Development of Microrobot with Optical Magnetic Dual Control for Regulation of Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
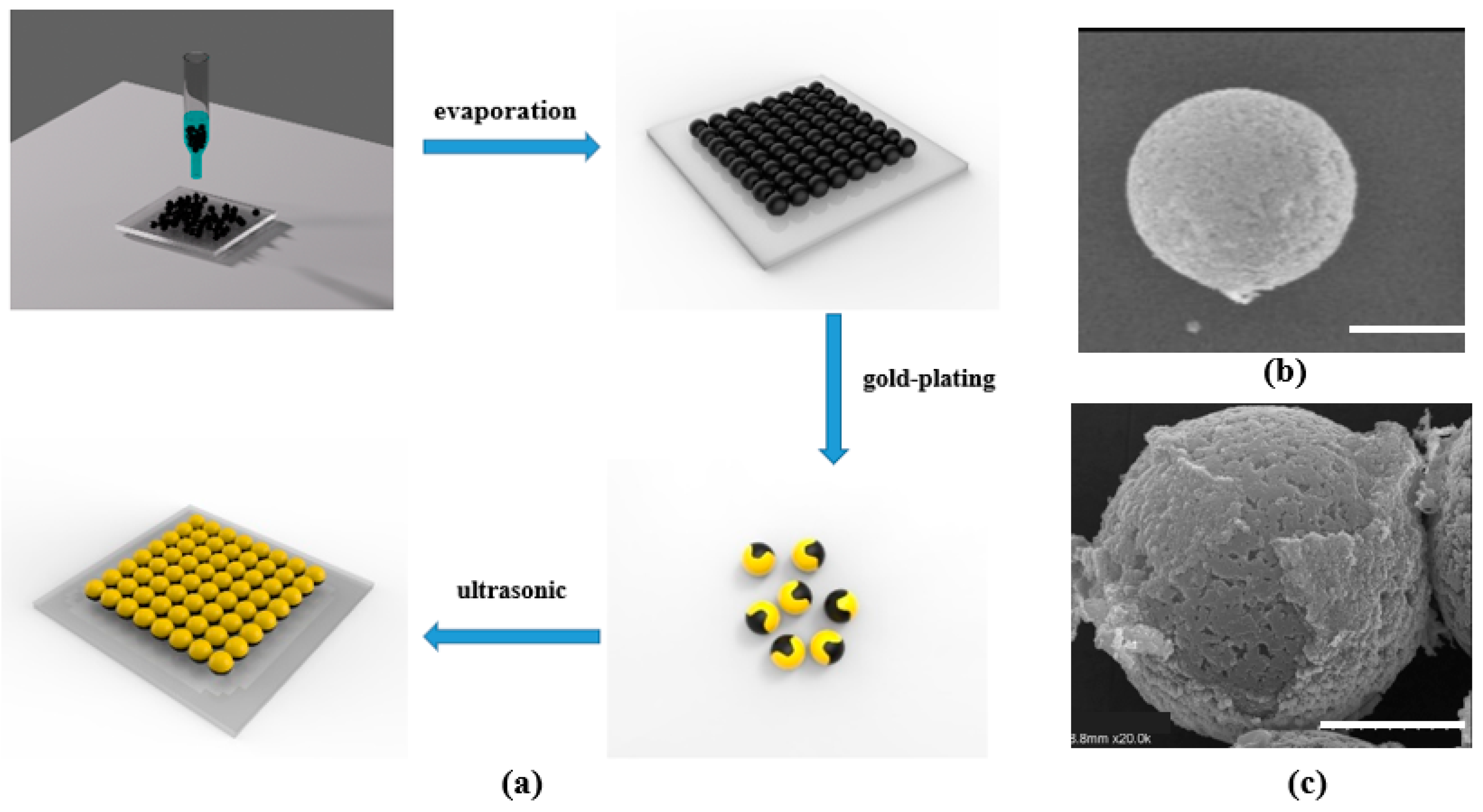
2.2. Fabrication of the Microrobots
2.3. Structure of the Optical Magnetic Dual-Control System
2.4. Functionalized Microrobots
- Add 2 mL of microrobot solution with a concentration of 0.17% to a dish and let it air-dry for 1 h;
- Add 2 mL of 1 mmol/L 3-MPA solution to the dish and incubate it at room temperature for 4 h, then rinse off the excess solution with deionized water and let it air-dry for 1 h;
- Add 2 mL of 5 mmol/L EDC/NHS mixed solution to the culture dish and incubate it at room temperature for 3 h, then rinse off the excess solution with deionized water and let it air-dry for 1 h;
- Add 1 mL of 100 μg/mL S. cerevisiae antibodies solution to the dish and incubate it at room temperature for 40 min, then wash off the excess antibodies with 0.1 mM PBS and let it air-dry for 1 h;
- Add 1% BSA solution to the dish to cover the microrobots and incubate the dish at 4 °C for 30 min, then wash the microrobots with 0.1 mM PBS and let it air-dry;
- Add a concentration of 100 μg/mL antibody solution to the dish and incubate it at 4 °C for 30 min, then wash the microrobots with 0.1 mM PBS to remove any residue solution. The modification process is now complete.
3. Results and Discussion
3.1. Magnetic Field Drive System Verification
3.2. Optical Control System Verification
3.3. Optical and Magnetic Dual-Drive System Verification
3.4. Drug Loading Verification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, D.; Bai, R.; Zhou, W. Angiogenin maintains gut microbe homeostasis by balancing α-Proteobacteria and Lachnospiraceae. Gut 2020, 70, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Ron, S.; Shai, F.; Ron, M. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar]
- Cho, J.H.; Kim, M.K.; Lee, H.S. Fatty Acid Composition of Safflower Seed Oil and Growth-Promoting Effect of Safflower Seed Extract toward Beneficial Intestinal Bacteria. Food. Sci. Biotechnol. 2002, 11, 480–483. [Google Scholar]
- Belkaid, Y.; Hand, T. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Zhang, X.; Chen, W. MicroRNAs Regulate Intestinal Immunity and Gut Microbiota for Gastrointestinal Health: A Comprehensive Review. Genes 2020, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.L.; Lyon, D.E.; Yoon, S.L. The Microbiome and Cancer. Cancer Nurs. 2015, 13, 11800–11812. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. Microbiology 2019, 7, e7502. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, X.; Guo, W. The relationship between frequently used glucose-lowering agents and gut microbiota in type 2 diabetes mellitus. J. Diabetes Res. 2018, 2018, 1890978. [Google Scholar] [CrossRef]
- Preidis, G.A.; Hill, C.; Guerrant, R.L. Probiotics, enteric and diarrheal diseases, and global health. Gastroenterology 2011, 140, 8–14.e9. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, D.; Liang, S. Recent Advances in Field-Controlled Micro–Nano Manipulations and Micro–Nano Robots. Adv. Intell. Syst. 2022, 4, 2100116. [Google Scholar] [CrossRef]
- Luo, M.; Feng, Y.; Wang, T. Micro-/nanorobots at work in active drug delivery. Adv. Funct. Mater. 2018, 28, 1706100. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Sen Gupta, S.; Bhattacharya, T. Gut microbiomes of Indian children of varying nutritional status. PLoS ONE 2014, 9, e95547. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, R.; Wu, H. A review on artificial micro/nanomotors for cancer-targeted delivery, diagnosis, and therapy. Nano Micro Lett. 2020, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, T.; Pardridge, W. Preface: Targeted drug delivery to the brain (blood-brain barrier, efflux, endothelium, biological transport). J. Drug Target. 2000, 8, 353–355. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Wei, F.; Zhong, T.; Zhan, Z. Self-assembled Micro-nanorobots: From Assembly Mechanisms to Applications. ChemNanoMat 2021, 7, 238–252. [Google Scholar] [CrossRef]
- Li, J.; Esteban-Fernández de Ávila, B.; Gao, W. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef]
- Zhang, L.; Abbott, J.J.; Dong, L. Artificial bacterial flagella: Fabrication and magnetic control. Appl. Phys. Lett. 2009, 94, 064107. [Google Scholar] [CrossRef]
- Sitti, M. Miniature soft robots—Road to the clinic. Nat. Rev. Mater. 2018, 3, 74–75. [Google Scholar] [CrossRef]
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef] [PubMed]
- Solovev, A.A.; Mei, Y.; Bermúdez Ureña, E. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small 2009, 5, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; An, M.; Liu, Y. Advances in Chemically Powered Micro/Nanorobots for Biological Applications: A Review. Adv. Funct. Mater. 2023, 33, 2209883. [Google Scholar] [CrossRef]
- Wang, H.J.; Sun, Z.; Zhao, C. Movement control of autonomous mobile robot powered by ultrasonic motors. Zhendong Ceshi Yu Zhenduan/J. Vib. Meas. Diagn. 2006, 26, 181–184. [Google Scholar]
- Gao, Y.; Xiong, Z.; Wang, J. Light hybrid micro/nano-robots: From propulsion to functional signals. Nano Res. 2022, 15, 5355–5375. [Google Scholar] [CrossRef]
- Palagi, S.; Mark, A.G.; Reigh, S.Y. Structured light enables biomimetic swimming and versatile locomotion of photoresponsive soft microrobots. Nat. Mater. 2016, 15, 647–653. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, K.; Huang, C. Self-healing graphene oxide-based nanocomposite hydrogels serve as near-infrared light-driven valves. Sens. Actuat. B Chem. 2019, 298, 126908. [Google Scholar] [CrossRef]
- Sul, O.J.; Falvo, M.R.; Taylor, R.M. Thermally actuated untethered impact-driven locomotive microdevices. Appl. Phys. Lett. 2006, 89, 20. [Google Scholar] [CrossRef]
- Xuanying, L.; Zhenbo, L.; Ling, M. Manufacturing and Wireless Driving of a Permanent Magnetic Micro-robot. Jiqiren/Robot 2013, 35, 513–520. [Google Scholar]
- Chen, X.Z.; Hoop, M.; Mushta, F. Recent developments in magnetically driven micro-and nanorobots. Appl. Mater. Today 2017, 9, 37–48. [Google Scholar] [CrossRef]
- Meng, F.L.; Matsunaga, D.; Golestanian, R. Clustering of magnet ic swimmers in a poiseuille flow. Phys. Rev. Lett. 2018, 120, 1. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.M.; Fujita, S.; Mhanna, R. Magnetic helical mi croswimmers functionalized with lipoplexes for targeted gene delivery. Adv. Funct. Mater. 2015, 25, 1666–1671. [Google Scholar] [CrossRef]
- Yu, J.F.; Wang, B.; Du, X.Z. Ultra-extensible ribbon-like magnetic microswarm. Nat. Commun. 2018, 9, 3260. [Google Scholar] [CrossRef]
- Patil, G.; Ghosh, A. Anomalous behavior of highly active heli cal swimmers. Front. Phys. 2021, 8, 628276. [Google Scholar] [CrossRef]
- Fernandez-Medina, M.; Ramos-Docampo, M.A.; Hovorka, O. Recent advances in nano- and micromotors. Adv. Funct. Mater. 2020, 30, 12. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, R.; Gao, Y. Programming the lifestyles of engineered bacteria for cancer therapy. Natl. Sci. Rev. 2023, 10, 5. [Google Scholar] [CrossRef]
- Gao, W.; Feng, X.; Pei, A. Seawater-driven magnesium based Janus micromotors for environmental remediation. Nanoscale 2013, 5, 4696–4700. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, G.; Chen, C. One-step fabrication of dual optically/magnetically modulated walnut-like micromotor. Langmuir 2019, 35, 2801–2807. [Google Scholar] [CrossRef]
- Nocentini, S.; Parmeggiani, C.; Martella, D. Optically driven soft micro robotics. Adv. Opt. Mater. 2018, 6, 1800207. [Google Scholar] [CrossRef]
- Deng, Z.; Mou, F.; Tang, S. Swarming and collective migration of micromotors under near infrared light. Appl. Mater. Today 2018, 13, 45–53. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Zhang, D. Calligraphy/painting based on a bioinspired light-driven micromotor with concentration-dependent motion direction reversal and dynamic swarming behavior. ACS Appl. Mater. Interfaces 2019, 11, 40533–40542. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, X.; Du, Y.; Liu, F.; Li, G. Development of Microrobot with Optical Magnetic Dual Control for Regulation of Gut Microbiota. Micromachines 2023, 14, 2252. https://doi.org/10.3390/mi14122252
Lan X, Du Y, Liu F, Li G. Development of Microrobot with Optical Magnetic Dual Control for Regulation of Gut Microbiota. Micromachines. 2023; 14(12):2252. https://doi.org/10.3390/mi14122252
Chicago/Turabian StyleLan, Xiaotian, Yijie Du, Fei Liu, and Gongxin Li. 2023. "Development of Microrobot with Optical Magnetic Dual Control for Regulation of Gut Microbiota" Micromachines 14, no. 12: 2252. https://doi.org/10.3390/mi14122252
APA StyleLan, X., Du, Y., Liu, F., & Li, G. (2023). Development of Microrobot with Optical Magnetic Dual Control for Regulation of Gut Microbiota. Micromachines, 14(12), 2252. https://doi.org/10.3390/mi14122252





