Analysis of Photocatalytic Properties of NS-CQDs/g-C3N4 Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalyst
2.2. Visible Light Catalytic Degradation of Rhodamine B Catalyst Performance Evaluation
2.3. Catalyst Performance Evaluation
2.4. Optical Performance Testing
2.5. Free Radical Capture Experiments
3. Results and Discussion
3.1. XRD and FT-IR Analysis
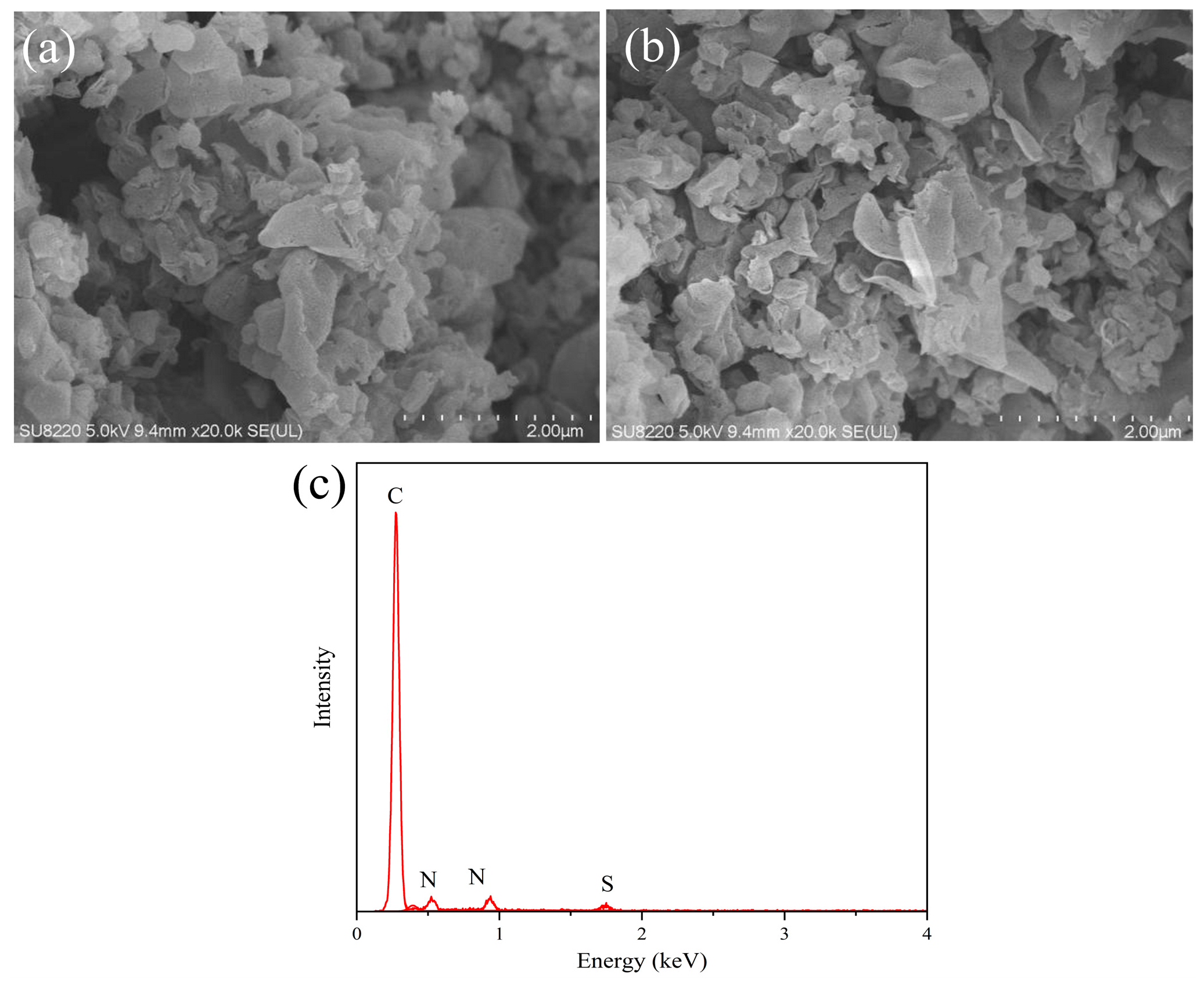
3.2. SEM and EDS Analysis
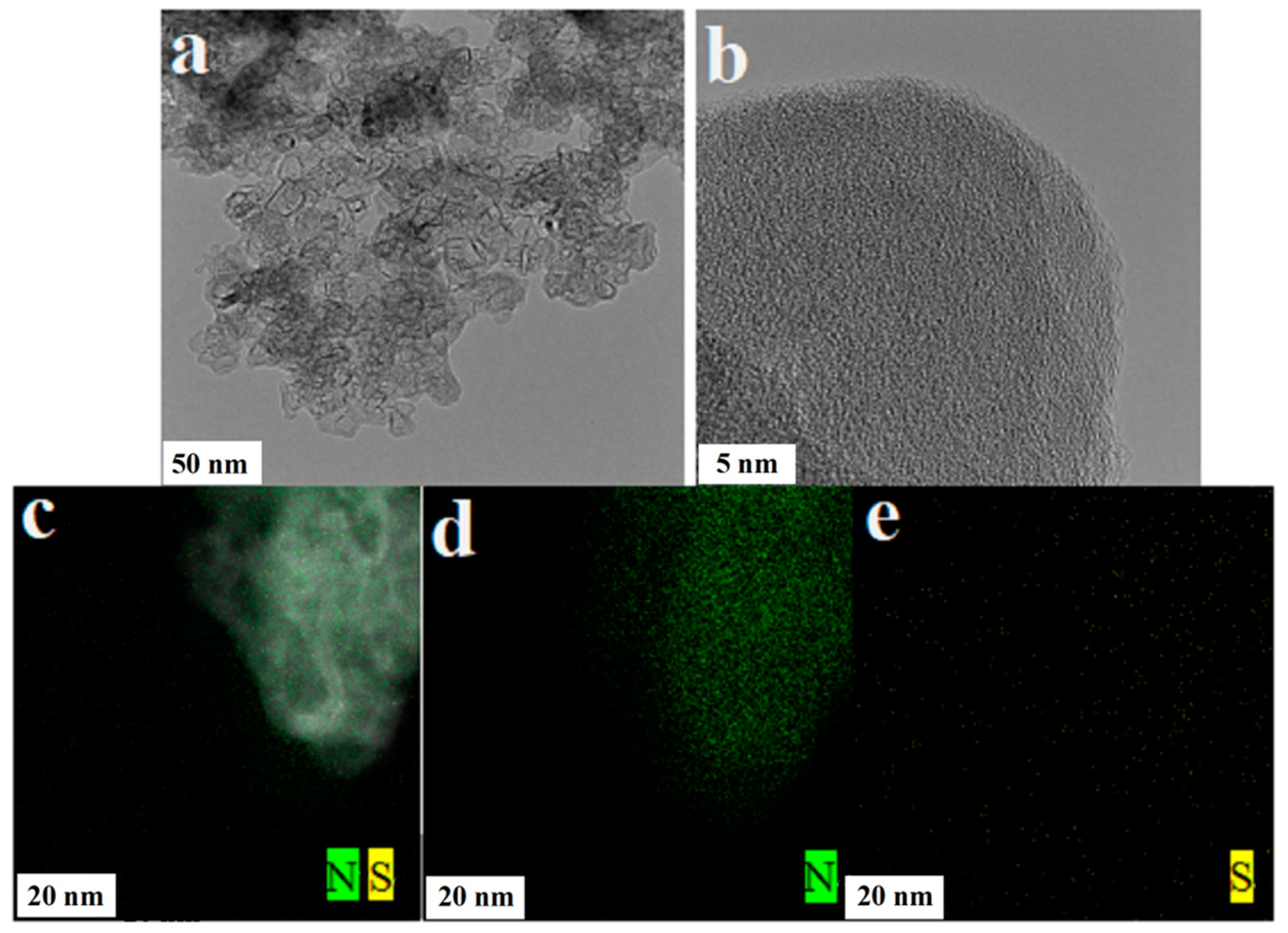
3.3. TEM and Mapping
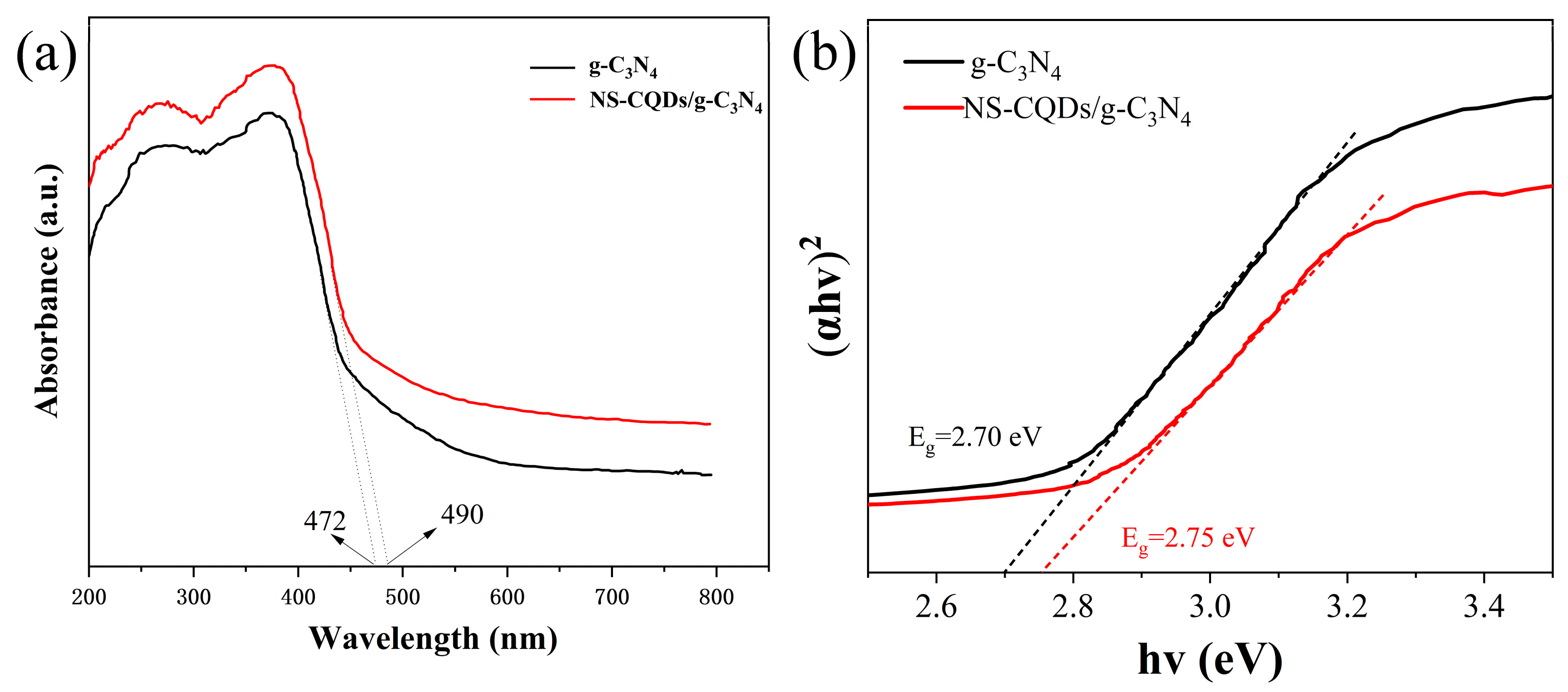
3.4. UV-Vis DRS Analysis
3.5. PL Analysis
3.6. Optical Performance Analysis
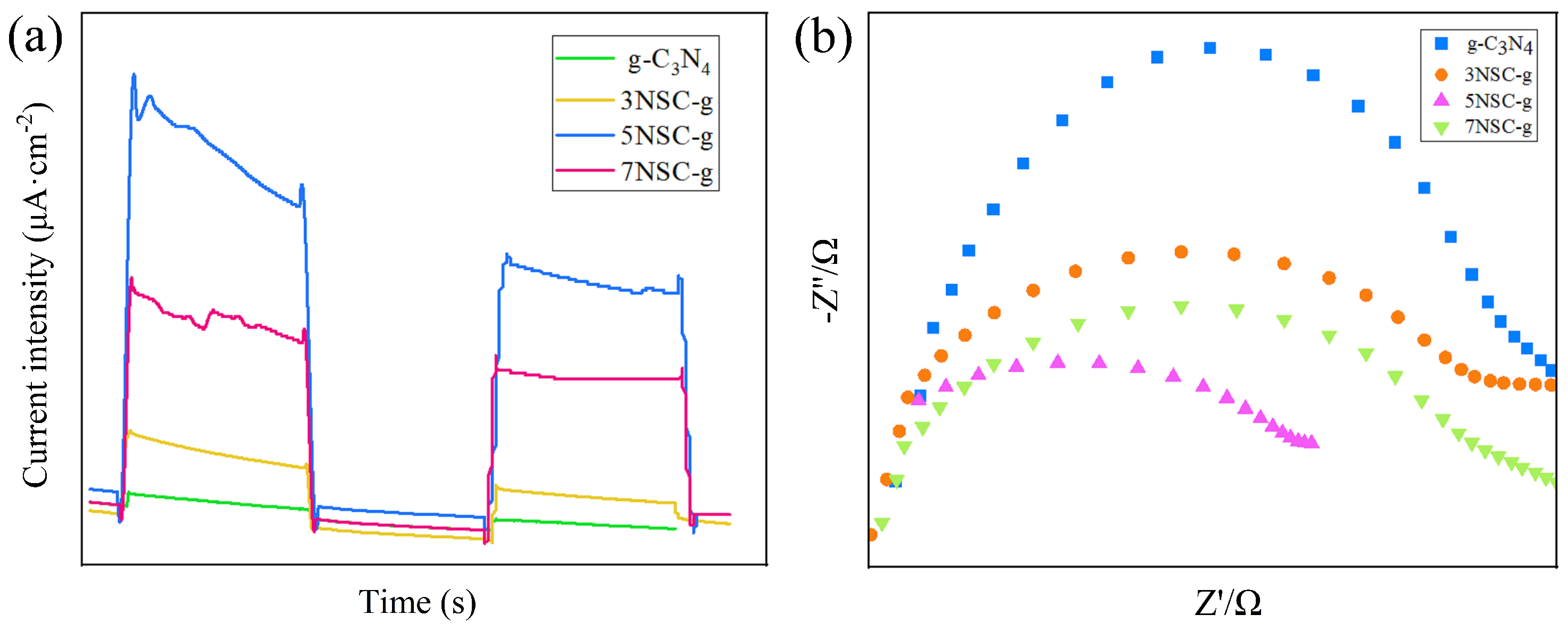
3.7. Effect of Different NS-CQDs Doping on RhB Degradation
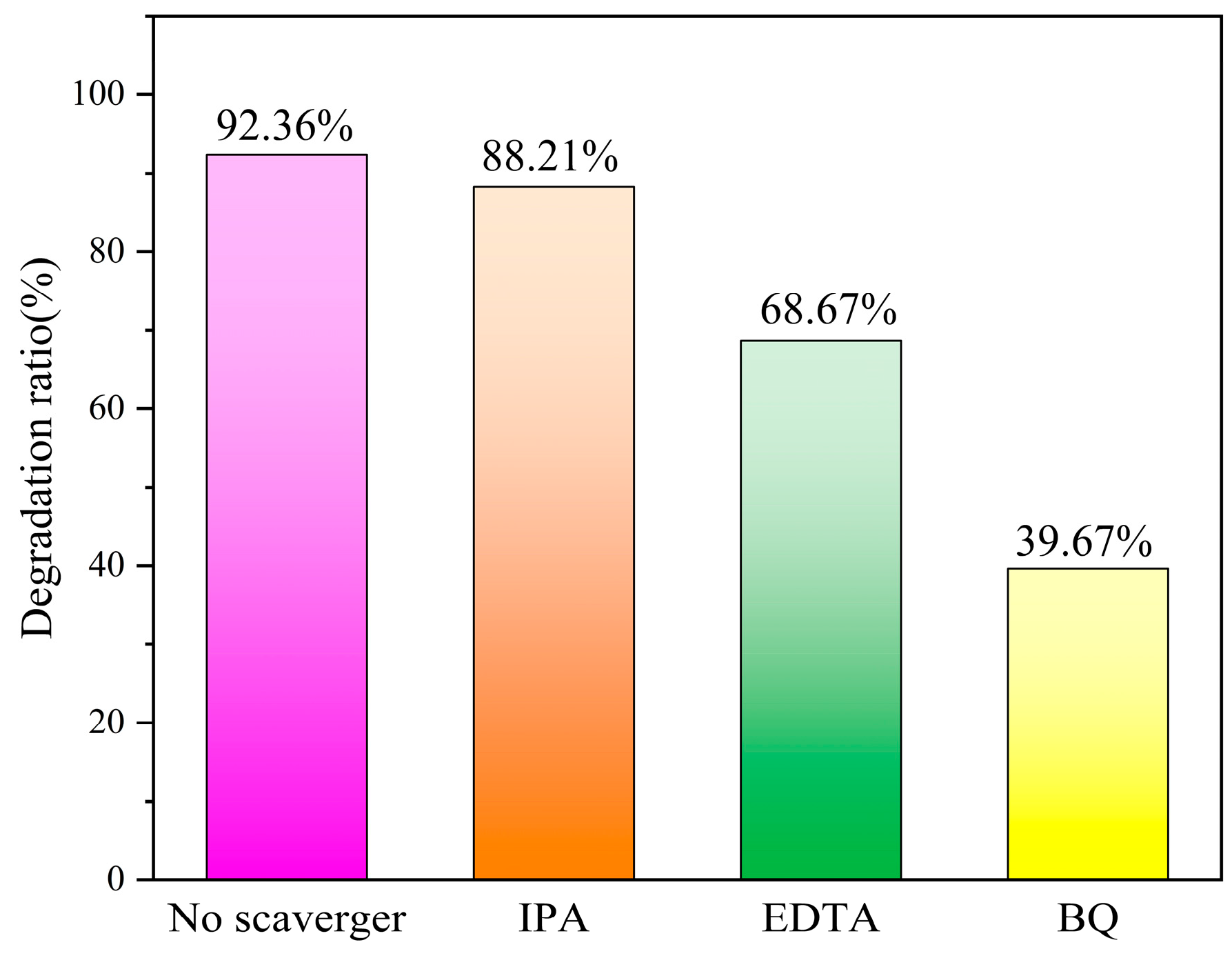
3.8. Free Radical Capture Experiments
3.9. Analysis of Photocatalytic Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusior, A.; Michalec, K.; Jelen, P.; Radecka, M. Shaped Fe2O3 nanoparticles–synthesis and enhanced photocatalytic degradation towards RhB. Appl. Surf. Sci. 2019, 476, 342–352. [Google Scholar] [CrossRef]
- Cao, Q.W.; Zheng, Y.F.; Song, X.C. Enhanced visible-light-driven photocatalytic degradation of RhB by AgIO3/WO3 composites. J. Taiwan Inst. Chem. Eng. 2017, 70, 359–365. [Google Scholar] [CrossRef]
- Khalid, N.; Mazia, U.; Tahir, M.; Niaz, N.; Javid, M.A. Photocatalytic degradation of RhB from an aqueous solution using Ag3PO4/N-TiO2 heterostructure. J. Mol. Liq. 2020, 313, 113522. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Ye, W.; Ma, J.; Gao, D. Flower-like N-doped MoS2 for photocatalytic degradation of RhB by visible light irradiation. Nanotechnology 2016, 27, 225403. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Xie, J.; Guo, N.; Liu, A.; Cao, Y.; Hu, J.; Jia, D. Simple solid-state synthesis of BiOCl/Bi2O2CO3 heterojunction and its excellent photocatalytic degradation of RhB. J. Alloys Compd. 2019, 784, 377–385. [Google Scholar] [CrossRef]
- Zhao, J.; He, Q.; Zhang, X.; Guo, X.; Song, Q.; Liu, Y.; Yao, B.; Zhang, Q.; Dionysiou, D.D. Fabrication of CQDs/Bi5Nb3O15 nanocomposites for photocatalytic degradation of veterinary pharmaceutical sarafloxacin. Catal. Today 2020, 355, 716–726. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Zhai, B.; Liang, Y. Enhanced photocatalytic degradation of RhB by two-dimensional composite photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 429–435. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. A review on the factors affecting the photocatalytic degradation of hazardous materials. Mater. Sci. Eng. Int. J. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Jin, T.; Liu, C.; Chen, F.; Qian, J.; Qiu, Y.; Meng, X.; Chen, Z. Synthesis of g-C3N4/CQDs composite and its photocatalytic degradation property for Rhodamine B. Carbon. Lett. 2022, 32, 1451–1462. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ge, Y.; Li, H.; Ji, H.; Xu, H.; Zhang, Q.; Li, H.; Li, M. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl. Catal. B Environ. 2015, 168, 51–61. [Google Scholar] [CrossRef]
- Gao, H.; Zheng, C.; Yang, H.; Niu, X.; Wang, S. Construction of a CQDs/Ag3PO4/BiPO4 heterostructure photocatalyst with enhanced photocatalytic degradation of rhodamine B under simulated solar irradiation. Micromachines 2019, 10, 557. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X.; Xiong, T.; Zuo, M.; Luo, L.; Qin, P.; Lei, M.; Liang, Y.; Gong, X.; Zou, D. Photocatalytic degradation of tetracycline by N-CQDs modified Sg-C3N4 nanotubes and its product toxicity evaluation. Sep. Purif. Technol. 2023, 314, 123533. [Google Scholar] [CrossRef]
- Yu, H.; Huang, J.; Jiang, L.; Shi, Y.; Yi, K.; Zhang, W.; Zhang, J.; Chen, H.; Yuan, X. Enhanced photocatalytic tetracycline degradation using N-CQDs/OV-BiOBr composites: Unraveling the complementary effects between N-CQDs and oxygen vacancy. Chem. Eng. J. 2020, 402, 126187. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Zhang, H.; Huang, R.; Qi, W.; Su, R.; He, Z. Controllable synthesis of a sponge-like Z-scheme N, S-CQDs/Bi2MoO6@ TiO2 film with enhanced photocatalytic and antimicrobial activity under visible/NIR light irradiation. J. Hazard. Mater. 2022, 429, 128310. [Google Scholar] [CrossRef]
- Chang, L. N, S-Doped Carbon Quantum Dots/TiO₂ Nanocomposites for Visible-Light-Driven Photocatalytic Degradation of Water Pollutants; Memorial University of Newfoundland: St. John’s, NL, Canada, 2022. [Google Scholar]
- Yan, W.; Yan, L.; Jing, C. Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: Structure, photoactivity and degradation mechanisms. Appl. Catal. B Environ. 2019, 244, 475–485. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Xu, P.; Li, Y.; Duan, J.; Zhang, G.; Hu, L.; Wang, X.; Zhang, W. Visible-light-driven photo-Fenton reactions using Zn1−1.5 xFexS/g-C3N4 photocatalyst: Degradation kinetics and mechanisms analysis. Appl. Catal. B Environ. 2020, 266, 118653. [Google Scholar] [CrossRef]
- Kočí, K.; Reli, M.; Troppová, I.; Šihor, M.; Kupková, J.; Kustrowski, P.; Praus, P. Photocatalytic decomposition of N2O over TiO2/g-C3N4 photocatalysts heterojunction. Appl. Surf. Sci. 2017, 396, 1685–1695. [Google Scholar] [CrossRef]
- Ma, L.; Wang, G.; Jiang, C.; Bao, H.; Xu, Q. Synthesis of core-shell TiO2@g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Surf. Sci. 2018, 430, 263–272. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhou, H.; Li, T.; Zhang, L. A Z-scheme mechanism of N-ZnO/g-C3N4 for enhanced H2 evolution and photocatalytic degradation. Appl. Surf. Sci. 2019, 466, 133–140. [Google Scholar] [CrossRef]
- Monga, D.; Basu, S. Enhanced photocatalytic degradation of industrial dye by g-C3N4/TiO2 nanocomposite: Role of shape of TiO2. Adv. Powder Technol. 2019, 30, 1089–1098. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@ g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Chen, W.; Huang, J.; He, Z.C.; Ji, X.; Zhang, Y.F.; Sun, H.L.; Wang, K.; Su, Z.W. Accelerated photocatalytic degradation of tetracycline hydrochloride over CuAl2O4/g-C3N4 pn heterojunctions under visible light irradiation. Sep. Purif. Technol. 2021, 277, 119461. [Google Scholar] [CrossRef]
- Fakheri, M.; Fatemi, S.; Rahimi Kakolaki, R. Comparative study of one-pot and facile methods to synthesize codoped CQDs with low band gap and photovoltaic properties. Can. J. Chem. Eng. 2023, 101, 4480–4492. [Google Scholar] [CrossRef]


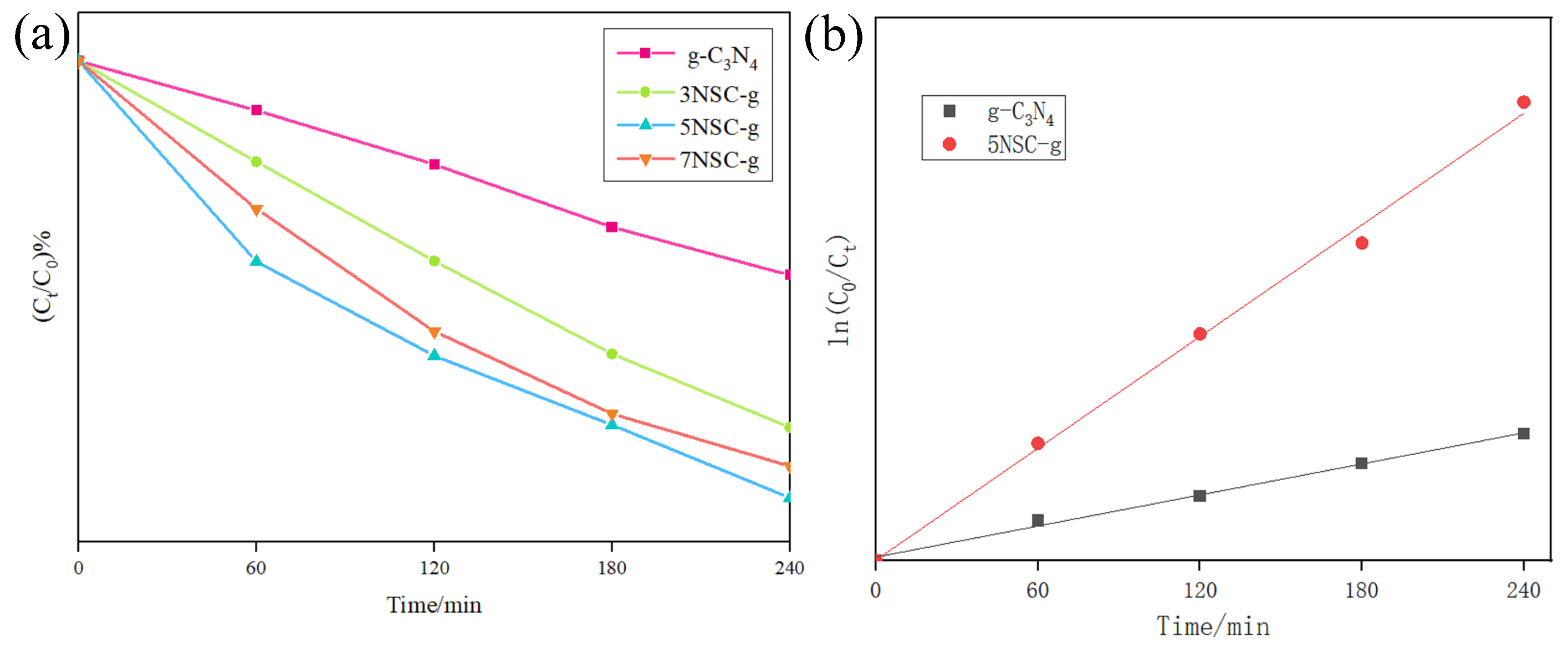

| Sample Type | Regression Equation | k | R2 |
|---|---|---|---|
| g-C3N4 | y = 0.0029x + 0.0186 | 0.0029 | 0.9955 |
| 5NSC-g | y = 0.0103x + 0.0023 | 0.0103 | 0.9962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, H.; Jia, Z.; Lv, J.; Lv, Y.; Wu, J. Analysis of Photocatalytic Properties of NS-CQDs/g-C3N4 Composites. Micromachines 2023, 14, 2143. https://doi.org/10.3390/mi14122143
Wang Y, Chen H, Jia Z, Lv J, Lv Y, Wu J. Analysis of Photocatalytic Properties of NS-CQDs/g-C3N4 Composites. Micromachines. 2023; 14(12):2143. https://doi.org/10.3390/mi14122143
Chicago/Turabian StyleWang, Yu, Hongyue Chen, Zijian Jia, Jingxue Lv, Yuguang Lv, and Jiang Wu. 2023. "Analysis of Photocatalytic Properties of NS-CQDs/g-C3N4 Composites" Micromachines 14, no. 12: 2143. https://doi.org/10.3390/mi14122143
APA StyleWang, Y., Chen, H., Jia, Z., Lv, J., Lv, Y., & Wu, J. (2023). Analysis of Photocatalytic Properties of NS-CQDs/g-C3N4 Composites. Micromachines, 14(12), 2143. https://doi.org/10.3390/mi14122143




