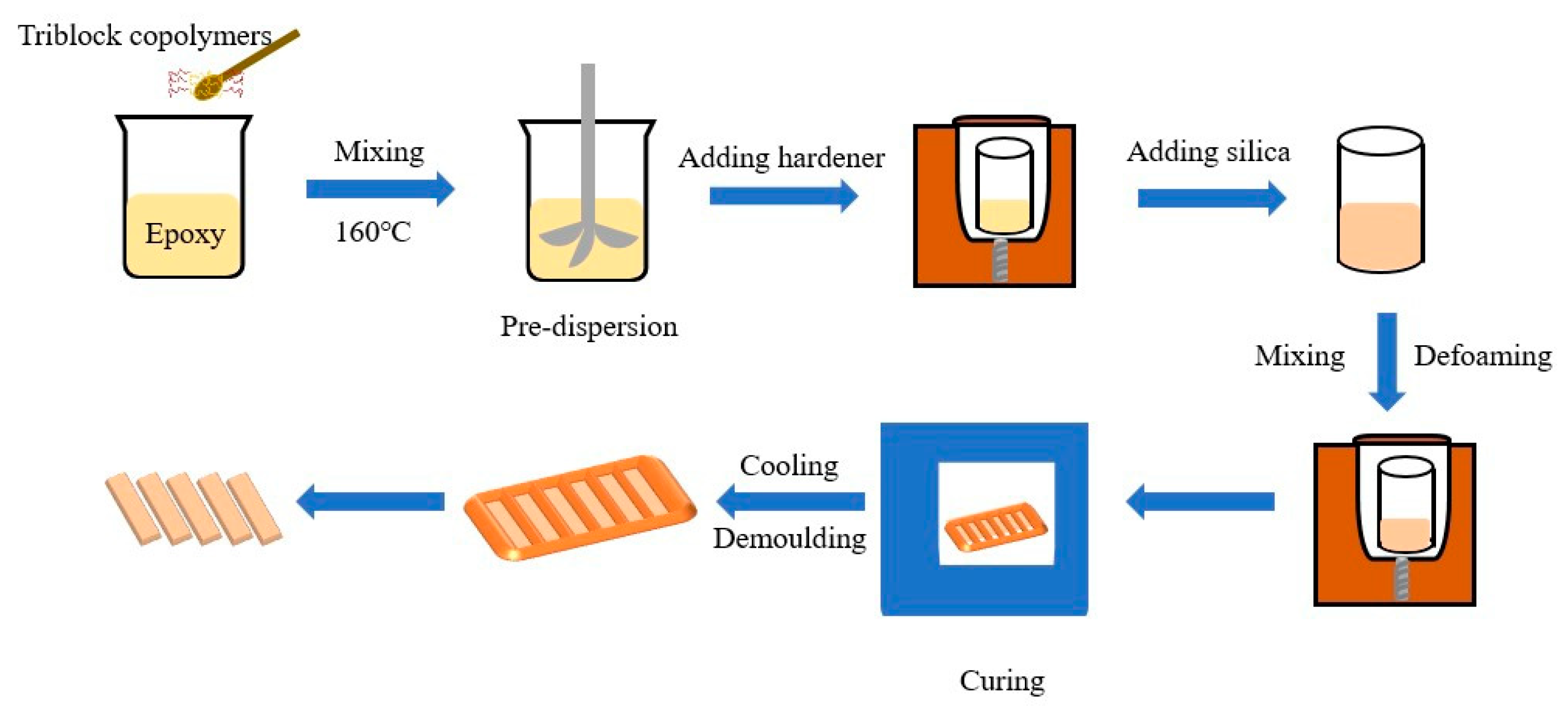
2.2. Preparation of Composites
2.3. Characterizations
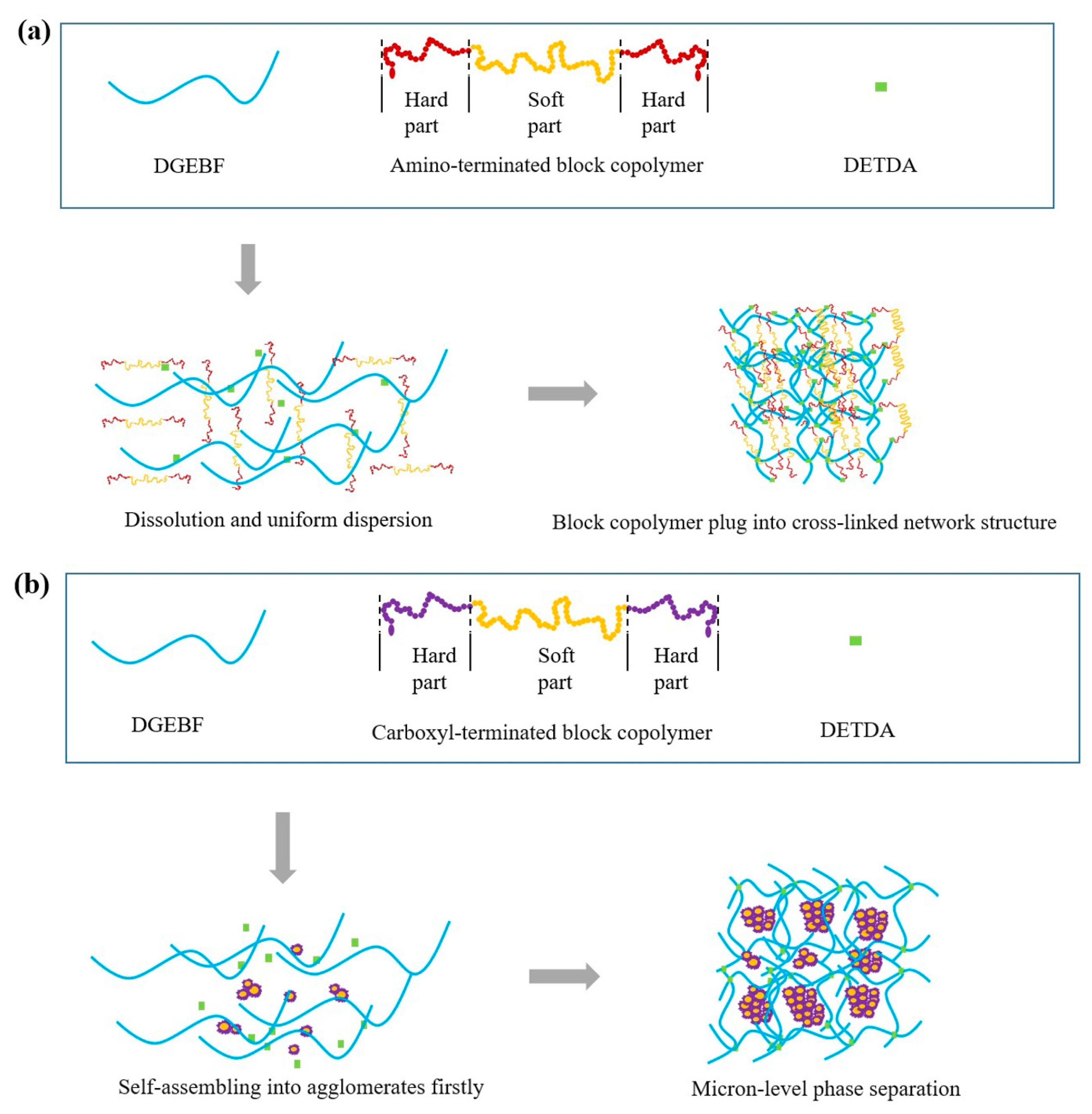
3. Results
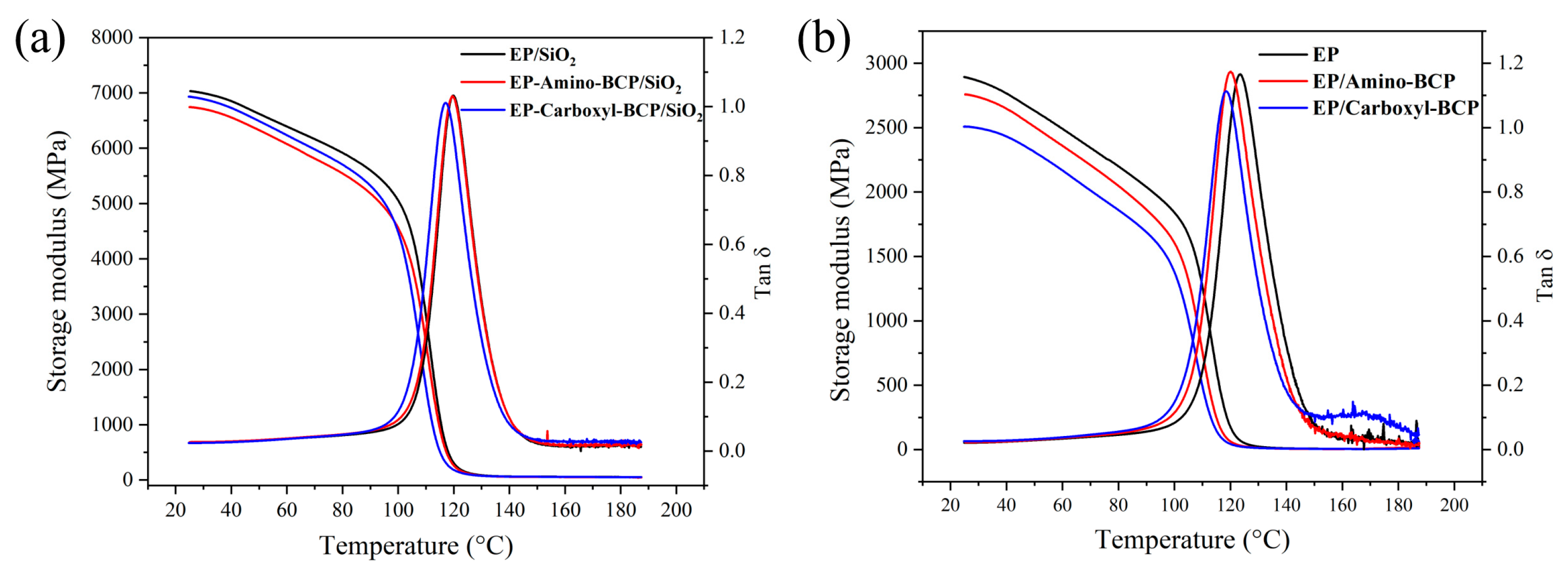
3.1. Dynamic Mechanical Property
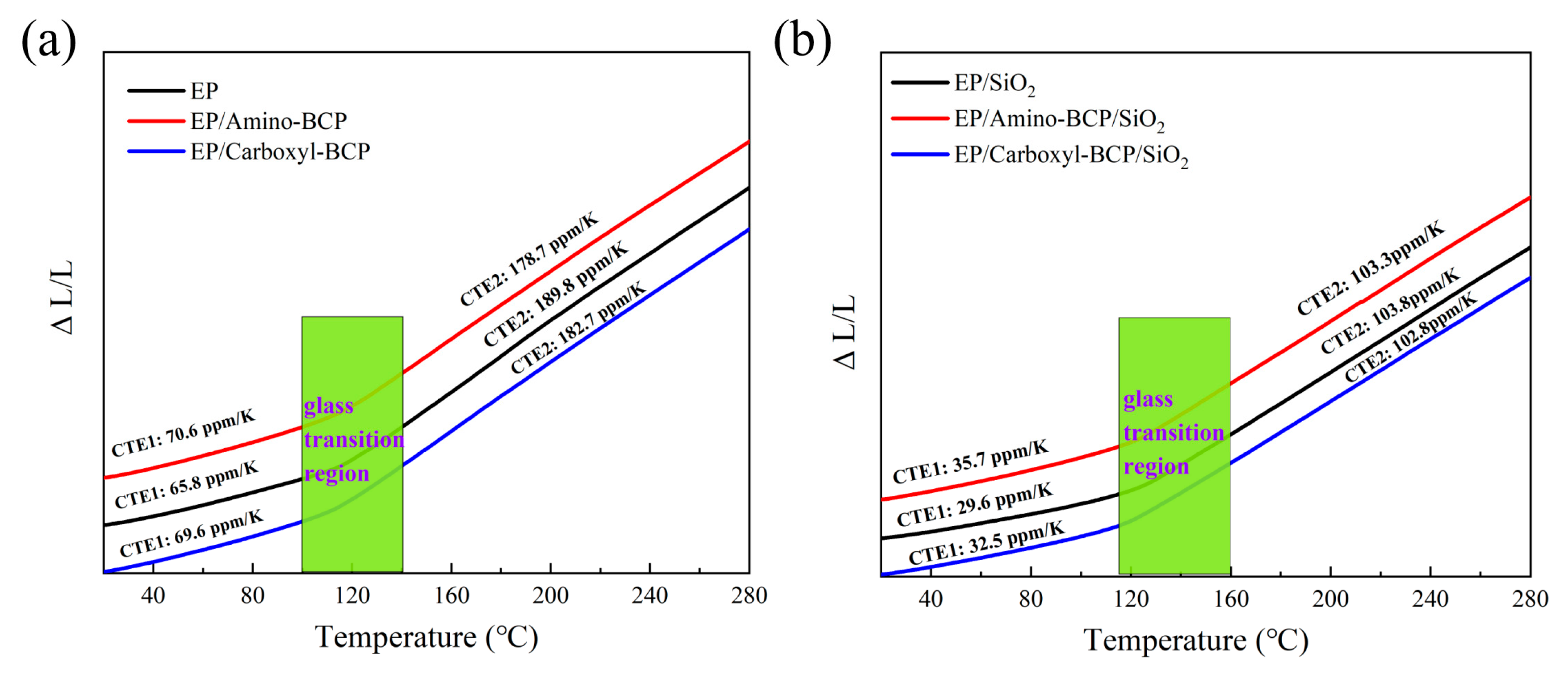
3.2. Coefficient of Thermal Expansion (CTE)
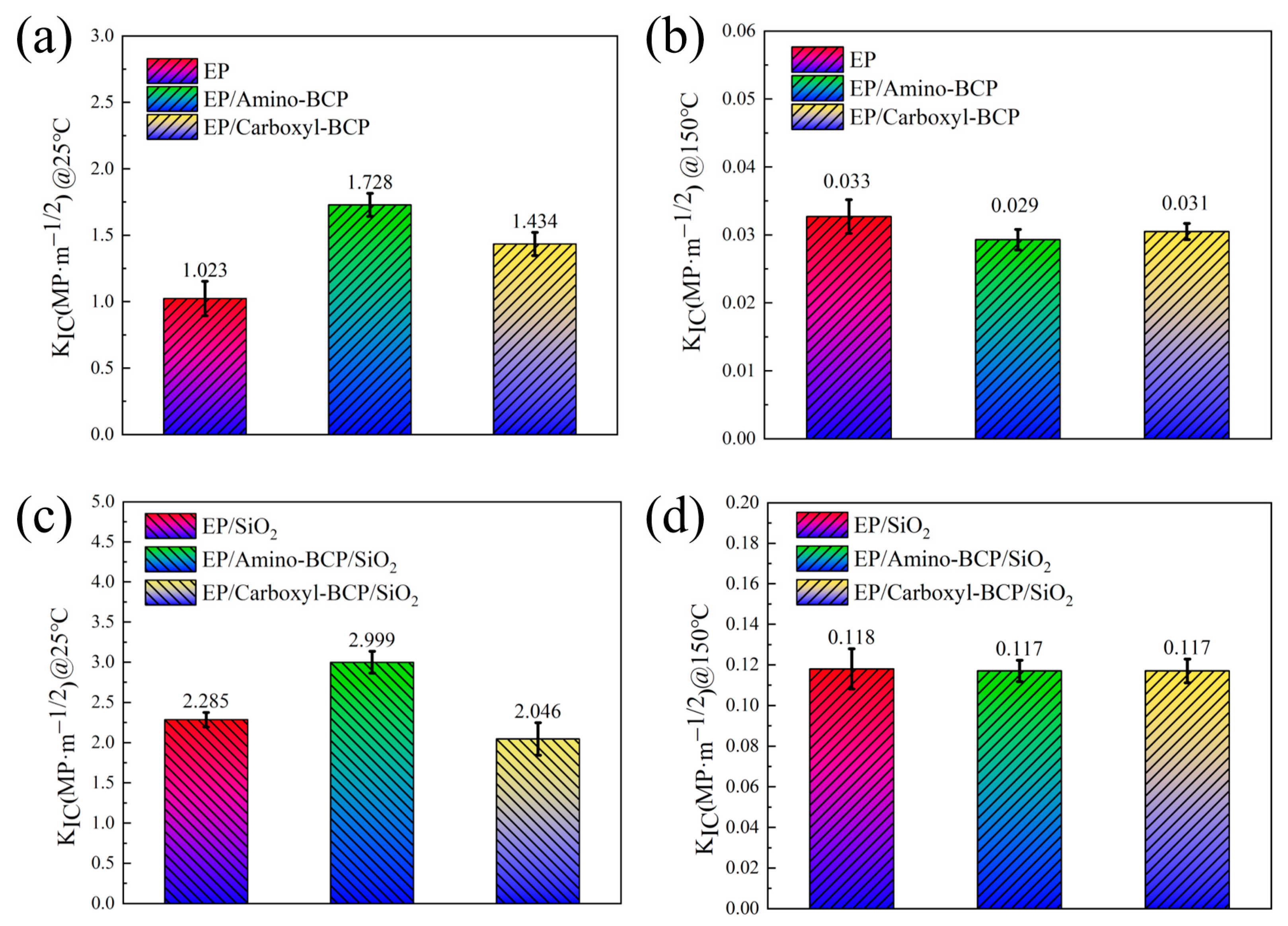
3.3. Fracture Toughness
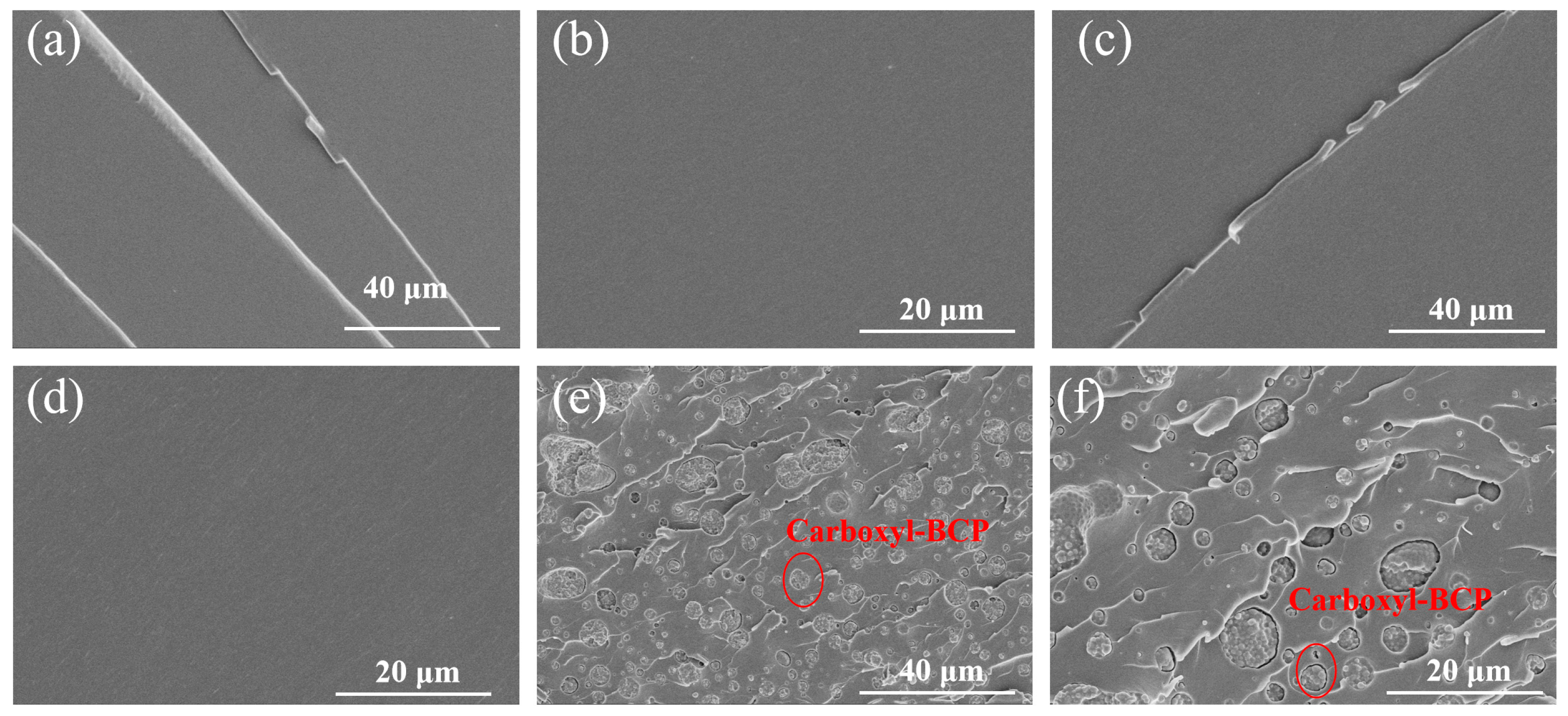
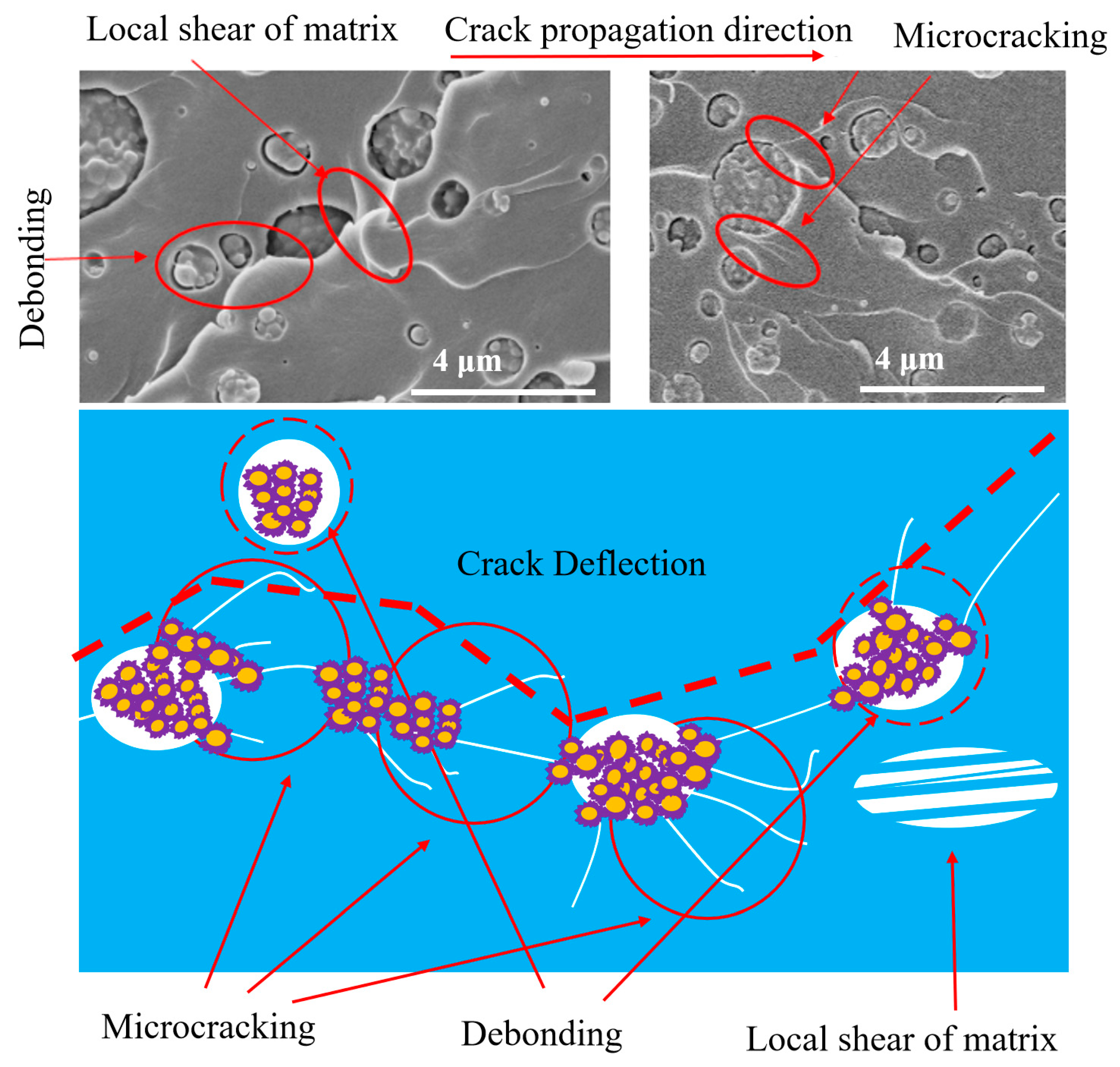
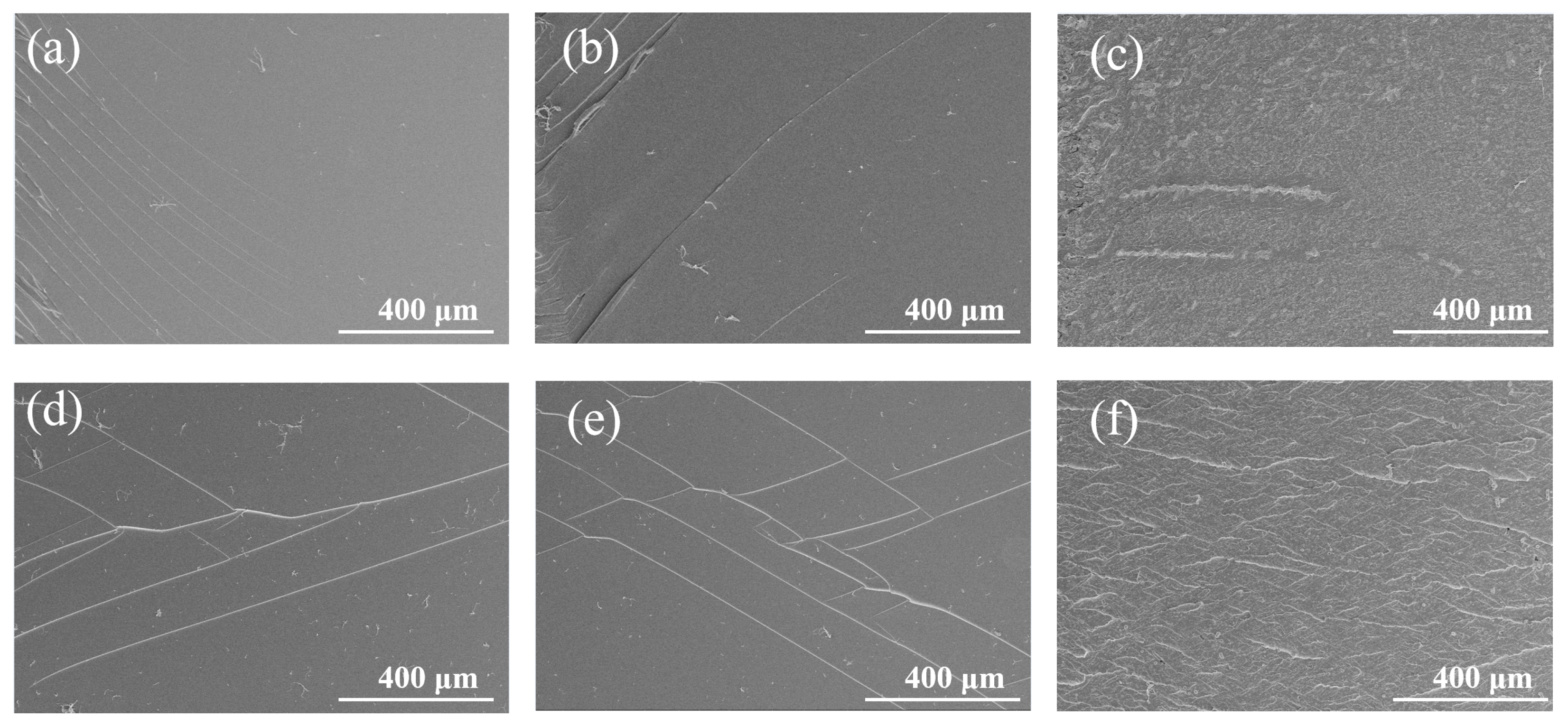
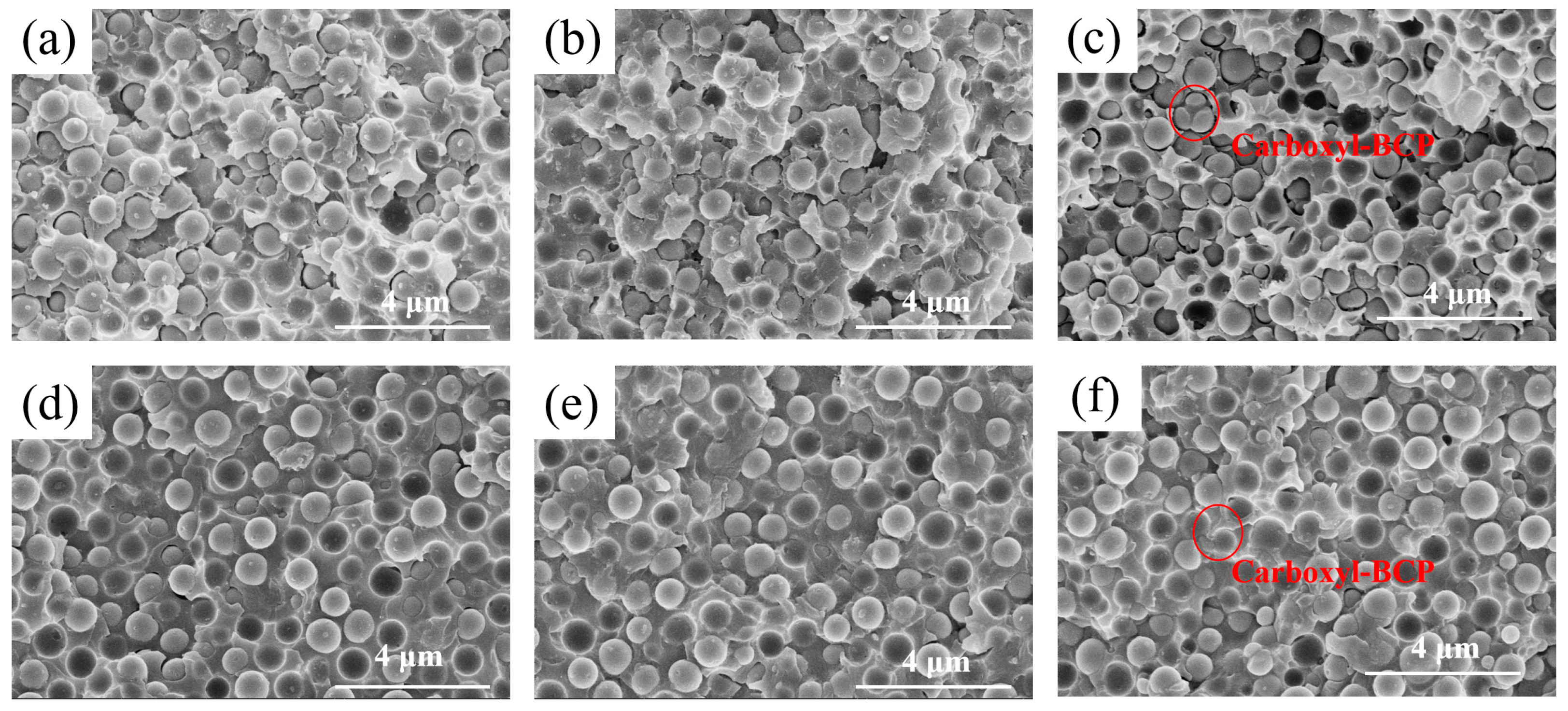
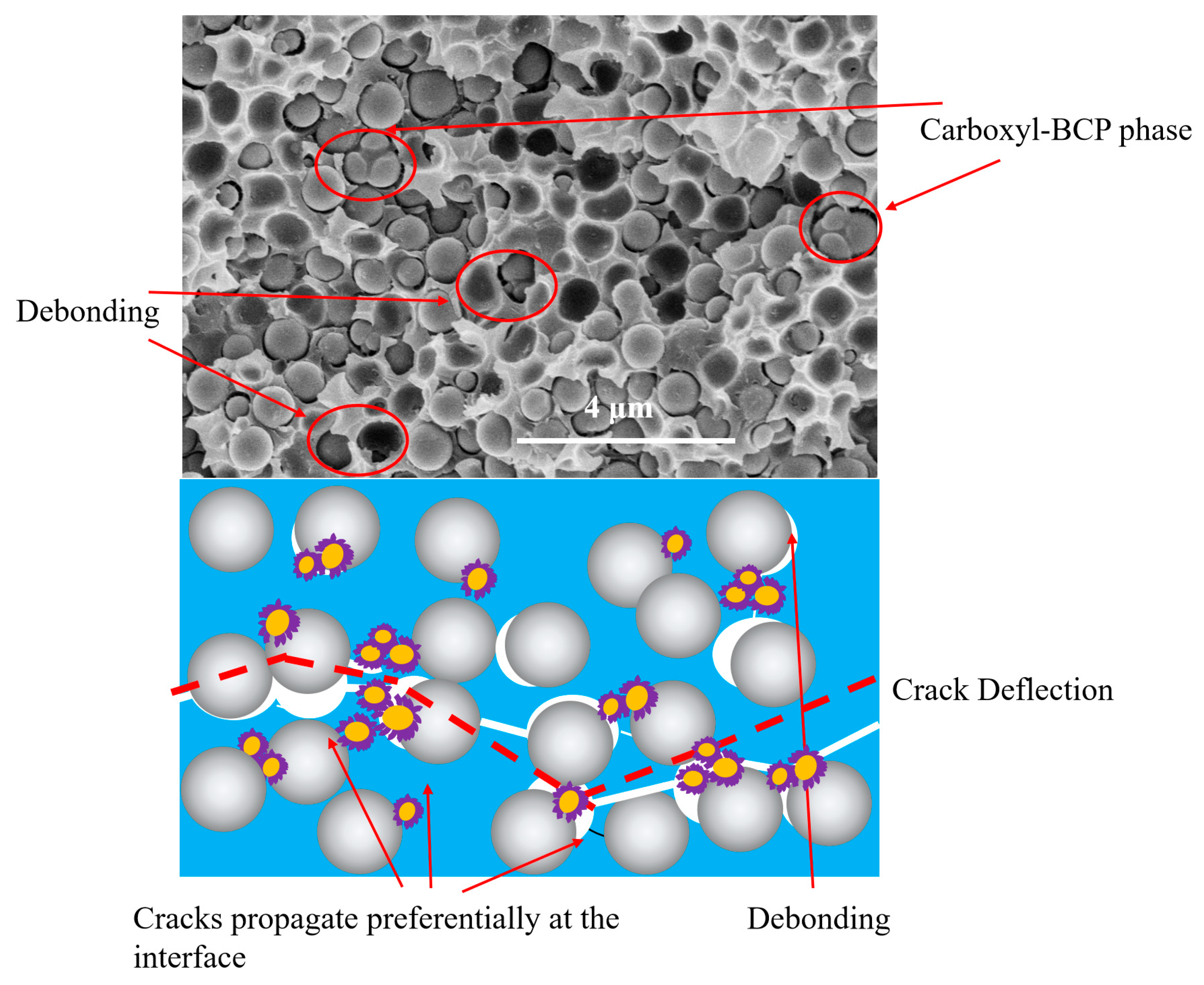
3.4. Fracture Surface Analysis
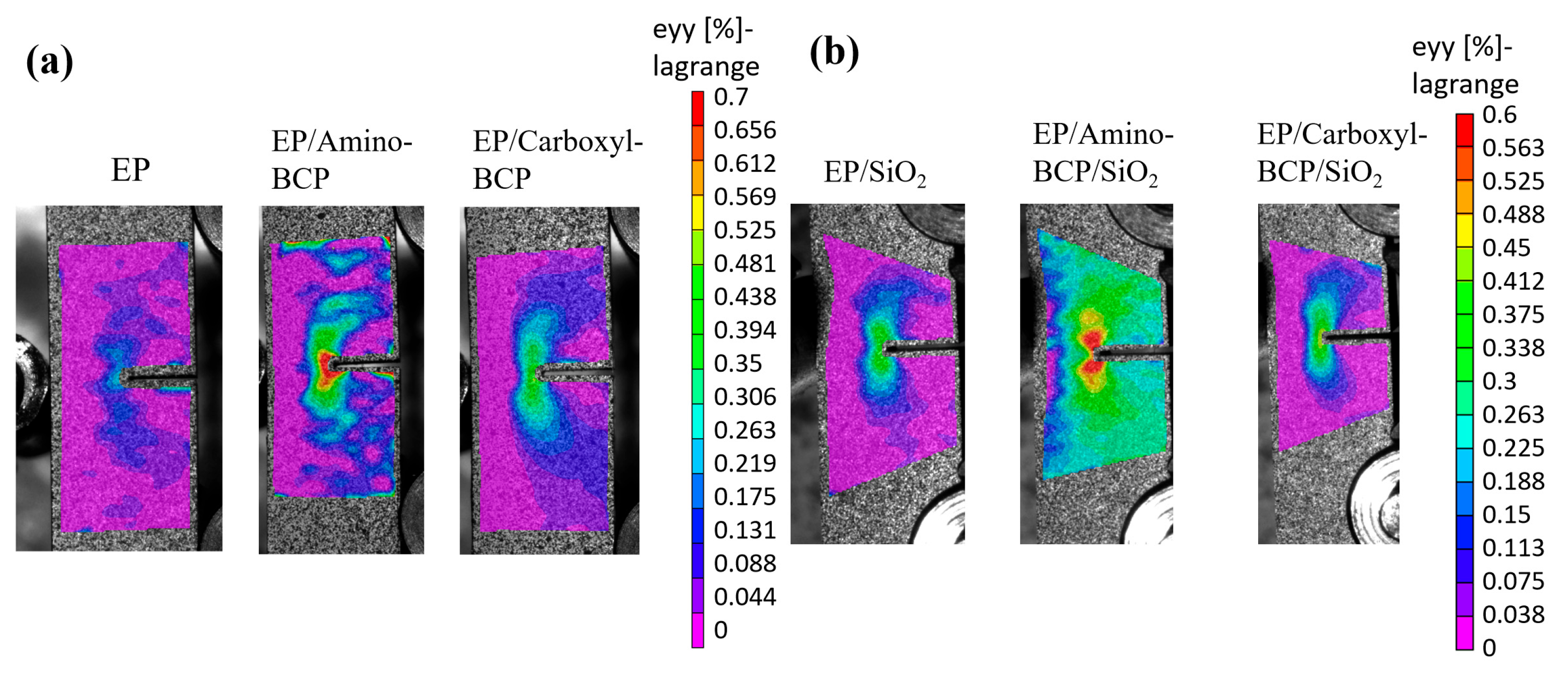
3.5. Strain Field at the Crack Tip
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zucchi, I.A.; Galante, M.J.; Williams, R.J.J. Comparison of morphologies and mechanical properties of crosslinked epoxies modified by polystyrene and poly(methyl methacrylate)) or by the corresponding block copolymer polystyrene-b-poly(methyl methacrylate). Polymer 2005, 46, 2603–2609. [Google Scholar] [CrossRef]
- Wetzel, B.; Rosso, P.; Haupert, F.; Friedrich, K. Epoxy nanocomposites-fracture and toughening mechanisms. Eng. Fract. Mech. 2006, 73, 2375–2398. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Liang, H.; Hao, L.; Wang, Y.; Du, B. Study on non-uniformity and dynamic fracture characteristics of GIL tri-post insulators considering Al2O3 sedimentation. High Volt. 2023, 8, 659–667. [Google Scholar] [CrossRef]
- Pecora, M.; Pannier, Y.; Lafarie-Frenot, M.; Gigliotti, M.; Guigon, C. Effect of thermo-oxidation on the failure properties of an epoxy resin. Polym. Test 2016, 52, 209–217. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, J.; Cao, M.; Wang, M.; Gu, B.; Sun, B. A mesoscale study of thermal expansion behaviors of epoxy resin and carbon fiber/epoxy unidirectional composites based on periodic temperature and displacement boundary conditions. Polym. Test 2016, 55, 44–60. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, L.; Ren, R.; Ma, X.; Chen, P. Thermal, mechanical properties and shape memory performance of a novel phthalide-containing epoxy resins. Polymer 2018, 140, 326–333. [Google Scholar] [CrossRef]
- Jung, H.; Park, Y.; Nah, C.; Lee, J.; Kim, K.; Lee, C.S. Evaluation of the Mechanical Properties of Polyether Sul-fone-Toughened Epoxy Resin for Carbon Fiber Composites. Fiber Polym. 2021, 22, 184–195. [Google Scholar] [CrossRef]
- Mathew, V.S.; Jyotishkumar, P.; George, S.C.; Gopalakrishnan, P.; Delbreilh, L.; Saiter, J.M.; Saikia, P.J.; Thomas, S. High Performance HTLNR/Epoxy Blend-Phase Morphology and Thermomechanical Properties. J. Appl. Polym. Sci. 2012, 125, 804–811. [Google Scholar] [CrossRef]
- An, H.; Liu, Z.; Tian, Q.; Li, J.; Zhou, C.; Liu, X.; Zhu, W. Thermal behaviors of nanoparticle reinforced epoxy resins for mi-croelectronics packaging. Microelectron. Reliab. 2019, 93, 39–44. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Kinloch, A.J.; Masania, K.; Taylor, A.C.; Sprenger, S. The mechanisms and mechanics of the toughening of epoxy polymers modified with silica nanoparticles. Polymer 2010, 51, 6284–6294. [Google Scholar] [CrossRef]
- Lee, M.; Paria, S.; Mondal, S.; Lee, G.; Shin, B.; Kim, S.; Park, S.; Nah, C. Amphiphilic block co-polymer and silica reinforced epoxy composite with excellent toughness and delamination resistance for durable electronic packaging application. Polymer 2022, 245, 124679. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Shaw, S.J.; Tod, D.A.; Hunston, D.L. Deformation and fracture behaviour of a rubber-toughened epoxy: 1. Mi-crostructure and fracture studies. Polymer 1983, 24, 1341–1354. [Google Scholar] [CrossRef]
- Kishi, H.; Shi, Y.B.; Huang, J.; Yee, A.F. Ductility and toughenability study of epoxy resins under multiaxial stress states. J. Mater. Sci. 1998, 33, 3479–3488. [Google Scholar] [CrossRef]
- Wang, M.; Fan, X.; Thitsartarn, W.; He, C. Rheological and mechanical properties of epoxy/clay nanocomposites with en-hanced tensile and fracture toughnesses. Polymer 2015, 58, 43–52. [Google Scholar] [CrossRef]
- Cho, D.; Hwang, J.H. Elastomeric Coating of Exfoliated Graphite Nanoplatelets with Amine-Terminated Poly(butadiene-co-acrylonitrile): Characterization and Its Epoxy Toughening Effect. Adv. Polym. Technol. 2013, 32, 21366. [Google Scholar] [CrossRef]
- Mijovic, J.; Shen, M.; Sy, J.W.; Mondragon, I. Dynamics and Morphology in Nanostructured Thermoset Network/Block Co-polymer Blends during Network Formation. Macromolecules 2000, 33, 5235–5244. [Google Scholar] [CrossRef]
- Grishchuk, S.; Sorochynska, L.; Vorster, O.C.; Karger-Kocsis, J. Structure, thermal, and mechanical properties of DDM-hardened epoxy/benzoxazine hybrids: Effects of epoxy resin functionality and ETBN toughening. J. Appl. Polym. Sci. 2013, 127, 5082–5093. [Google Scholar] [CrossRef]
- Konnola, R.; Parameswaranpillai, J.; Joseph, K. Mechanical, thermal, and viscoelastic response of novel in situ CTBN/POSS/epoxy hybrid composite system. Polym. Compos. 2016, 37, 2109–2120. [Google Scholar] [CrossRef]
- Song, X.; Xu, S. Curing kinetics of pre-crosslinked carboxyl-terminated butadiene acrylonitrile (CTBN) modified epoxy blends. J. Therm. Anal. Calorim. 2016, 123, 319–327. [Google Scholar] [CrossRef]
- Vijayan, P.P.; Puglia, D.; Vijayan, P.P.; Kenny, J.M.; Thomas, S. The role of clay modifier on cure characteristics and proper-ties of epoxy/clay/carboxyl-terminated poly(butadiene-co-acrylonitrile) (CTBN) hybrid. Mater. Technol. 2017, 32, 171–177. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Gilbert, A.H. Toughening tetrafunctional epoxy resins using polyetherimide. Polymer 1989, 30, 213–217. [Google Scholar] [CrossRef]
- Raghava, R.S. Role of matrix-particle interface adhesion on fracture toughness of dual phase epoxy-polyethersulfone blend. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1017–1031. [Google Scholar] [CrossRef]
- Keller, A.; Chong, H.M.; Taylor, A.C.; Dransfeld, C.; Masania, K. Core-shell rubber nanoparticle reinforcement and pro-cessing of high toughness fast-curing epoxy composites. Compos. Sci. Technol. 2017, 147, 78–88. [Google Scholar] [CrossRef]
- Jingqiang, S.; Yafeng, Z.; Jindong, Q.; Jianzheng, K. Core-shell particles with an acrylate polyurethane core as tougheners for epoxy resins. J. Mater. Sci. 2004, 39, 6383–6384. [Google Scholar] [CrossRef]
- Giannakopoulos, G.; Masania, K.; Taylor, A.C. Toughening of epoxy using core-shell particles. J. Mater. Sci. 2011, 46, 327–338. [Google Scholar] [CrossRef]
- Staudinger, U.; Satapathy, B.K.; Weidisch, R. Influence of block composition on crack toughness behaviour of sty-rene-b-(styrene-random-butadiene)-b-styrene triblock copolymers. Eur. Polym. J. 2008, 44, 1822–1833. [Google Scholar] [CrossRef]
- Cong, H.; Li, L.; Zheng, S. Formation of nanostructures in thermosets containing block copolymers: From self-assembly to reaction-induced microphase separation mechanism. Polymer 2014, 55, 1190–1201. [Google Scholar] [CrossRef]
- Rio, T.G.; Salazar, A.; Pearson, R.A.; Rodriguez, J. Fracture behaviour of epoxy nanocomposites modified with triblock co-polymers and carbon nanotubes. Compos. Part B-Eng. 2016, 87, 7. [Google Scholar] [CrossRef]
- Li, M.; Heng, Z.; Chen, Y.; Zou, H.; Liang, M. High Toughness Induced by Wormlike-Nanostructure in Epoxy Thermoset Containing Amphiphilic PDMS-PCL Block Copolymers. Ind. Eng. Chem. Res. 2018, 57, 13036–13047. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, B.; Mei, H.; Li, L.; Zheng, S. Toughening of epoxy thermosets with polysty-rene-block-polybutadiene-block-polystyrene triblock copolymer via formation of nanostructures. Polym. Eng. Sci. 2019, 59, 2387–2396. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Q.; Yu, Y.; Zhuang, Y.; Lv, Y.; Xiao, H.; Song, N.; Ni, L. Morphological evolution and mechanical properties of an “anchor chain” nanodomain structure of a reactive amphiphilic triblock copolymer in epoxy resin. Polym. Chem. 2020, 11, 3615–3626. [Google Scholar] [CrossRef]
- Silva, B.L.; Schuster, M.B.; Bello, R.H.; Becker, D.; Coelho, L.A.F. The role of carbon nanoparticles in epoxy-based nano-composites modified with (poly[polypropylene oxide]-block-poly[ethylene oxide]-block-poly[propylene oxide]) triblock co-polymers on phase morphology and mechanical properties. Polym. Compos. 2020, 41, 4243–4252. [Google Scholar] [CrossRef]
- Tao, L.; Sun, Z.; Min, W.; Ou, H.; Qi, L.; Yu, M. Improving the toughness of thermosetting epoxy resins via blending triblock copolymers. RSC Adv. 2020, 10, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Jheng, L.C.; Wang, I.H.; Hsieh, T.H.; Fan, C.T.; Hsiao, C.H.; Wu, C.P.; Leu, M.T.; Chang, T.Y. Toughening of epoxy thermo-sets with nano-sized or micron-sized domains of poly (ethylene oxide)-b-poly(butadiene-co-acrylonitrile)-b-poly(ethylene oxide) triblock copolymers synthesized using room temperature ester coupling reaction. J. Appl. Polym. Sci. 2021, 138, 50096. [Google Scholar] [CrossRef]
- Silva, B.L.; Schuster, M.B.; Becker, D.; Coelho, L.A.F. The Influence of the Molecular Architecture of the PEG: PPG Triblock Copolymer on the Properties of Epoxy Nanocomposites. Mater. Res. 2022, 25, e20210522. [Google Scholar] [CrossRef]
- Li, L.; Peng, W.; Liu, L.; Zheng, S. Toughening of epoxy by nanostructures with ABA triblock copolymers: An influence of organosilicon modification of block copolymer. Polym. Eng. Sci. 2022, 62, 392–404. [Google Scholar] [CrossRef]
- Cano, L.; Gutierrez, J.; Tercjak, A. Enhancement of the mechanical properties at the macro and nano scale of thermosetting systems modified with a polystyrene-block-polymethylmethacrylate block copolymer. RSC Adv. 2015, 5, 11. [Google Scholar] [CrossRef]
- Paz, E.; Abenojar, J.; Ballesteros, Y.; Forriol, F.; Dunne, N.; Del Real, J.C. Mechanical and thermal behaviour of an acrylic bone cement modified with a triblock copolymer. J. Mater. Sci. Mater. Med. 2016, 27, 72. [Google Scholar] [CrossRef]
- Ignaczak, W.; Wiśniewska, K.; Janik, J.; Fray, M.E. Mechanical and thermal properties of PP/PBT blends compatibilized with triblock thermoplastic elastomer. Pol. J. Chem. Technol. 2015, 17, 78–83. [Google Scholar] [CrossRef]
- Kishi, H.; Kunimitsu, Y.; Nakashima, Y.; Imade, J.; Oshita, S.; Morishita, Y.; Asada, M. Relationship between the mechanical properties of epoxy/PMMA-b-PnBA-b-PMMA block copolymer blends and their three-dimensional nanostructures. Express Polym. Lett. 2017, 11, 765–777. [Google Scholar] [CrossRef]
- Mao, H.I.; Hsu, T.S.; Chen, C.W.; Huang, K.W.; Rwei, S.P. Synthesis and characteristics of poly(ethylene terephthalate) with EO-PO-EO triblock copolymers: A thermal and mechanical property study. J. Appl. Polym. Sci. 2022, 139, 51605. [Google Scholar] [CrossRef]
- Asada, M.; Oshita, S.; Morishita, Y.; Nakashima, Y.; Kunimitsu, Y.; Kishi, H. Effect of miscible PMMA chain length on disor-dered morphologies in epoxy/PMMA-b-PnBA-b-PMMA blends by in situ simultaneous SAXS/DSC. Polymer 2016, 105, 8. [Google Scholar] [CrossRef]
- Klingler, A.; Wetzel, B. Fatigue crack propagation in triblock copolymer toughened epoxy nanocomposites. Polym. Eng. Sci. 2017, 57, 579–587. [Google Scholar] [CrossRef]
- Kishi, H.; Kunimitsu, Y.; Imade, J.; Oshita, S.; Morishita, Y.; Asada, M. Nano-phase structures and mechanical properties of epoxy/acryl triblock copolymer alloys. Polymer 2011, 52, 760–768. [Google Scholar] [CrossRef]
- George, S.M.; Puglia, D.; Kenny, J.M.; Parameswaranpillai, J.; Thomas, S. Reaction-Induced Phase Separation and Thermo-mechanical Properties in Epoxidized Styrene-block-butadiene-block-styrene Triblock Copolymer Modified Epoxy/DDM System. Ind. Eng. Chem. Res. 2014, 53, 6941–6950. [Google Scholar] [CrossRef]
- George, S.M.; Puglia, D.; Kenny, J.M.; Causin, V.; Parameswaranpillai, J.; Thomas, S. Morphological and Mechanical Char-acterization of Nanostructured Thermosets from Epoxy and Styrene-block-Butadiene-block-Styrene Triblock Copolymer. Ind. Eng. Chem. Res. 2013, 52, 9121–9129. [Google Scholar] [CrossRef]
- Chen, J.; Taylor, A.C. Epoxy modified with triblock copolymers: Morphology, mechanical properties and fracture mechanisms. J. Mater. Sci. 2012, 47, 4546–4560. [Google Scholar] [CrossRef]
- ASTM E1820-11; Standard Test Method for Measurement of Fracture Toughness. American Society for Testing and Materials: West Conshohocken, PA, USA, 2011.
- Carroll, J.D.; Abuzaid, W.; Lambros, J.; Sehitoglu, H. High resolution digital image correlation measurements of strain accu-mulation in fatigue crack growth. Int. J. Fatigue 2013, 57, 140–150. [Google Scholar] [CrossRef]
- Yang, Y.; Souare, P.M.; Sylvestre, J. Using Confocal Microscopy and Digital Image Correlation to Measure Local Strains Around a Chip Corner and a Crack Front. IEEE Trans. Device Mater. Reliab. 2020, 20, 97–105. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, M.; Xuan, F. Crack tip strain evolution and crack closure during overload of a growing fatigue crack. Frat. Integrità Strutt. 2017, 11, 143–148. [Google Scholar] [CrossRef]
| Sample | Abbreviation Label |
|---|---|
| Neat epoxy | EP |
| Epoxy/Amino-terminated BCP | EP/Amino-BCP |
| Epoxy/Carboxyl-terminated BCP | EP/Carboxyl-BCP |
| Epoxy/60 wt.% SiO2 | EP/SiO2 |
| Epoxy/Amino-terminated BCP/60 wt.% SiO2 | EP/Amino-BCP/SiO2 |
| Epoxy/Carboxyl-terminated BCP/60 wt.% SiO2 | EP/Carboxyl-BCP/SiO2 |
| CTE 1/(ppm/K) | CTE 2/(ppm/K) | |
|---|---|---|
| EP | 65.8 | 189.8 |
| EP/Amino-BCP | 70.6 | 178.7 |
| EP/Carboxyl-BCP | 69.6 | 182.7 |
| EP/SiO2 | 29.6 | 103.8 |
| EP/Amino-BCP/SiO2 | 35.7 | 103.3 |
| EP/Carboxyl-BCP/SiO2 | 32.5 | 102.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wu, W.; Yu, X.; Zhang, R.; Sun, R.; Cao, L.; Zhu, P. Effects of Block Copolymer Terminal Groups on Toughening Epoxy-Based Composites: Microstructures and Toughening Mechanisms. Micromachines 2023, 14, 2112. https://doi.org/10.3390/mi14112112
Li G, Wu W, Yu X, Zhang R, Sun R, Cao L, Zhu P. Effects of Block Copolymer Terminal Groups on Toughening Epoxy-Based Composites: Microstructures and Toughening Mechanisms. Micromachines. 2023; 14(11):2112. https://doi.org/10.3390/mi14112112
Chicago/Turabian StyleLi, Gang, Wenjie Wu, Xuecheng Yu, Ruoyu Zhang, Rong Sun, Liqiang Cao, and Pengli Zhu. 2023. "Effects of Block Copolymer Terminal Groups on Toughening Epoxy-Based Composites: Microstructures and Toughening Mechanisms" Micromachines 14, no. 11: 2112. https://doi.org/10.3390/mi14112112
APA StyleLi, G., Wu, W., Yu, X., Zhang, R., Sun, R., Cao, L., & Zhu, P. (2023). Effects of Block Copolymer Terminal Groups on Toughening Epoxy-Based Composites: Microstructures and Toughening Mechanisms. Micromachines, 14(11), 2112. https://doi.org/10.3390/mi14112112





