Electrophoretic Deposition of Multi-Walled Carbon Nanotube Coatings on CoCrMo Alloy for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the CoCrMo Substrate
2.2. Preparation of the MWCNT Suspension
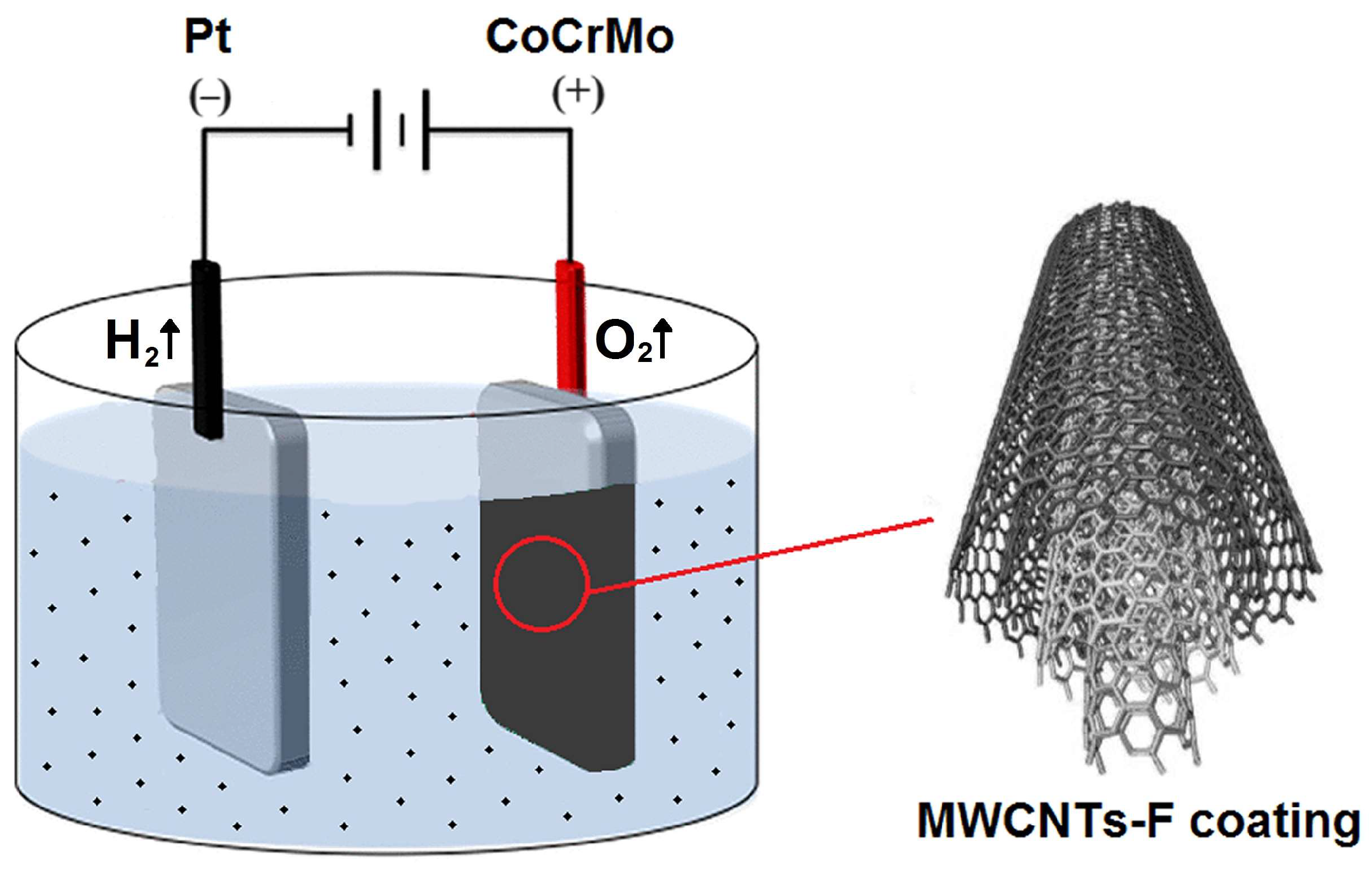
2.3. Electrophoretic Deposition Conditions of the MWNT Coating
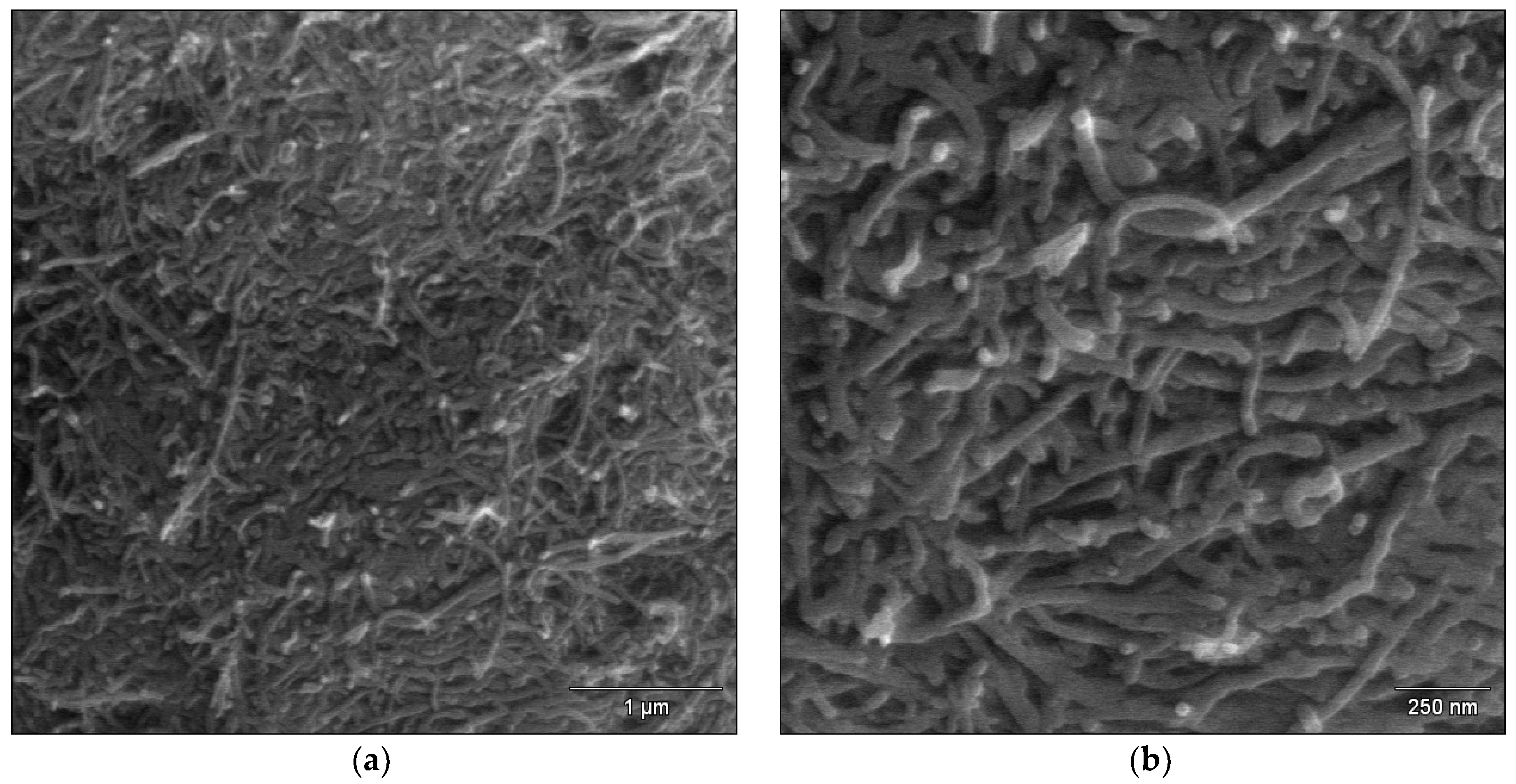
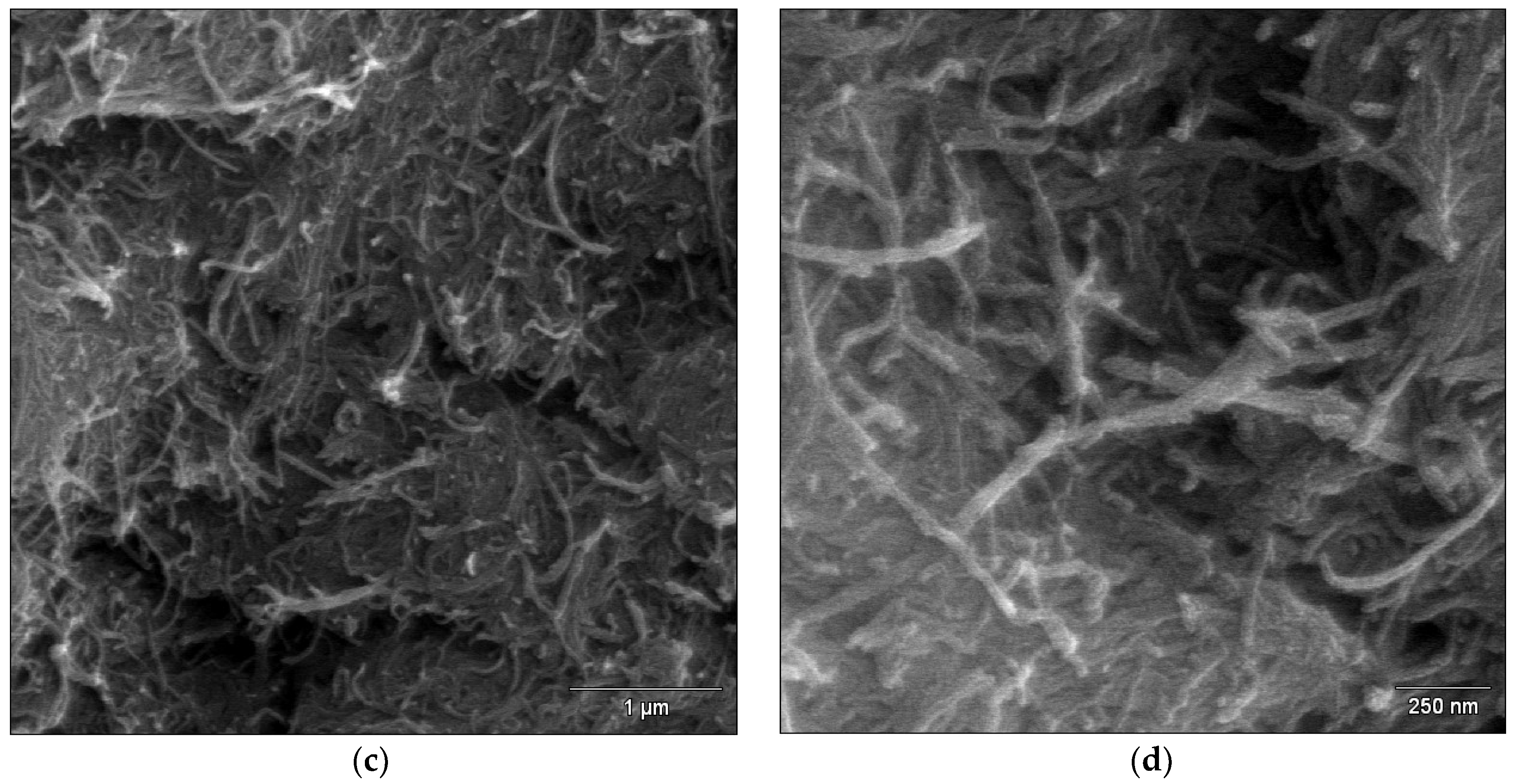
2.4. FE-SEM Study of the MWCNT Coatings
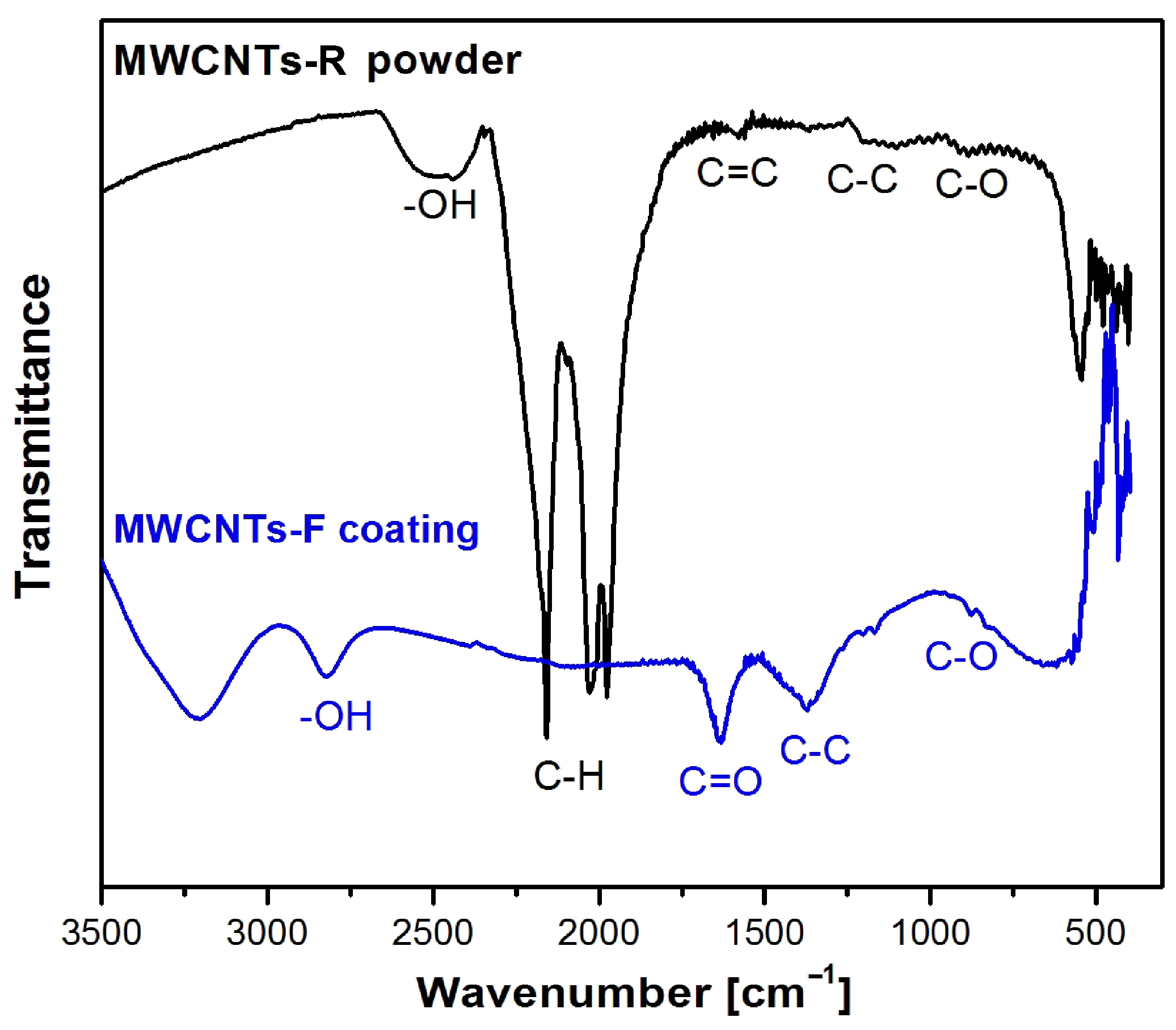
2.5. ATR-FTIR Study of the MWCNT Coatings
2.6. Corrosion Resistance Study of the MWCNT Coatings
3. Results and Discussion
3.1. Microstructure Characteristics of the MWNTs Coatings
3.2. Functional Groups Characteristics of the MWNTs Coatings
3.3. Open-Circuit Potential Measurements
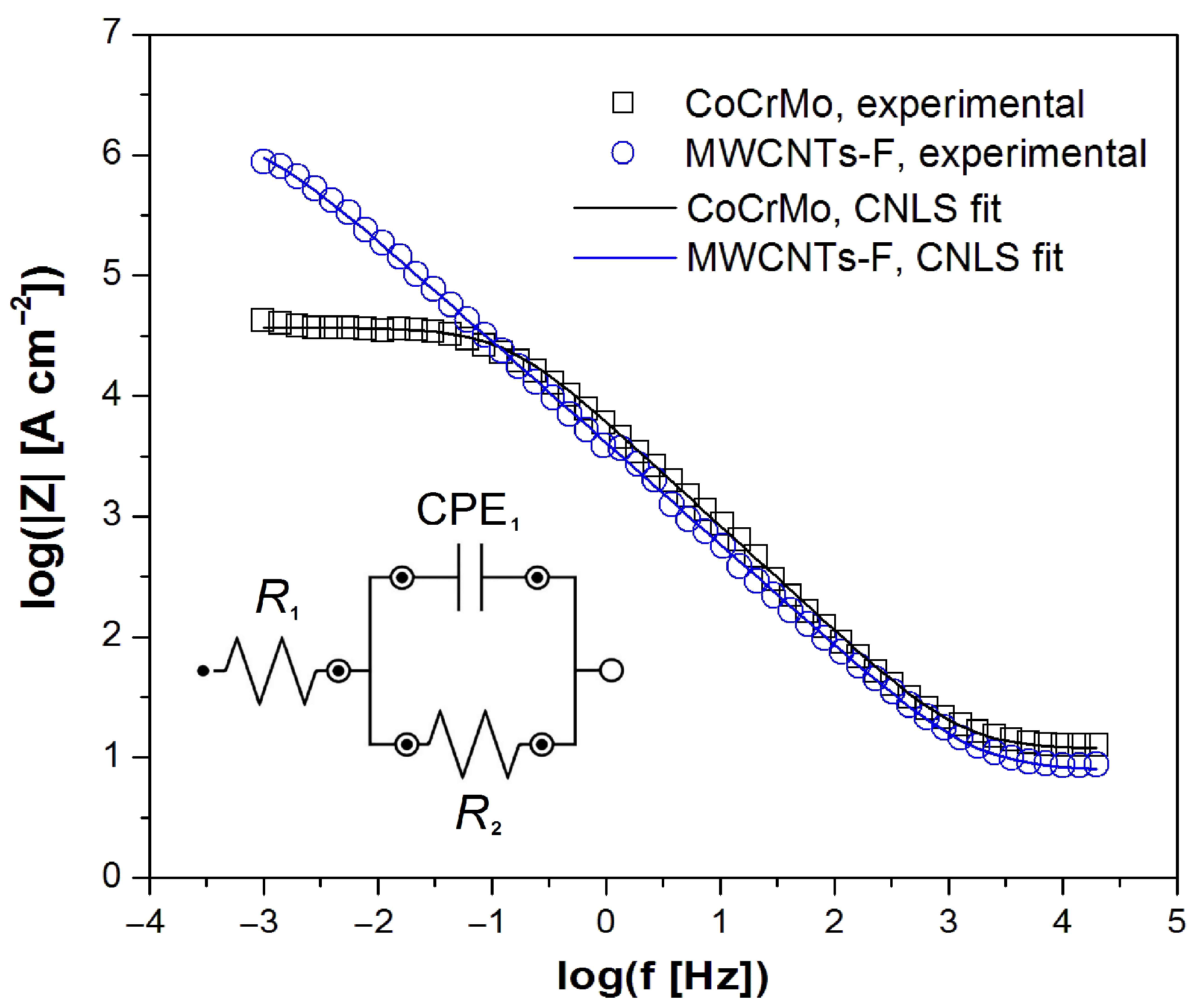
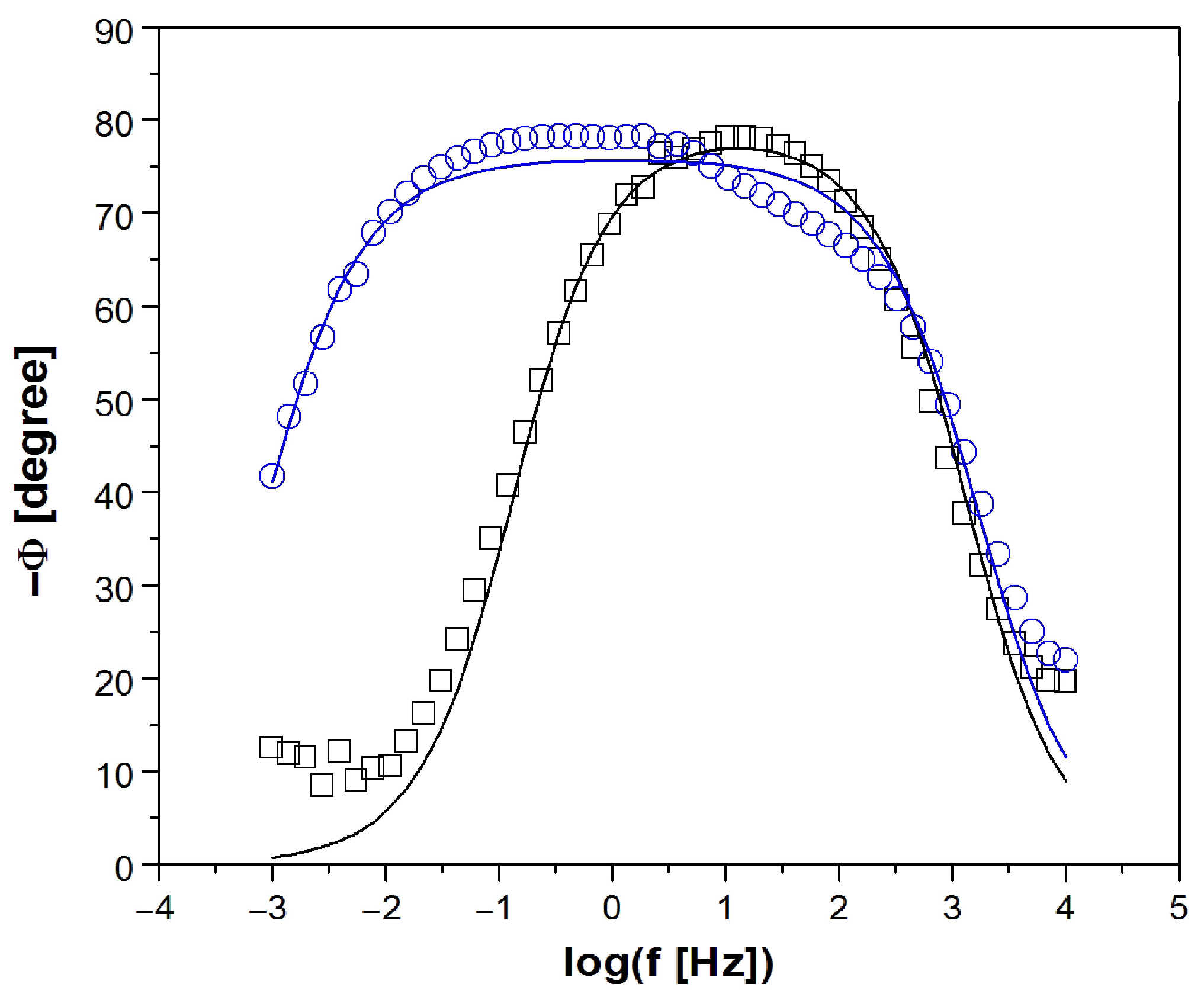
3.4. Electrochemical Impedance Spectroscopy Measurements
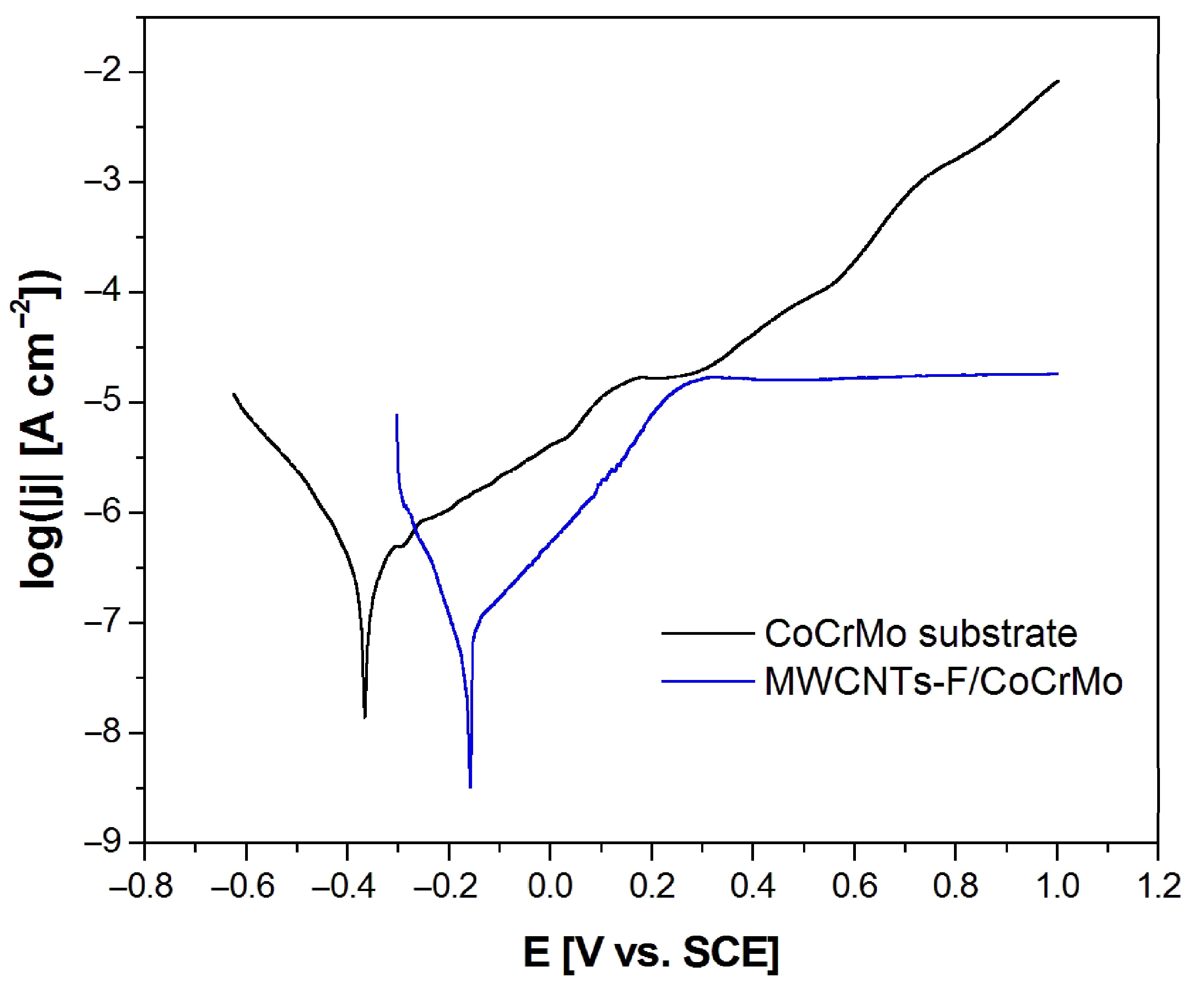
3.5. Potentiodynamic Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durgo, K.; Orešić, S.; Rinčić Mlinarić, M.; Fiket, Ž.; Jurešić, G.Č. Toxicity of metal ions released from a fixed orthodontic appliance to gastrointestinal tract cell lines. Int. J. Mol. Sci. 2023, 24, 9940. [Google Scholar] [CrossRef]
- Uriciuc, W.A.; Boșca, A.B.; Băbțan, A.M.; Vermeșan, H.; Cristea, C.; Tertiș, M.; Pășcuță, P.; Borodi, G.; Suciu, M.; Barbu-Tudoran, L.; et al. Study on the surface of cobalt-chromium dental alloys and their behavior in oral cavity as cast materials. Materials 2022, 15, 3052. [Google Scholar] [CrossRef] [PubMed]
- Mace, A.; Khullar, P.; Bouknight, C.; Gilbert, J.L. Corrosion properties of low carbon CoCrMo and additively manufactured CoCr alloys for dental applications. Dent. Mater. 2022, 38, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Wepner, L.; Färber, H.A.; Jaensch, A.; Weber, A.; Heuser, F.; Keilig, L.; Singer, L.; Bourauel, C.P. In vitro ion release of wires in removable orthodontic appliances. Materials 2021, 14, 3402. [Google Scholar] [CrossRef] [PubMed]
- Padrós, R.; Giner-Tarrida, L.; Herrero-Climent, M.; Punset, M.; Gil, F.J. Corrosion resistance and ion release of dental prosthesis of CoCr obtained by CAD-CAM milling, casting and laser sintering. Metals 2020, 10, 827. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Stach, S. Effect of autoclaving time on corrosion resistance of sandblasted Ti G4 in artificial saliva. Materials 2020, 13, 4154. [Google Scholar] [CrossRef] [PubMed]
- Szklarska, M.; Dercz, G.; Simka, W.; Łosiewicz, B. A.c. impedance study on the interfacial properties of passivated Ti13Zr13Nb alloy in physiological saline solution. Surf. Interface Anal. 2014, 46, 698–701. [Google Scholar] [CrossRef]
- Kajzer, W.; Szewczenko, J.; Kajzer, A.; Basiaga, M.; Jaworska, J.; Jelonek, K.; Nowińska, K.; Kaczmarek, M.; Orłowska, A. Physical properties of electropolished CoCrMo alloy coated with biodegradable polymeric coatings releasing heparin after prolonged exposure to artificial urine. Materials 2021, 14, 2551. [Google Scholar] [CrossRef] [PubMed]
- Aniołek, K.; Łosiewicz, B.; Kubisztal, J.; Osak, P.; Stróż, A.; Barylski, A.; Kaptacz, S. Mechanical properties, corrosion resistance and bioactivity of oxide layers formed by isothermal oxidation of Ti–6Al–7Nb alloy. Coatings 2021, 11, 505. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Łosiewicz, B. Functionalization of the NiTi shape memory alloy surface by HAp/SiO2/Ag hybrid coatings formed on SiO2-TiO2 glass interlayer. Materials 2020, 13, 1648. [Google Scholar] [CrossRef]
- Stróż, A.; Dercz, G.; Chmiela, B.; Stróż, D.; Łosiewicz, B. Electrochemical formation of second generation TiO2 nanotubes on Ti13Nb13Zr alloy for biomedical applications. Acta Phys. Pol. 2016, 130, 1079–1080. [Google Scholar] [CrossRef]
- Lelątko, J.; Goryczka, T.; Wierzchoń, T.; Ossowski, M.; Łosiewicz, B.; Rówiński, E.; Morawiec, H. Structure of low temperature nitrided/oxidized layer formed on NiTi shape memory alloy. Solid State Phenom. 2010, 163, 127–130. [Google Scholar] [CrossRef]
- Sorokina, L.I.; Tarasov, A.M.; Pepelyaeva, A.I.; Lazarenko, P.I.; Trifonov, A.Y.; Savchuk, T.P.; Kuzmin, A.V.; Tregubov, A.V.; Shabaeva, E.N.; Zhurina, E.S.; et al. The Composite TiO2–CuOx Layers Formed by Electrophoretic Method for CO2 Gas Photoreduction. Nanomaterials 2023, 13, 2030. [Google Scholar] [CrossRef]
- Shakir, S.; Abd-ur-Rehman, H.M.; Zahid, R.; Iwamoto, M.; Periasamy, V. Multistep electrophoretic deposition of TiO2 film and its surface modification for dye sensitized solar cells. J. Alloys Compd. 2020, 837, 155579. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.; Bae, T.; Kim, K.; Watari, F.; Uo, M. Carbon nanotube coating on titanium substrate modified with TiO2 nanotubes. J. Wuhan Univ. Technol. 2011, 26, 867–871. [Google Scholar] [CrossRef]
- Chen, K.; Mitra, S. Controlling the Dissolution Rate of Hydrophobic Drugs by Incorporating Carbon Nanotubes with Different Levels of Carboxylation. Appl. Sci. 2019, 9, 1475. [Google Scholar] [CrossRef]
- Park, J.-E.; Jang, Y.-S.; Bae, T.-S.; Lee, M.-H. Biocompatibility Characteristics of Titanium Coated with Multi Walled Carbon Nanotubes—Hydroxyapatite Nanocomposites. Materials 2019, 12, 224. [Google Scholar] [CrossRef]
- Oliveira, T.M.B.F.; Morais, S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Appl. Sci. 2018, 8, 1925. [Google Scholar] [CrossRef]
- Yang, G.; Cheng, F.; Zuo, S.; Zhang, J.; Xu, Y.; Hu, Y.; Hu, X. Growing Carbon Nanotubes In Situ Surrounding Carbon Fiber Surface via Chemical Vapor Deposition to Reinforce Flexural Strength of Carbon Fiber Composites. Polymers 2023, 15, 2309. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wei, Y.; Han, G.; Zhang, J.; Sun, G.; Hu, X.; Cheng, F. Comparison of Impact Resistance of Carbon Fibre Composites with Multiple Ultra-Thin CNT, Aramid Pulp, PBO and Graphene Interlayers. Compos. Part Appl. Sci. Manuf. 2022, 155, 106815. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, F.; Wang, L.; Xu, Y.; Zhou, Z.; Liu, X.; Hu, Y.; Hu, X. Reinforcement Study of Anodizing Treatment with Various Temperatures on Aluminum Substrates for Stronger Adhesive Bonding with Carbon Fiber Composites. Surf. Coat. Technol. 2023, 462, 129473. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kozma, G.; Kónya, Z. Multi-Walled Carbon Nanotubes; Springer Handbook of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 147–188. [Google Scholar] [CrossRef]
- Morais, S. Multi-Walled Carbon Nanotubes. Appl. Sci. 2019, 9, 2696. [Google Scholar] [CrossRef]
- Terada, M.; Abe, S.; Akasaka, T.; Uo, M.; Kitagawa, Y.; Watari, F. Multiwalled carbon nanotube coating on titanium. Biomed. Mater. Eng. 2009, 19, 45–52. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Hu, Y.; Kang, L.; Zhao, Q.; Zhong, H.; Zhang, S.; Yang, L.; Wang, Z.; Lin, J.; Li, Q.; Zhang, Z.; et al. Growth of high-density horizontally aligned SWNT arrays using Trojan catalysts. Nat. Commun. 2015, 6, 6099. [Google Scholar] [CrossRef]
- Mierczynski, P.; Dubkov, S.V.; Bulyarskii, S.V.; Pavlov, A.A.; Skorik, S.N.; Trifonov, A.Y.; Mierczynska, A.; Kitsyuk, E.P.; Gavrilov, S.A.; Maniecki, T.P.; et al. Growth of carbon nanotube arrays on various CtxMey alloy films by chemical vapour deposition metod. J. Mater. Sci. Technol. 2018, 34, 3472–3480. [Google Scholar] [CrossRef]
- Park, J.E.; Park, I.S.; Bae, T.S.; Lee, M.H. Electrophoretic deposition of carbon nanotubes over TiO2 nanotubes: Evaluation of surface properties and biocompatibility. Bioinorg. Chem. Appl. 2014, 2014, 236521. [Google Scholar] [CrossRef]
- Atiq Ur Rehman, M.; Chen, Q.; Braem, A.; Shaffer, M.S.P.; Boccaccini, A.R. Electrophoretic deposition of carbon nanotubes: Recent progress and remaining challenges. Int. Mater. Rev. 2020, 66, 533–562. [Google Scholar] [CrossRef]
- Zhao, P.; LeSergent, L.J.; Farnese, J.; Wen, J.Z.; Ren, C.L. Electrophoretic deposition of carbon nanotubes on semi-conducting and non-conducting substrates. Electrochem. Commun. 2019, 2019, 106558. [Google Scholar] [CrossRef]
- Cho, J.; Konopka, K.; Rożniatowski, K.; García-Lecina, E.; Shaffer, M.S.P.; Boccaccini, A.R. Characterisation of carbon nanotube films deposited by electrophoretic deposition. Carbon 2009, 47, 58–67. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Kubisztal, J. The effect of a composite chitosan-silver(I) ion coating on the corrosion resistance of the cobalt-chromium-molybdenum alloy in saline solution. Prog. Chem. Appl. Chitin Deriv. 2023, 28, 75–88. [Google Scholar] [CrossRef]
- Marsh, D.H.; Rance, G.A.; Zaka, M.H.; Whitby, R.J.; Khlobystov, A.N. Comparison of the Stability of Multiwalled Carbon Nanotube Dispersions in Water. Phys. Chem. Chem. Phys. 2007, 9, 5490–5496. [Google Scholar] [CrossRef] [PubMed]
- Kharisov, B.I.; Kharissova, O.V.; Vázquez Dimas, A. The Dispersion, Solubilization and Stabilization in “Solution” of Single-Walled Carbon Nanotubes. RSC Adv. 2016, 6, 68760–68787. [Google Scholar] [CrossRef]
- AFNOR/NF Standard S90-701; Matériel Médico-Chirurgical—Biocompatibilité Des Matériaux et Dispositifs Médicaux—Méthodes d’extraction. French Standardization Association: Paris, France, 1988.
- ISO 10271:2021; Dentistry—Corrosion Test Methods for Metallic Materials. ISO: Geneva, Switzerland, 2021.
- Boukamp, B.A. A Linear Kronig-Kramers transform test for immittance data validation. J. Electrochem. Soc. 1995, 142, 1885–1894. [Google Scholar] [CrossRef]
- Sukor, R.; Yusof, N.; Azri, F.; Hajian, R. Modification Strategy of Screen-Printed Carbon Electrode with Functionalized Multi-Walled Carbon Nanotube and Chitosan Matrix for Biosensor Development. Asian J. Chem. 2017, 29, 31–36. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Rybolt, T.; Jordan, H. Interactions and Binding Energies in Carbon Nanotube Bundles. Appl. Nano 2021, 2, 128–147. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of Carbon Nanotubes in Drug Delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, H.; Xu, R. Research on the dispersion of carbon nanotubes by ultrasonic oscillation, surfactant and centrifugation respectively and fiscal policies for its industrial development. Ultrason. Sonochem. 2018, 48, 30–38. [Google Scholar] [CrossRef]
- Azhar, M.; Rauf, S.; Catanante, G.; Nawaz, M.H.; Nunes, G.; Marty, J.; Hayat, A. One Step Assembly of Thin Films of Carbon Nanotubes on Screen Printed Interface for Electrochemical Aptasensing of Breast Cancer Biomarker. Sensors 2016, 2016, 651. [Google Scholar] [CrossRef]
- Bhaghavathi Parambath, V.; Nagar, R.; Vedarajan, R.; Rajalakshmi, N.; Dhathathreyan, K.S.; Sundara, R. Synthesis of Graphene-Multiwalled Carbon Nanotubes Hybrid Nanostructure by Strengthened Electrostatic Interaction and Its Lithium Ion Battery Application. J. Mater. Chem. 2012, 22, 9949–9956. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer Science + Business Media: New York, NY, USA, 2014; ISBN 978-1-4614-8932-0. [Google Scholar]

| Powder Characteristics | Apparent density | 50–150 kg m−3 |
| Mean agglomerate size | 200–500 μm | |
| Weight loss at 105 °C | <1% | |
| MWNTs-R Characteristics | C content | >90 wt.% |
| Free amorphous carbon | Not detectable (SEM/TEM) | |
| Mean number of walls | 5–15 | |
| Outer mean diameter | 30–50 nm | |
| Length | 0.5–2 μm |
| Electrode | R1 (Ω cm2) | CPE1-T (F cm−2 sϕ−1) | CPE1-ϕ | R2 (Ω cm2) |
|---|---|---|---|---|
| CoCrMo | 11.72(92) | 0.74(9) × 10−5 | 0.880(14) | 3.71(21) × 104 |
| MWCNTs-F | 7.89(19) | 0.82(9) × 10−5 | 0.841(11) | 1.61(16) × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łosiewicz, B.; Osak, P.; Górka-Kulikowska, K. Electrophoretic Deposition of Multi-Walled Carbon Nanotube Coatings on CoCrMo Alloy for Biomedical Applications. Micromachines 2023, 14, 2122. https://doi.org/10.3390/mi14112122
Łosiewicz B, Osak P, Górka-Kulikowska K. Electrophoretic Deposition of Multi-Walled Carbon Nanotube Coatings on CoCrMo Alloy for Biomedical Applications. Micromachines. 2023; 14(11):2122. https://doi.org/10.3390/mi14112122
Chicago/Turabian StyleŁosiewicz, Bożena, Patrycja Osak, and Karolina Górka-Kulikowska. 2023. "Electrophoretic Deposition of Multi-Walled Carbon Nanotube Coatings on CoCrMo Alloy for Biomedical Applications" Micromachines 14, no. 11: 2122. https://doi.org/10.3390/mi14112122
APA StyleŁosiewicz, B., Osak, P., & Górka-Kulikowska, K. (2023). Electrophoretic Deposition of Multi-Walled Carbon Nanotube Coatings on CoCrMo Alloy for Biomedical Applications. Micromachines, 14(11), 2122. https://doi.org/10.3390/mi14112122






