Increasing the Performance of {[(1-x-y) LiCo0.3Cu0.7] (Al and Mg doped)] O2}, xLi2MnO3, yLiCoO2 Composites as Cathode Material in Lithium-Ion Battery: Synthesis and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cathode Electrode Preparation
2.2. Electrochemical Testing
2.3. State of Charge (SOC) Measurements
2.4. Applied Equipment
2.5. X-ray Diffraction
2.6. Charge and Discharge Measurement
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lithium Ion Batteries Outlook and Alternative Energy Vehicle (HEVs, PHEVs) Technologies, Markets, Competitors and Opportunities: 2010–2012; Analysis and Forecasts Market Research: Rockville, MD, USA, 2010.
- Yoshio, M.; Brodd, R.J.; Kozawa, A. Lithium-Ion Batteries: Science and Technologies; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Scrosati, B.; Garche, J. Lithium Batteries: Status, Prospects and Future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Daniel, C.; Besenhard, J.O. Handbook of Battery Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Meyers, R.A. Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2012. [Google Scholar]
- Rossen, E.; Jones, C.; Dahn, J. Structure and Electrochemistry of LixMnyNi1−yO2. Solid State Ion. 1992, 57, 311–318. [Google Scholar] [CrossRef]
- Spahr, M.E. Characterization of Layered Lithium Nickel Manganese Oxides Synthesized by a Novel Oxidative Coprecipitation Method and Their Electrochemical Performance as Lithium Insertion Electrode Materials. J. Electrochem. Soc. 1998, 145, 1113–1121. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered Lithium Insertion Material of LiNi1/2Mn1/2O2: A Possible Alternative to LiCoO2 for Advanced Lithium-Ion Batteries. Chem. Lett. 2001, 30, 744–745. [Google Scholar] [CrossRef]
- Makimura, Y.; Ohzuku, T. Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithiumion batteries. J. Power Sources 2003, 119–121, 156–160. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, A.; Lee, J. Synthesis and characterization of LiNi1−x−yCoxMnyO2 as the cathode materials of secondary lithium batteries. J. Power Sources 1999, 81–82, 416–419. [Google Scholar] [CrossRef]
- Yoshio, M.; Noguchi, H.; Itoh, J.I.; Okada, M.; Mouri, T. Preparation and properties of LiCoyMnxNi1−x−yO2 as a cathode for lithium ion batteries. J. Power Sources 2000, 90, 176–181. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered Lithium Insertion Material of LiCo1/3Ni1/3Mn1/3O2 for Lithium-Ion Batteries. Chem. Lett. 2001, 30, 642–643. [Google Scholar] [CrossRef]
- Belharouak, I.; Sun, Y.K.; Liu, J.; Amine, K. Li(Ni1/3Co1/3Mn1/3)O2 as a suitable cathode for high power applications. J. Power Sources 2003, 123, 247–252. [Google Scholar] [CrossRef]
- Ngala, J.K.; Chernova, N.A.; Ma, M.; Mamak, M.; Zavalij, P.Y.; Whittingham, M.S. The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound. J. Mater. Chem. 2004, 14, 214–220. [Google Scholar] [CrossRef]
- Yoon, W.S.; Chung, K.Y.; McBreen, J.; Yang, X.Q. A comparative study on structural changes of LiCo1/3Ni1/3Mn1/3O2 and LiNi0.8Co0.15Al0.05O2 during first charge using in situ XRD. Electrochem. Commun. 2006, 8, 1257–1262. [Google Scholar] [CrossRef]
- Ammundsen, B.; Paulsen, J.; Davidson, I.; Liu, R.S.; Shen, C.H.; Chen, J.M.; Jang, L.Y.; Lee, J.F. Redox Mechanism of Layered Li1.2Cr0.4Mn0.4O2 Cathode Material. J. Electrochem. Soc. 2002, 149, A431–A436. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, C.S.; Vaughey, J.T.; Li, N.; Hackney, S.A. Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 2005, 15, 2257–2267. [Google Scholar] [CrossRef]
- Zhang, L.; Noguchi, H.; Yoshio, M. Synthesis and electrochemical properties of layered Li–Ni–Mn–O compounds. J. Power Sources 2002, 110, 57–64. [Google Scholar] [CrossRef]
- Shin, S.S.; Sun, Y.K.; Amine, K. Synthesis and electrochemical properties of Li[Li(1−2x)/3NixMn(2−x)/3]O2 as cathode materials for lithium secondary batteries. J. Power Sources 2002, 112, 634–638. [Google Scholar] [CrossRef]
- Zhang, H. Synthesis and Characterization of Li Ni0.7-x MgxCo0.3O2 (0 ≤ x ≤ 0.1) Cathode Materials for Lithium-Ion Batteries Prepared by a Sol-Gel Method. Adv. Mater. Sci. Eng. 2014, 7, 746341. [Google Scholar]
- Ehrlich, G.M.; Linden, D. Handbook of Lithium-Ion Batteries; McGraw-Hill: New York, NY, USA, 2002; p. 35. [Google Scholar]
- Yang, Z.-G.; Zhang, J.-L.; Kintner-Meyer, M.C.W.; Lu, X.-C.; Choi, D.-W.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577. [Google Scholar] [CrossRef] [PubMed]

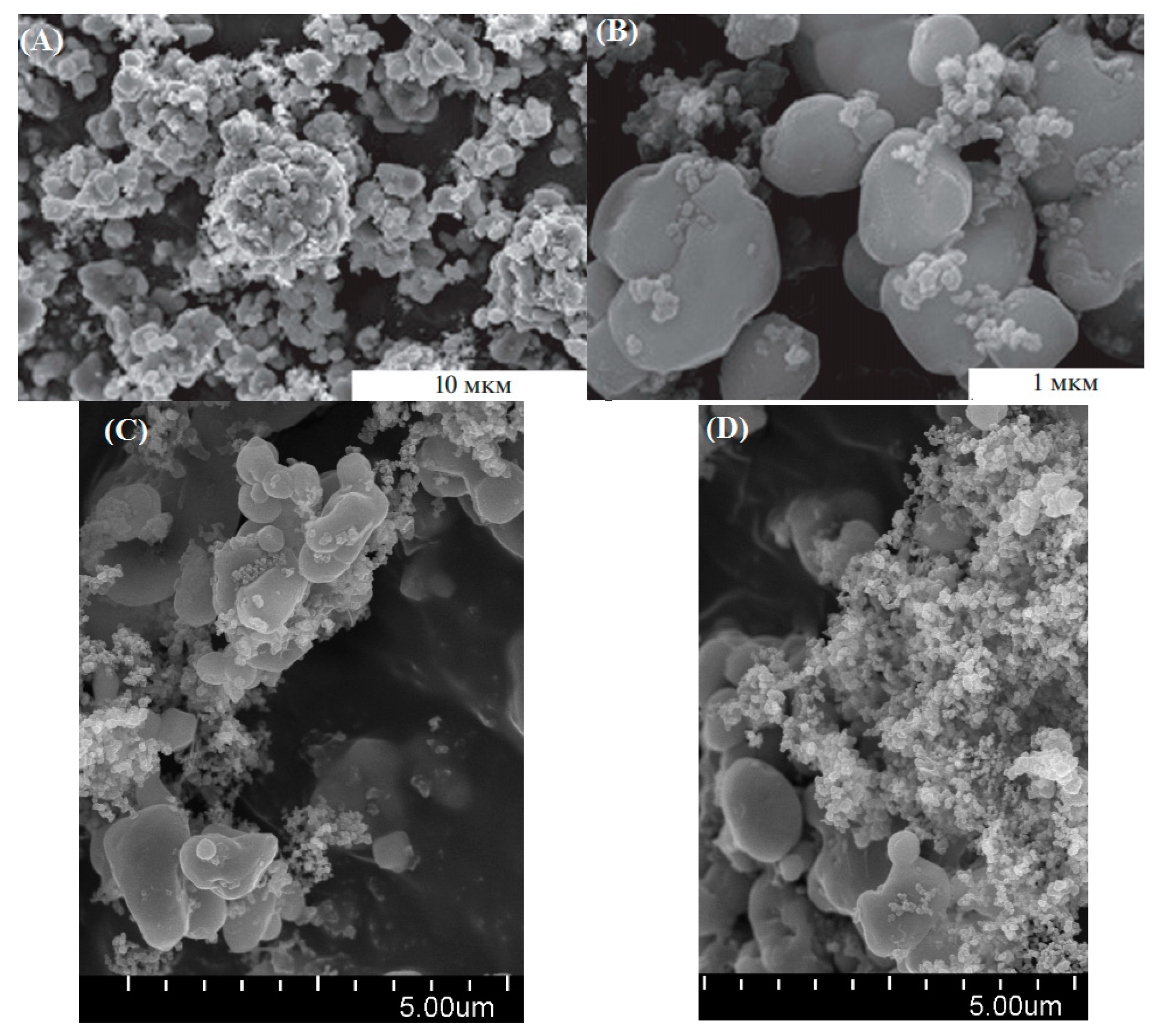
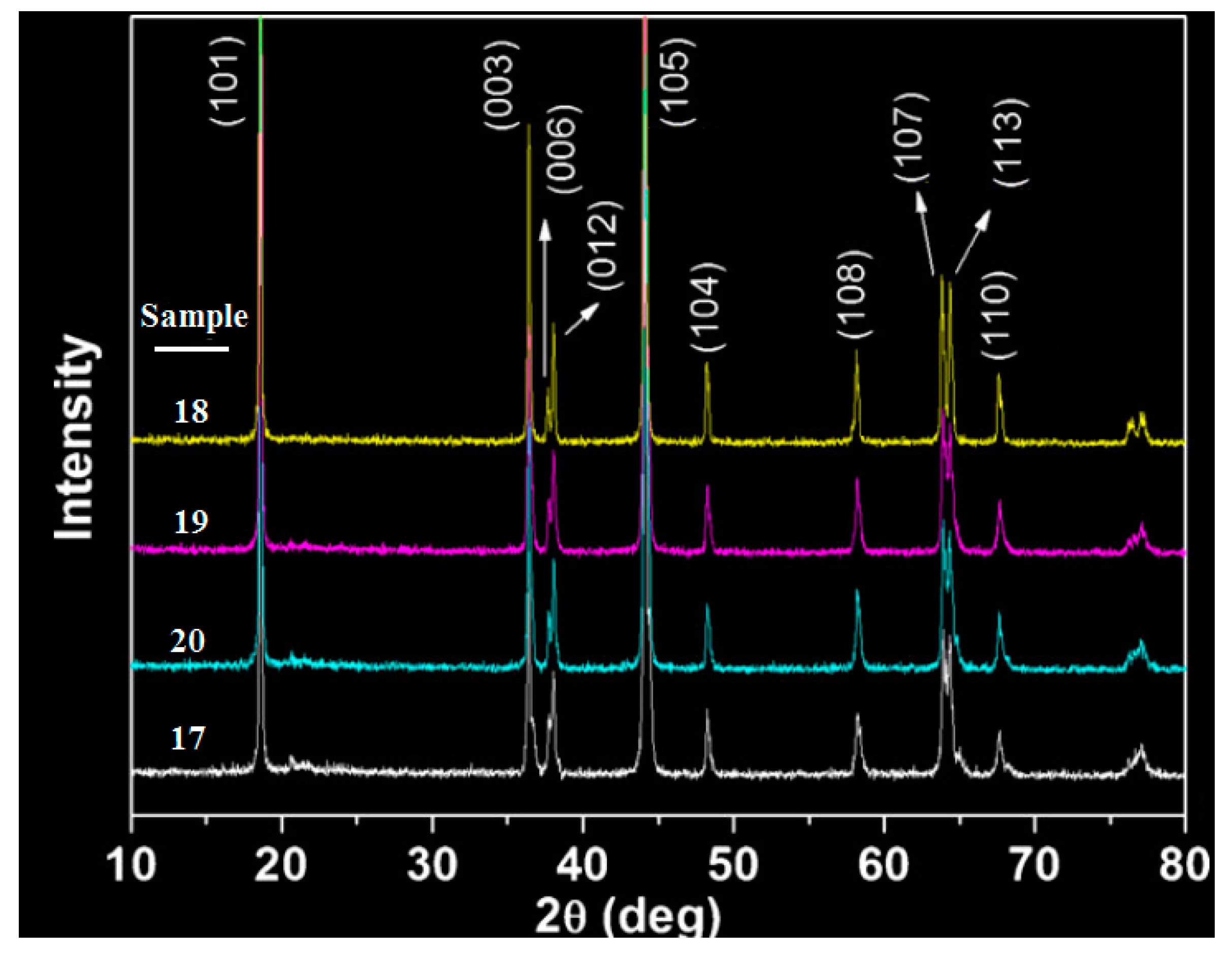

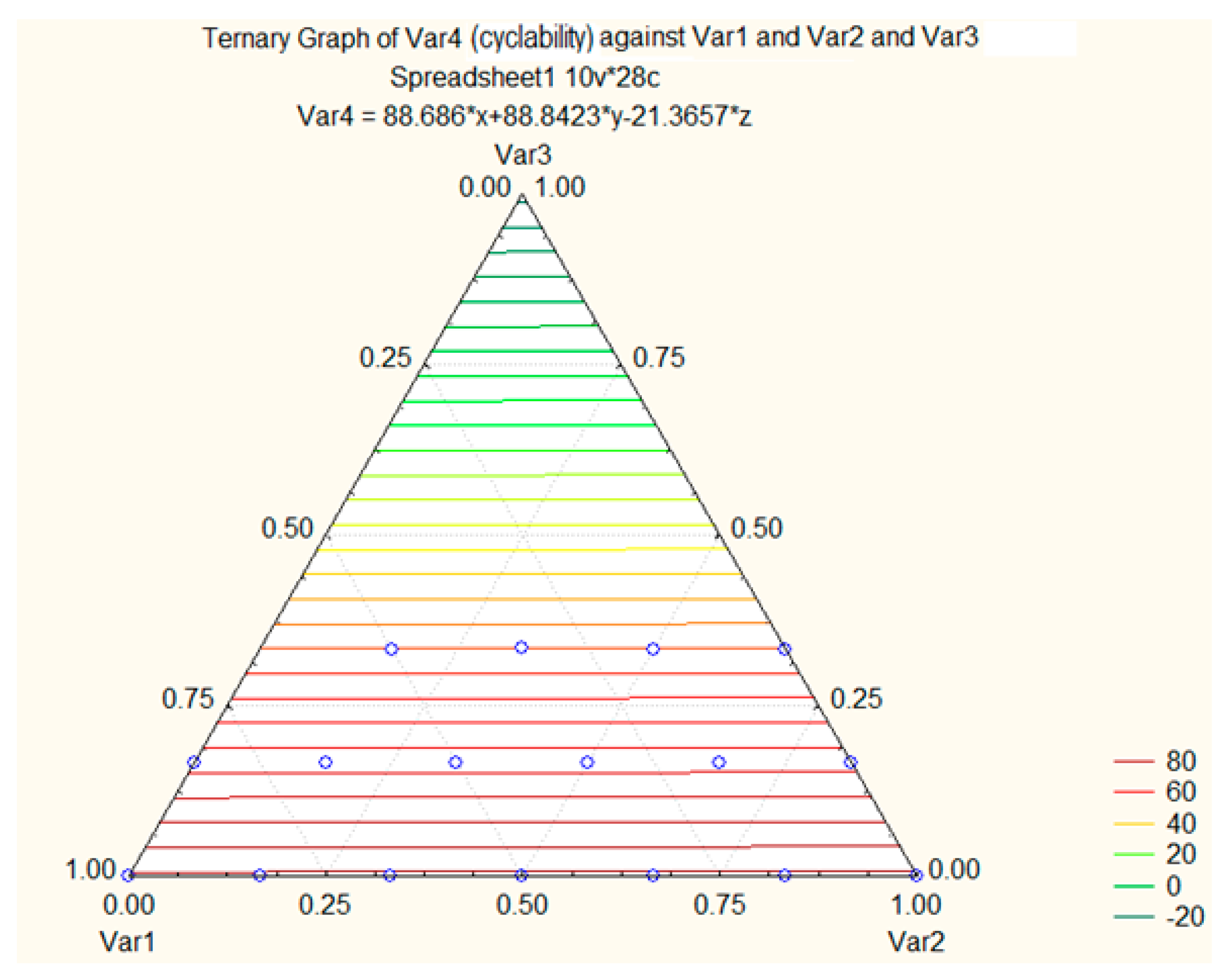
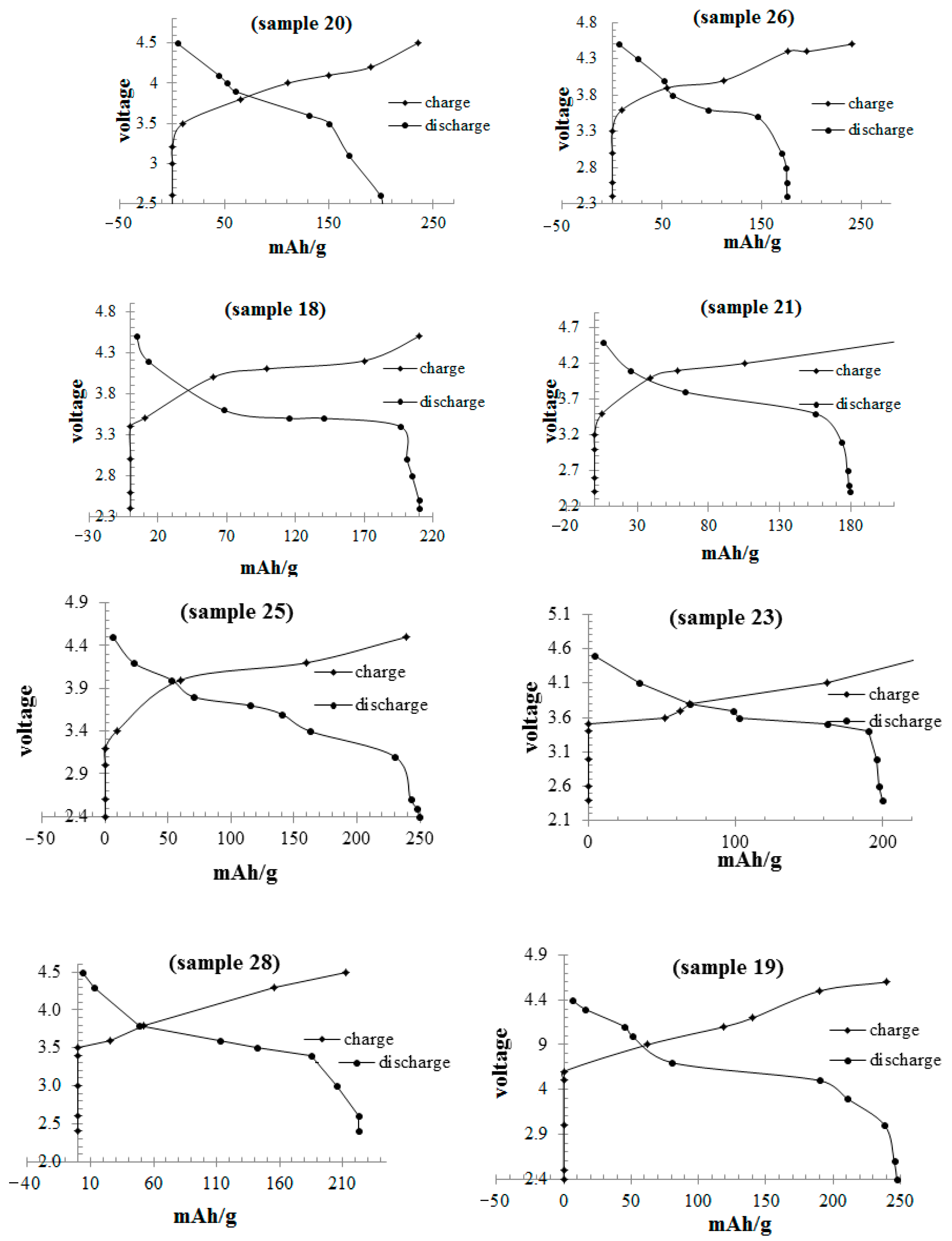
| Material | Potential vs. Li/Li+ | Capacity (mAh/g) | Energy (Wh/Kg) | Advantage | Disadvantage |
|---|---|---|---|---|---|
| LiCoO2 | 3.95 | 140 | 546 | Low | High |
| Cobalt, Nickel, Aluminum (CAN) | 3.85 | 182–202 | 684–765 | Slow reaction inside electrolyte High voltage and capacity Good performance | Expensive cost |
| Cobalt, Nickel, Manganese (CNM) | 3.86 | 162–175 | 615–655 | High capacity and operating voltages Slow reaction inside electrolyte Explosion | Expensive cost |
| LiMn2O4 (LMO) | 4.14 | 105–125 | 415–495 | Low safety due to oxygen release Suitable performance and voltage | Low cost |
| LiFePO4 (LFP) | 3.40 | 155–175 | 515–585 | Low voltage Low capacity | Low cost |
| Sample | Composition | Al Doped | Mg Doped |
|---|---|---|---|
| 1 | LiCu0.7Co0.3O2 | LiCu0.7Co0.2Al0.1O2 | LiCu0.6Mg0.1Co0.3O2 |
| 2 | Li1.167Cu0.583Co0.25Mn0.167O2 | Li1.167Cu0.583Co0.167Al0.083Mn0.167O2 | Li1.167Cu0.5Mg0.083Co0.25Mn0.167O2 |
| 3 | LiCu0.583Co0.417O2 | LiCu0.583Co0.334Al0.083O2 | LiCu0.5Mg0.083Co0.42O2 |
| 4 | Li1.333Cu0.467Co0.203Mn0.333O2 | Li1.333Cu0.467Co0.133Al0.067Mn0.333O2 | Li1.333Cu0.4Mg0.067Co0.2Mn0.333O2 |
| 5 | Li1.167Cu0.467Co0.366Mn0.167O2 | Li1.167Cu0.467Co0.299Al0.067Mn0.167O2 | Li1.167Cu0.4Mg0.067Co0.366Mn0.167O2 |
| 6 | LiCu0.467Co0.533O2 | LiCu0.467Co0.466Al0.067O2 | LiCu0.4Mg0.067Co0.533O2 |
| 7 | Li1.5Cu0.35Co0.15Mn0.5O2 | Li1.5Cu0.35Co0.1Al0.05Mn0.5O2 | Li1.5Cu0.3Mg0.05Co0.15Mn0.5O2 |
| 8 | Li1.333Cu0.35Co0.317Mn0.333O2 | Li1.333Cu0.35Co0.267Al0.05Mn0.333O2 | Li1.333Cu0.3Mg0.05Co0.317Mn0.333O2 |
| 9 | Li1.167Cu0.35Co0.483Mn0.167O2 | Li1.167Cu0.35Co0.433Al0.05Mn0.167O2 | Li1.167Cu0.3Mg0.05Co0.483Mn0.167O2 |
| 10 | Li Cu0.35Co0.6O2 | LiCu0.35Co0.6Al0.05O2 | LiCu0.3Mg0.05Co0.65O2 |
| 11 | Li1.667Cu0.233Co0.1Mn0.667O2 | Li1.667Cu0.233Co0.067Al0.033Mn0.667O2 | Li1.667Cu0.2Mg0.033Co0.1Mn0.667O2 |
| 12 | Li1.5Cu0.233Co0.267Mn0.5O2 | Li1.5Cu0.233Co0.234Al0.033Mn0.5O2 | Li1.5Cu0.2Mg0.033Co0.267Mn0.5O2 |
| 13 | Li1.333Cu0.233Co0.434Mn0.333O2 | Li1.333Cu0.233Co0.4Al0.033Mn0.333O2 | Li1.333Cu0.2Mg0.033Co0.434Mn0.333O2 |
| 14 | Li1.167Cu0.233Co0.6Mn0.167O2 | Li0.167Cu0.233Co0.567Al0.033Mn0.167O2 | Li1.167Cu0.2Mg0.033Co0.6Mn0.167O2 |
| 15 | LiCu0.233Co0.767O2 | LiCu0.233Co0.734Al0.033O2 | LiCu0.2Mg0.033Co0.767O2 |
| 16 | Li1.835Cu0.116Co0.049Mn0.835O2 | Li1.833Cu0.117Co0.033Al0.017Mn0.833O2 | Li1.835Cu0.1Mg0.017Co0.049Mn0.835O2 |
| 17 | Li1.667Cu0.116Co0.217Mn0.667O2 | Li1.667Cu0.117Co0.199Al0.017Mn0.667O2 | Li1.667Cu0.1Mg0.017Co0.217Mn0.667O2 |
| 18 | Li1.5 Cu0.116Co0.384Mn0.5O2 | Li1.5Cu0.117Co0.366Al0.017Mn0.5O2 | Li1.5Cu0.1Mg0.017Co0.384Mn0.5O2 |
| 19 | Li1.333Cu0.116Co0.551Mn0.333O2 | Li1.333Cu0.117Co0.533Al0.017Mn0.333O2 | Li1.333Cu0.1Mg0.017Co0.551Mn0.333O2 |
| 20 | Li1.167Cu0.116Co0.717Mn0.167O2 | Li1.167Cu0.117Co0.699Al0.017Mn0.167O2 | Li1.167Cu0.1Mg0.017Co0.717Mn0.167O2 |
| 21 | LiCu0.116Co0.884O2 | LiCu0.117Co0.866Al0.017O2 | LiCu0.1Mg0.017Co0.884O2 |
| 22 | Li2MnO2 | Li2MnO2 | Li2MnO2 |
| 23 | Li1.833Co0.167Mn0.833O2 | Li1.833Co0.167Mn0.833O2 | Li1.833Co0.167Mn0.833O2 |
| 24 | Li1.667Co0.333Mn0.667O2 | Li1.667Co0.333Mn0.667O2 | Li1.667Co0.333Mn0.667O2 |
| 25 | Li1.5Co0.5Mn0.5O2 | Li1.5Co0.5Mn0.5O2 | Li1.5Co0.5Mn0.5O2 |
| 26 | Li1.333 Co0.667Mn0.333O2 | Li1.333Co0.667Mn0.333O2 | Li1.333Co0.667Mn0.333O2 |
| 27 | Li1.167Co0.833Mn0.167O2 | Li1.167Co0.833Mn0.167O2 | Li1.167Co0.833Mn0.167O2 |
| 28 | LiCoO2 | LiCoO2 | LiCoO2 |
| Samples | Li2MnO3 | LiCoO2 | LiCu0.7Co0.3(Al Doped)]O2 | Blend | Capacity | Cyclability |
|---|---|---|---|---|---|---|
| 1- | 0 | 0 | 1 | Pure | 102.5 | 81 |
| 2- | 1/6 = 0.167 | 0 | 0.833 | Binary | 95.5 | 67 |
| 3- | 0 | 0.167 | 0.833 | Binary | 98.5 | 73 |
| 4- | 1/3 = 0.333 | 0 | 0.667 | Binary | 135.5 | 90 |
| 5- | 1/6 = 0.167 | 0.167 | 0.667 | Ternary | 130.5 | 82 |
| 6- | 0 | 0.333 | 0.667 | Binary | 96.5 | 71 |
| 7- | 1/2 = 0.500 | 0 | 0.500 | Binary | 152.5 | 75 |
| 8- | 1/3 = 0.333 | 0.167 | 0.500 | Ternary | 179.5 | 68 |
| 9- | 1/6 = 0.167 | 0.333 | 0.500 | Ternary | 156.5 | 83 |
| 10- | 0 | 0.500 | 0.500 | Binary | 171.5 | 99 |
| 11- | 2/3 = 0.667 | 0 | 0.333 | Binary | 102.6 | 95 |
| 12- | 1/2 = 0.500 | 0.167 | 0.333 | Ternary | 153.1 | 90 |
| 13- | 1/3 = 0.333 | 0.333 | 0.333 | Ternary | 173.4 | 78 |
| 14- | 1/6 = 0.167 | 0.500 | 0.333 | Ternary | 174.5 | 83 |
| 15- | 0 | 0.667 | 0.333 | Binary | 116.8 | 93 |
| 16- | 5/6 = 0.833 | 0 | 0.167 | Binary | 182.5 | 81 |
| 17- | 2/3 = 0.667 | 0.167 | 0.167 | Ternary | 173.6 | 95 |
| 18- | 1/2 = 0.500 | 0.333 | 0.167 | Ternary | 250.5 | 105 |
| 19- | 1/3 = 0.333 | 0.5 | 0.167 | Ternary | 137.5 | 93 |
| 20- | 1/6 = 0.167 | 0.667 | 0.167 | Ternary | 248.1 | 52 |
| 21- | 0 | 0.833 | 0.167 | Binary | 181.5 | 93 |
| 22- | 1 | 0 | 0 | Pure | 71.6 | 96 |
| 23- | 5/6 = 0.833 | 0.167 | 0 | Binary | 181.5 | 91 |
| 24- | 2/3 = 0.667 | 0.333 | 0 | Binary | 174.2 | 95 |
| 25- | 1/2 = 0.500 | 0.500 | 0 | Binary | 193.5 | 92 |
| 26- | 1/3 = 0.333 | 0.667 | 0 | Binary | 192.5 | 58 |
| 27- | 1/6 = 0.167 | 0.833 | 0 | Binary | 235.5 | 98 |
| 28- | 0 | 1 | 0 | Pure | 250.2 | 99 |
| Sample | a (nm) | b (nm) | C (nm) | Capacity | Cyclability |
|---|---|---|---|---|---|
| 17 (Ternary) | 0.2215 | 0.2432 | 1.012 | 171.5 | 85 |
| 18 (Ternary) | 0.2435 | 0.2546 | 1.093 | 250.5 | 105 |
| 19 (Ternary) | 0.2923 | 0.2612 | 1.048 | 220.2 | 95 |
| 20 (Ternary) | 0.3041 | 0.2883 | 1.422 | 185.5 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahriari, S.; Mollaamin, F.; Monajjemi, M. Increasing the Performance of {[(1-x-y) LiCo0.3Cu0.7] (Al and Mg doped)] O2}, xLi2MnO3, yLiCoO2 Composites as Cathode Material in Lithium-Ion Battery: Synthesis and Characterization. Micromachines 2023, 14, 241. https://doi.org/10.3390/mi14020241
Shahriari S, Mollaamin F, Monajjemi M. Increasing the Performance of {[(1-x-y) LiCo0.3Cu0.7] (Al and Mg doped)] O2}, xLi2MnO3, yLiCoO2 Composites as Cathode Material in Lithium-Ion Battery: Synthesis and Characterization. Micromachines. 2023; 14(2):241. https://doi.org/10.3390/mi14020241
Chicago/Turabian StyleShahriari, Sara, Fatemeh Mollaamin, and Majid Monajjemi. 2023. "Increasing the Performance of {[(1-x-y) LiCo0.3Cu0.7] (Al and Mg doped)] O2}, xLi2MnO3, yLiCoO2 Composites as Cathode Material in Lithium-Ion Battery: Synthesis and Characterization" Micromachines 14, no. 2: 241. https://doi.org/10.3390/mi14020241
APA StyleShahriari, S., Mollaamin, F., & Monajjemi, M. (2023). Increasing the Performance of {[(1-x-y) LiCo0.3Cu0.7] (Al and Mg doped)] O2}, xLi2MnO3, yLiCoO2 Composites as Cathode Material in Lithium-Ion Battery: Synthesis and Characterization. Micromachines, 14(2), 241. https://doi.org/10.3390/mi14020241







