Microfiber-Patterned Versatile Perfusable Vascular Networks
Abstract
:1. Introduction
2. Materials and Methods
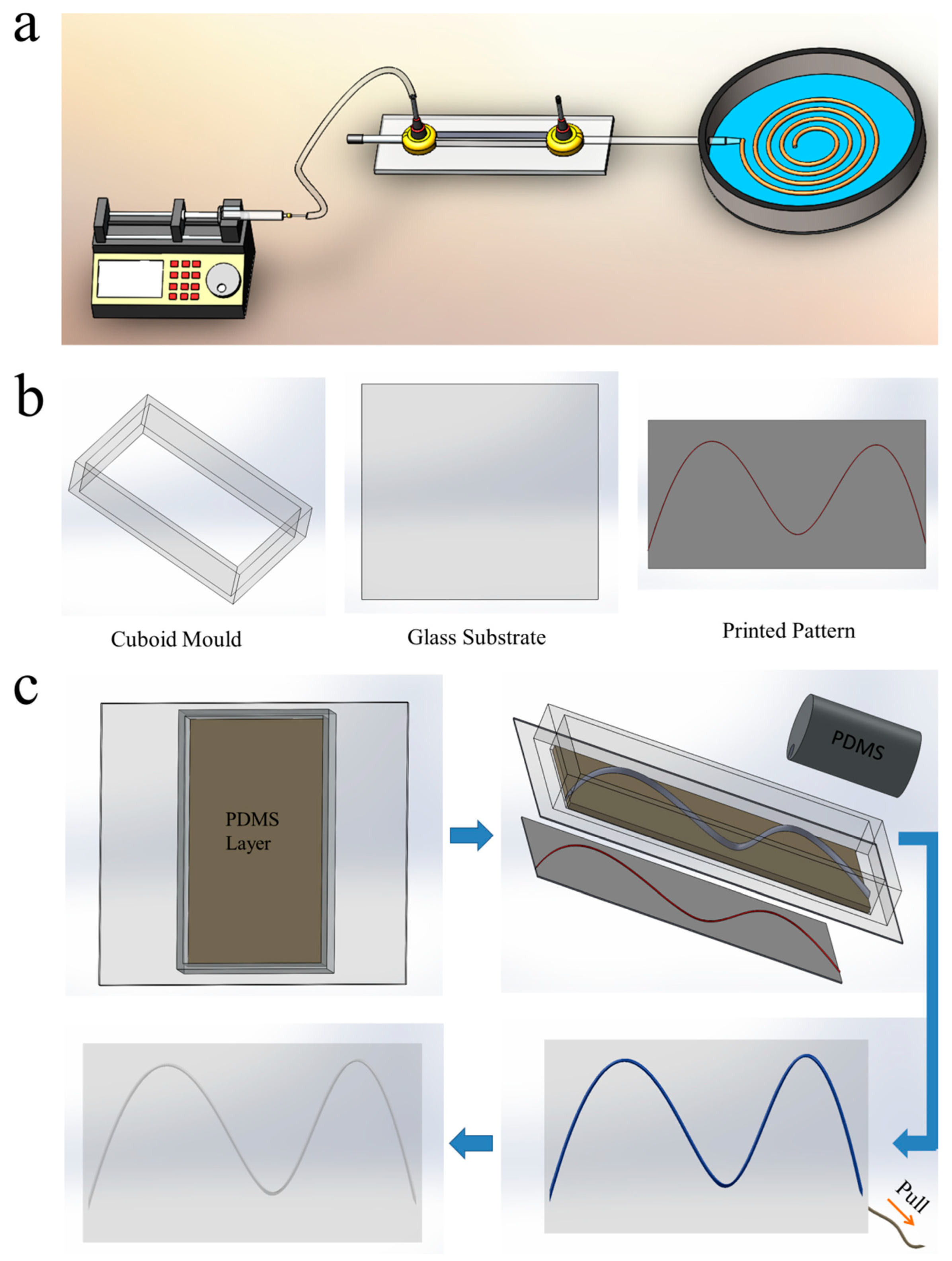
2.1. Fabrication of Alginate Fiber via Microfluidic Device
2.2. Fabrication of Vascular Networks via Microfiber-Patterned Method
2.3. Characterization of Vascular Network
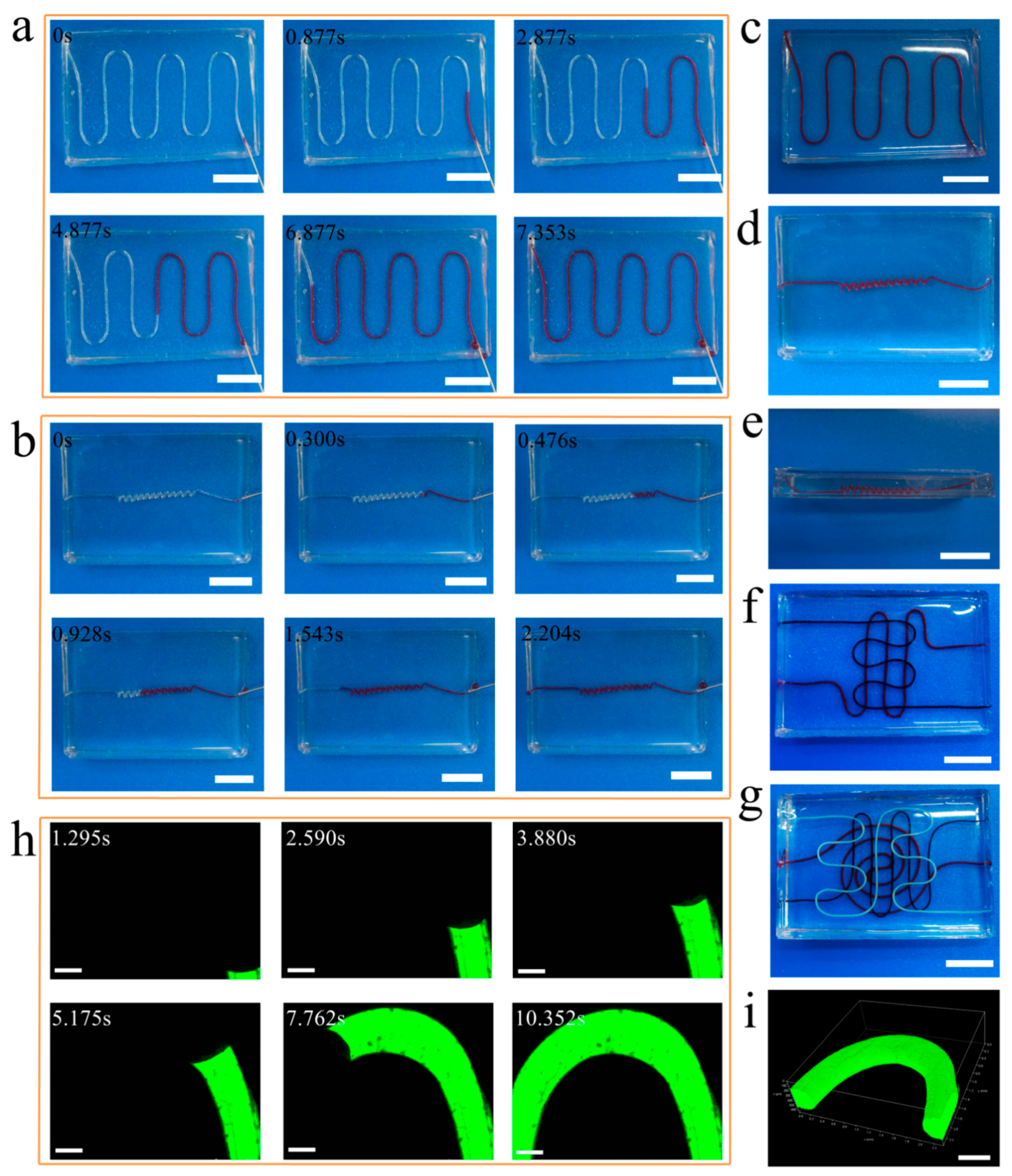
2.4. Perfusable Ability of Vascular Networks
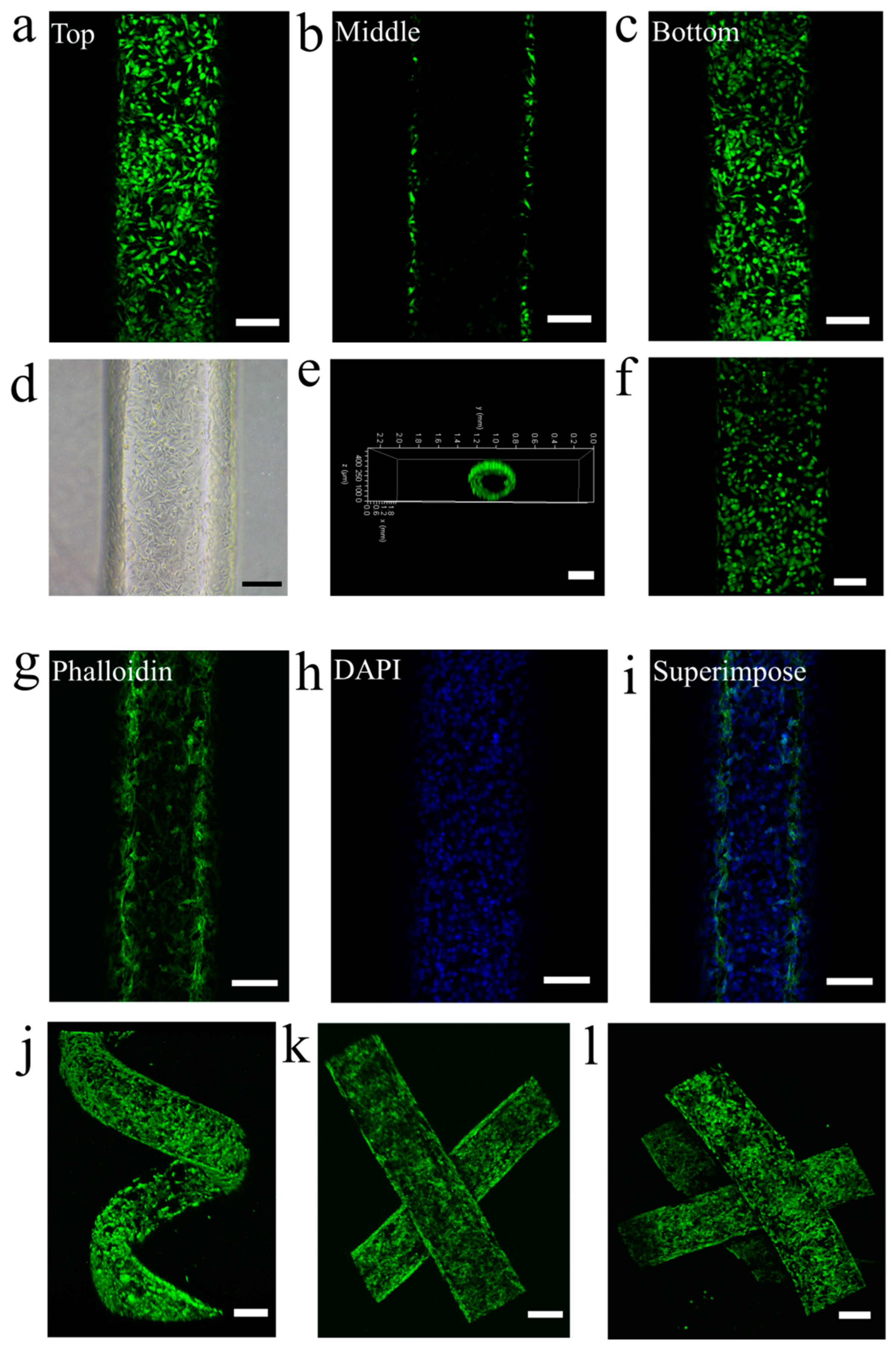
2.5. Investigation of the Endothelialization of Artificial Blood Vessel
3. Results and Discussion
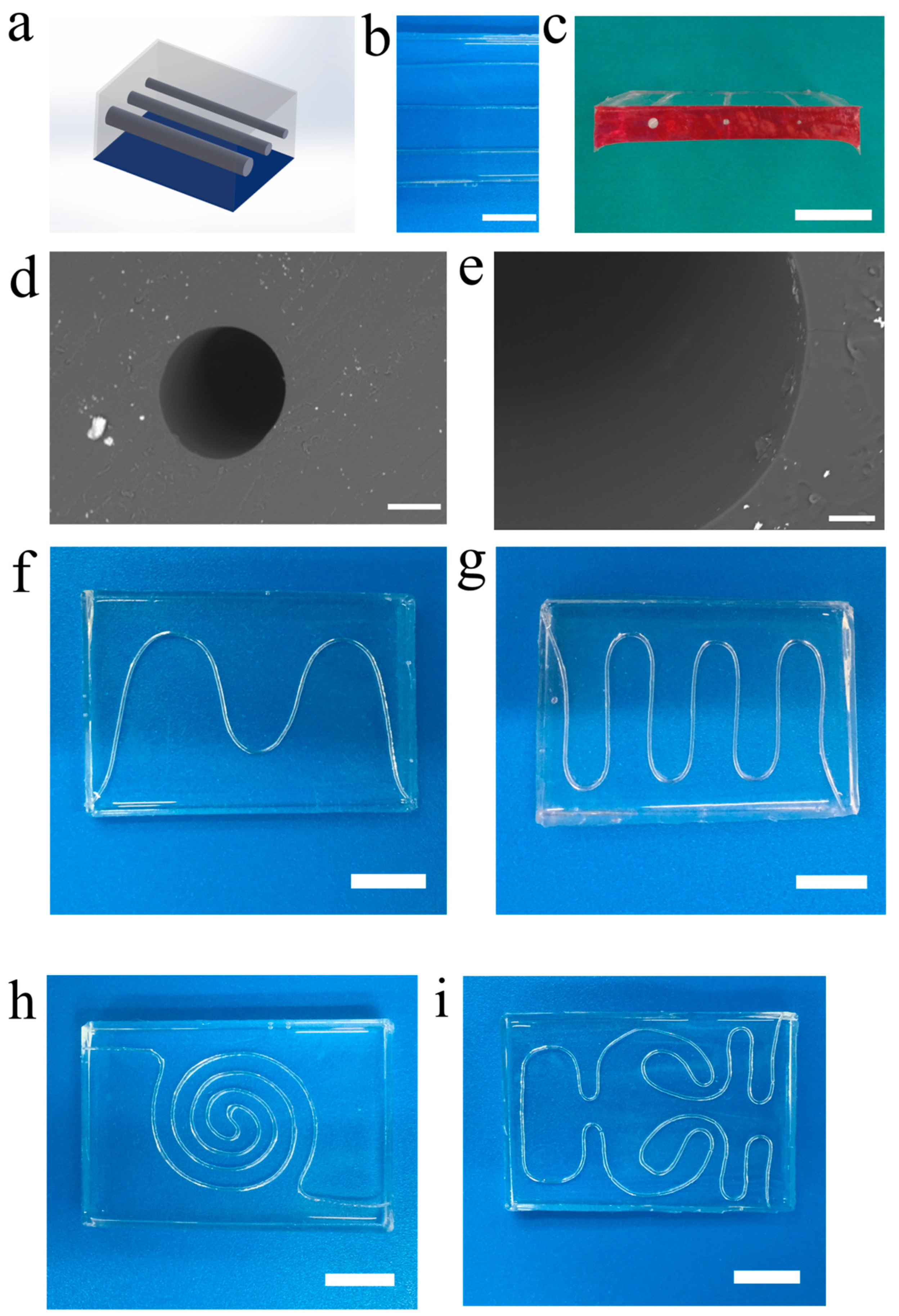
3.1. Engineering Vascular Networks via Microfiber-Patterned Method
3.2. 1D and 2D Vascular Networks
3.3. 3D and Multilayered Vascular Networks
3.4. Perfusable Ability of Vascular Networks
3.5. Endothelialization of Vascular Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.Y.; Salacinski, H.J.; Sales, K.; Butler, P.; Seifalian, A.M. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: A review. Biomaterials 2005, 26, 1857–1875. [Google Scholar] [CrossRef] [PubMed]
- Koike, N.; Fukumura, D.; Gralla, O.; Au, P.; Schechner, J.S.; Jain, R.K. Tissue engineering—Creation of long-lasting blood vessels. Nature 2004, 428, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef]
- Roper, C.S.; Schubert, R.C.; Maloney, K.J.; Page, D.; Ro, C.J.; Yang, S.S.; Jacobsen, A.J. Scalable 3D bicontinuous fluid networks: Polymer heat exchangers toward artificial organs. Adv. Mater. 2015, 27, 2479–2484. [Google Scholar] [CrossRef] [PubMed]
- Yasotharan, S.; Pinto, S.; Sled, J.G.; Bolz, S.S.; Günther, A. Artery-on-a-chip platform for automated, multimodal assessment of cerebral blood vessel structure and function. Lab Chip 2015, 15, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable scaold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 2016, 15, 669–680. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Chung, M.; Jeon, L.J. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef]
- Schimek, K.; Busek, M.; Brincker, S.; Groth, B.; Hoffmann, S.; Lauster, R.; Lindner, G.; Lorenz, A.; Menzel, U.; Sonntag, F.; et al. Integrating biological vasculature into a multi-organchip microsystem. Lab Chip 2013, 13, 3588–3598. [Google Scholar] [CrossRef]
- Dereli-Korkut, Z.; Akaydin, H.D.; Ahmed, A.H.R.; Jiang, X.; Wang, S. Three dimensional microfluidic cell arrays for ex vivo drug screening with mimicked vascular flow. Anal. Chem. 2014, 86, 2997–3004. [Google Scholar] [CrossRef]
- Choi, C.K.; Kim, J.B.; Jang, E.H.; Youn, Y.N.; Ryu, W.H. Curved biodegradable microneedles for vascular drug delivery. Small 2012, 8, 2483–2488. [Google Scholar] [CrossRef]
- Chapurina, Y.; Vinogradov, V.V.; Vinogradov, A.V.; Sobolev, V.E.; Dudanov, I.P.; Vinogradov, V.V. Synthesis of thrombolytic sol−gel coatings: Toward drug-entrapped vascular grafts. J. Med. Chem. 2015, 58, 6313–6317. [Google Scholar] [CrossRef] [PubMed]
- Hoganson, D.M.; Finkelstein, E.B.; Owens, G.E.; Hsiao, J.C.; Eng, K.Y.; Kulig, K.M.; Kim, E.S.; Kniazeva, T.; Pomerantseva, I.; Neville, C.M.; et al. A bilayer small diameter in vitro vascular model for evaluation of drug induced vascular injury. Biomicrofluidics 2016, 10, 054116. [Google Scholar] [CrossRef]
- Bhadriraju, K.; Chen, C.S. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov. Today 2002, 7, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, A.J.; Hibino, N.; Best, C.A.; Yi, T.; Lee, Y.U.; Kraynak, C.A.; Kimerer, L.K.; Krieger, A.; Kim, P.; Breuer, C.K.; et al. 3D-printed biodegradable polymeric vascular grafts. Adv. Healthc. Mater. 2016, 5, 319–325. [Google Scholar] [CrossRef]
- Cui, H.T.; Zhu, W.; Holmes, B.; Zhang, L.G. Biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration. Adv. Sci. 2016, 3, 1600058. [Google Scholar] [CrossRef]
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.S.; Vincent, P.A.; Dai, G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35, 8092–8102. [Google Scholar] [CrossRef]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef]
- Wu, W.; Hansen, C.J.; Aragon, A.M.; Geubelle, P.H.; White, S.R.; Lewis, J.A. Direct-write assembly of biomimetic microvascular networks for efficient fluid transport. Soft Matter 2010, 6, 739–742. [Google Scholar] [CrossRef]
- Hansen, C.J.; Wu, W.; Toohey, K.S.; Sottos, N.R.; White, S.R.; Lewis, J.A. Self-Healing Materials with Interpenetrating Microvascular Networks. Adv. Mater. 2009, 21, 4143–4147. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef]
- Therriault, D.; White, S.R.; Lewis, J.A. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat. Mater. 2003, 2, 265–271. [Google Scholar] [CrossRef]
- Toohey, K.S.; Hansen, C.J.; Lewis, J.A.; White, S.R.; Sottos, N.R. Delivery of two-part self-healing chemistry via microvascular networks. Adv. Funct. Mater. 2009, 19, 1399–1405. [Google Scholar] [CrossRef]
- Wu, W.; DeConinck, A.; DeConinck, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 13, H178–H183. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Jin, Z.H.; Gan, B.W.; Lv, S.W.; Xie, M.; Huang, W.H. Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template. Lab Chip 2014, 14, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Bellan, L.M.; Singh, S.P.; Henderson, P.W.; Porri, T.J.; Craighead, H.G.; Spector, J.A. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter 2009, 5, 1354–1357. [Google Scholar] [CrossRef]
- Gergely, R.C.R.; Pety, S.J.; Krull, B.P.; Patrick, J.F.; Doan, T.Q.; Coppola, A.M.; Thakre, P.R.; Sottos, N.R.; Moore, J.S.; White, S.R. Multidimensional vascularized polymers using degradable sacrificial templates. Adv. Funct. Mater. 2015, 25, 1043–1052. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, R.; Jiang, B.; Zhao, Y.; Zhang, W.; Jiang, X. An early-stage atherosclerosis research model based on microfluidics. Small 2016, 12, 2022–2034. [Google Scholar] [CrossRef] [PubMed]
- Gillette, B.M.; Jensen, J.A.; Tang, B.; Yang, G.J.; Bazargan-Lari, A.; Zhong, M.; Sia, S.K. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat. Mater. 2008, 7, 636–640. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wang, L. Microfiber-Patterned Versatile Perfusable Vascular Networks. Micromachines 2023, 14, 2201. https://doi.org/10.3390/mi14122201
Tian Y, Wang L. Microfiber-Patterned Versatile Perfusable Vascular Networks. Micromachines. 2023; 14(12):2201. https://doi.org/10.3390/mi14122201
Chicago/Turabian StyleTian, Ye, and Liqiu Wang. 2023. "Microfiber-Patterned Versatile Perfusable Vascular Networks" Micromachines 14, no. 12: 2201. https://doi.org/10.3390/mi14122201




