1. Introduction
The world population is projected to grow to around 10 billion in 2050. This leaves us with a future food mi, demanding an increase in food production of up to 56% by 2050 [
1]. However, environmental stresses such as extreme weather events or agricultural malpractices, including fertiliser overuse and soil tilling, cause significant losses in agriculture [
2]. In particular, soil degradation is estimated to cause economic losses that reach GBP 1.2 billion per year in the UK only [
3]. As such, a rising population as well as the changing climate require new solutions for agriculture that offer growth in a sustainable way. By detecting the physiological stress of plants early on, production losses can be minimised through the implementation of preventative measures against the stress source [
4]. To date, several technologies that detect plant stressors have been developed. Some examples of non-invasive devices for analysing plant health include proximal optical sensors to monitor nitrogen, imaging methods to detect plant diseases, as well as smartphone-based methods [
5].
Recently, some imaging approaches have been reported for the determination of H
2O
2 in plants using implanted carbon nanotubes and measuring their infrared absorption [
6]. However, this approach is complex, since it requires plant leaf infiltration with carbon nanotubes. Moreover, although a release of hydrogen peroxide has been observed in plants subjected to biotic and abiotic stress, this biomarker is not selective towards the source of stress [
7], and its detection requires expensive equipment for the measurement of infrared (IR) reflectance, limiting the applicability of this technology within real-world settings. Recent studies have also explored electrochemical methods, employing printed electronics and flexible materials such as silver/reduced graphene nanocomposites for the determination of toxic gases [
8]. Although electrochemical-based methods are less expensive compared with traditional optical approaches and they reduce the needs for expensive equipment, the sensing performance, in terms of sensitivity and selectivity, tends to be relatively low [
8]. Li et al. [
9] developed a real-time electrochemical sensor for the detection of leaf volatiles using a carbon nanotube (CNT)/graphite composite. The final device could quantify the concentrations of organic volatiles, such as methyl jasmonate, within 20 s. However, the device showed significant interference from other common metabolites, such as 1-hexenal and 2-phenylethanol, increasing the complexity of the data analysis, and the limit of detection was relatively high in the range of 5 ppm.
Another approach to monitor plant health that is becoming increasingly popular is remote sensing for the detection or prediction of plant diseases in crop fields [
10]. Typically, unmanned aerial vehicles (UAVs) are used to facilitate aerial imaging with cameras and sensors. These include the less expensive red, green, blue (RGB) detectors, multi- or hyper-spectral cameras, or thermal cameras capable of detecting stress-induced changes in plant metabolism. These technologies are becoming cheaper and more sophisticated, but are still far from affordable, in particular for small-scale farmers. Background noise and varying field conditions or symptom phenotypes present further challenges to application of these sensors. In addition, some applicability of electrophysiological monitoring for water stress could be demonstrated [
11]. However, most of these techniques using optical mechanisms cannot detect external biotic and abiotic stresses of individual plants. Moreover, there is often a lack of high temporal resolution, hindering the prompt and effective reaction to plant stresses.
The limitations observed in electrochemical and optical sensors could potentially be addressed by the combination of multiple low-cost devices in the form of electronic noses. Electronic noses, or e-noses, are devices capable of sensing odours by mimicking the human olfaction system. These systems incorporate multiple sensors with varying selectivities and sensitivities, which can be used to profile complex gas mixtures. In this case, gases react with each sensor contained in the e-nose to trigger a distinct signal change. In typical metal-oxide based sensors, the increase in gas concentrations leads to a change in electrical resistance, which can be determined by the output voltage. However, electronic noses often require complex circuit assembly, involving metal oxides with heating elements, and printed circuit boards, and often, they cannot be easily modified for the tailored detection of new biomarkers. As such, despite the wide range of commercially available sensing devices, the flexibility of such systems to be adapted for specific applications such as plant stress monitoring is limited. Furthermore, complex manufacturing processes lead to the relatively high costs of these devices, in the range of USD 200 [
12], and they generally require specialised personnel to assemble the devices and analyse the results.
The human olfactory system can discriminate trillions of odours with only hundreds of olfactory receptors by representing them as a combinatorial code [
13]. An emergent technology related to e-noses was developed by Suslick et al. [
14] and provides an alternative to current devices. These are optoelectronic noses that produce a unique pattern of colorimetric changes in response to a given analyte (
Figure 1).
Optoelectronic noses make use of multiple chemically responsive dyes such as metalloproteins or Reichardt reagents that induce a colour change upon their exposure to certain volatiles. These dyes can be further modified through the incorporation of reactive chemical species, such as silver nitrate, to change the mechanism of action and selectivity [
15]. Consequently, exposure to a particular volatile leads to a set of colour changes on the multiplexed sensor, and these can subsequently be analysed and classified as a specific colour fingerprint. This method results in high sensitivity and good discrimination between similar analytes. Moreover, it offers a low-cost and simple solution to the detection of a wide range of compounds. Thus far, applications include the detection of pesticides, explosives, or the early diagnosis of lung cancer [
16,
17]. However, their application in the prevention of crop losses has been limited. Recently, Li et al. [
5] developed an optoelectronic nose based on gold nanoparticles for the detection of Phytophora infestans infections on tomato plants. This device could diagnose tomato leaves within 1 day of inoculation by quantifying the amounts of 2-hexen-1-al. Although this device showed a high sensitivity, it required functionalised thin gold nanorods (around 20 nm), increasing the price of the final setup, and the sensors were only tested on individual leaves instead of whole plants.
As mentioned, one of the key limitations of optoelectronic noses is the high cost, resulting from the use of sometimes expensive dyes and spectrophotometric meters for analysing the results [
18]. This work reports a low-cost optoelectronic nose as an alternative to current approaches in the study of plant responses to abiotic stress. These sensors can detect a variety of specific gas analytes based on colorimetric changes caused by interactions between chromophores in a dye with the analyte. The devices can be easily fabricated by drop casting the selective dyes mixtures onto filter paper. We focused on the detection of volatile organic compounds (VOC), such as 2-hexenal, which are known to be released from soil or plant leaves under certain conditions of biotic or abiotic stress [
19]. Initially, a systematic study on how different reactive dyes can be combined to enhance the sensitivity towards certain analytes was conducted. Common chromophores, including methyl red or Reichardt’s dye, were deposited onto porous filter paper using different solvents. The impact of the solvent on the sensing performance of the sensors was determined. Moreover, the selectivity of the sensor was enhanced by combination of the solution with molecular pores, such as cyclodextrin, and optically active materials, such as graphene quantum dots. Finally, the VOC profiles of
Marchantia polymorpha, a model liverwort plant widely used in research, were screened to inform us about their health status. Two different abiotic stress sources were tested, i.e., nutrient deficiency and high salinity, and the changes in volatile profiles in each case were determined. The final devices were able to determine the stress response within 1 day of exposure to each stress source, and the price of each device was as low as GBP 1. Finally, to allow a standardisation of measurements, we developed a spectrometer case and incorporated a live display of colour data.
2. Materials and Methods
2.1. Materials
All compounds and reagents were purchased from Sigma Aldrich unless otherwise specified; silver nitrate, cyclodextrin, methyl red, acetone, ethanol, 2-hexen-1-al, acetic acid, blue graphene quantum dots, bromocresol green, phenyl red, Reichardt’s dye, nickel(III) phthalocyanine tetrasulfonic acid tetrasodium salt, and MnTBAP chloride. The Gamborg’s growth medium used was Gamborg’s B-5 Basal Medium with minimal organics, and was purchased from Sigma-Aldrich. Filter paper was purchased from Amersham Protran. Components for the spectrometer include a rotor (Small Reduction Stepper Motor - 5VDC 32-Step 1/16 Gearing, from The Pi Hut), an LED ring (16-pixel RGB LED ring Rainbow FC-102 for Arduino), and the Wio Terminal (from Seeed Studio).
2.2. Fabrication of Sensors and Deposition of Dyes
Solutions containing multiple colorimetric dyes with proven performance for gas detection, as well as combinations of dyes with different solvents and matrices, were tested. In each case, the dye solutions were prepared by mixing 5 mM of each dye in 1.5 mL Eppendorf tubes. To lower manufacturing costs, disposable sensors were produced by deposition of multiple dyes onto filter paper by drop casting. Filter paper was cut into circular pieces with a diameter of about 5.5 cm and the space was divided into 8 sections of equal size. On each of the 8 sections, 20 µL of dye was drop-casted, which formed a coloured spot of about 1–2 cm diameter. The manufacturing process of these sensors was fast and took less than one minute per sensor paper. To ensure low interference from solvent residues, each multiplexed sensing paper was left to dry for at least 4 h. A list of reactive dye combinations used in this study is provided in
Figure 2. These dyes were combined with different solvents, as well as analyte-binding compounds such as cyclodextrin (CD), to tune the selectivity of the reactions.
2.3. Testing of Sensitive Dye Response towards Different Gases
Initially, to demonstrate the selective reactivity of the sensing dyes in the presence of different volatiles, a series of tests were conducted with the following gases: water vapour, ethanol, acetic acid, and acetone. This experiment allowed us to determine potential interference due to water in our experiments, since it is ubiquitously present in all environments, and the changes in sensitivity of each dye towards different chemical functionalities. Moreover, the chosen organic volatiles (acetone, acetic acid, and ethanol) allowed us to study the impact of different functional groups on the response of the final devices. In each case, the sensing papers were subjected to 10 ppm of each gas inside a sealed pyrex borosilicate bottle, and the changes in colour were determined by a low-cost TCS34725 (Adafruit)-based spectrometer after 2 h.
After conducting this initial test using simple organic volatiles and water, the sensors were tested in the presence of trans-2-hexen-1-al, indole-3-acetic acid (auxin), and tryptophol. These molecules are involved in the growth and stress response of plants. In particular, trans-2-hexen-1-al is a volatile organic compound (VOC) emitted by wounded or stressed plants, indole-3-acetic acid is the most common naturally occurring phytohormone of the auxin class, and tryptophol is a quorum sensing molecule (QSM) found during fungal infections. We tested the sensitivity of the dyes in the presence of 10 ppm of each gas separately. The exact values were calculated using the following formula (1):
where
c denotes concentration in ppm,
p denotes purity,
d denotes density in g/mL,
V1 denotes the volume injected in µL, M denotes molecular weight, and
V2 denotes the volume in the bottle in L. The colorimetric sensor arrays were left in sealed 1 L glass bottles for about 2 h to react with the gases. Color changes in the drop-casted dyes were then determined. A simple and low-cost RGB colour sensor (Adafruit TCS34725) was used, connected to an Arduino board for data collection. This sensor component also had an in-built white LED next to its RGB sensor. The TCS34725 contained a 3 × 4 photodiode array, consisting of red-filtered, green-filtered, blue-filtered, and clear (unfiltered) photodiodes. This RGB sensor quantifies the level of reflected light from the white LED source and measures the following wavelengths: 465 nm (blue), 525 nm (green), and 615 nm (red).
Connections to the colour sensor were soldered to allow for stable electrical contacts and to hold the components firmly in place. During the measurement, a paper sensor was placed onto the colour sensor, so that the LED of the colour sensor evenly illuminated the sample. RGB values for each coloured spot on the filter paper were recorded. The measurements were recorded using the microcontroller Wio Terminal, which could potentially be used to automate the whole data extraction and calculation process.
2.4. Dye Screening
A total of 19 dye mixtures were tested in this work. The composition of each mixture can be found in
Figure 2. To select the most successful dyes, the data analysis method proposed by Li et al. was adopted [
5]. In this case, the Euclidean distance of all colour measurements (each dye separately) from the control was calculated. These were compared to control sensing papers that had not been exposed to any of the gases. The Euclidean distance was determined as the distance between two points in three-dimensional colour space. Given two points, where R =
x, G =
y, and B =
z (test RGB values = (
x1,
y1,
z1) and control RGB values = (
x0,
y0,
z0)), the distance (
d) between them could be calculated as (2):
To screen for dyes that were most responsive towards each analyte, the average response of the sensors towards all tested gases was first calculated. Individual gases measurements were then subtracted from this average and plotted. This graph allowed us to select dye combinations that produced higher absolute signals after exposure to each analyte. Dyes which showed the largest colour changes were chosen to maximise sensitivity. The three dyes with the most sensitive response to trans-2-hexen-1-al were selected, as well those with the best performance for each of the other gases except for water vapour. The final sensing papers used in the biological studies comprised a total of 8 dyes. Water vapour-sensitive dyes were excluded to avoid unnecessary cross-talk, since it is present at varying levels in most tested environments, and alone provides little information about plant stress responses.
The optimised sensor papers were manufactured and tested in triplicate by exposure to each of the gases (acetic acid, acetone, ethanol, 2-hexen-1-al, indole-3-acetic acid, and tryptophol). After each experiment, 8 different Euclidean distance measurements were obtained. Given the high dimensionality of the data obtained, different gas volatile profiles could not be easily classified. To study the capability of our device to differentiate the studied gases, principal component analysis (PCA) was used. The analysis software BioVinci 2.0 was employed, allowing a reduction in data into two dimensions for the visualisation of plant subgroups. Moreover, the response of the sensing strips subjected to different concentrations of trans-2-hexen-1-al was tested. This allowed the determination of the detection limit of our sensors, and to also potentially quantify the gas concentrations over time during the in vivo experiments. In this case, the sensors were tested by exposure to analyte concentrations of 0.5, 1, 2.5, 5, 10, and 25 ppm. Similar to the previous test, the sensors were left for about 2 h to react with the gas in each case.
2.5. Testing In Vivo Using Plants
Finally, the performance of paper sensors was tested in vivo using living plants. Marchantia polymorpha was chosen as the model plant, since they are easy and fast to grow, as well as to allow for simple testing procedures. Wild-type Marchantia polymorpha accessions Cam-1 (male) and Cam-2 (female) were used in the experiments. Gemmae and thallus cuttings of plants were grown and maintained in 0.5× Gamborg’s media (Gamborg’s B5 medium plus vitamins, Duchefa Biochemie G0210, pH 5.8) and 1.2% (w/v) agar (Melford capsules, A20021) at a continuous temperature of 21 °C under continuous light (light intensity of 150 μmol/m²/s). Plates containing 100 μg/mL of cefotaxime were used in the earliest two batches of tissue culture to prevent bacterial and fungal contamination.
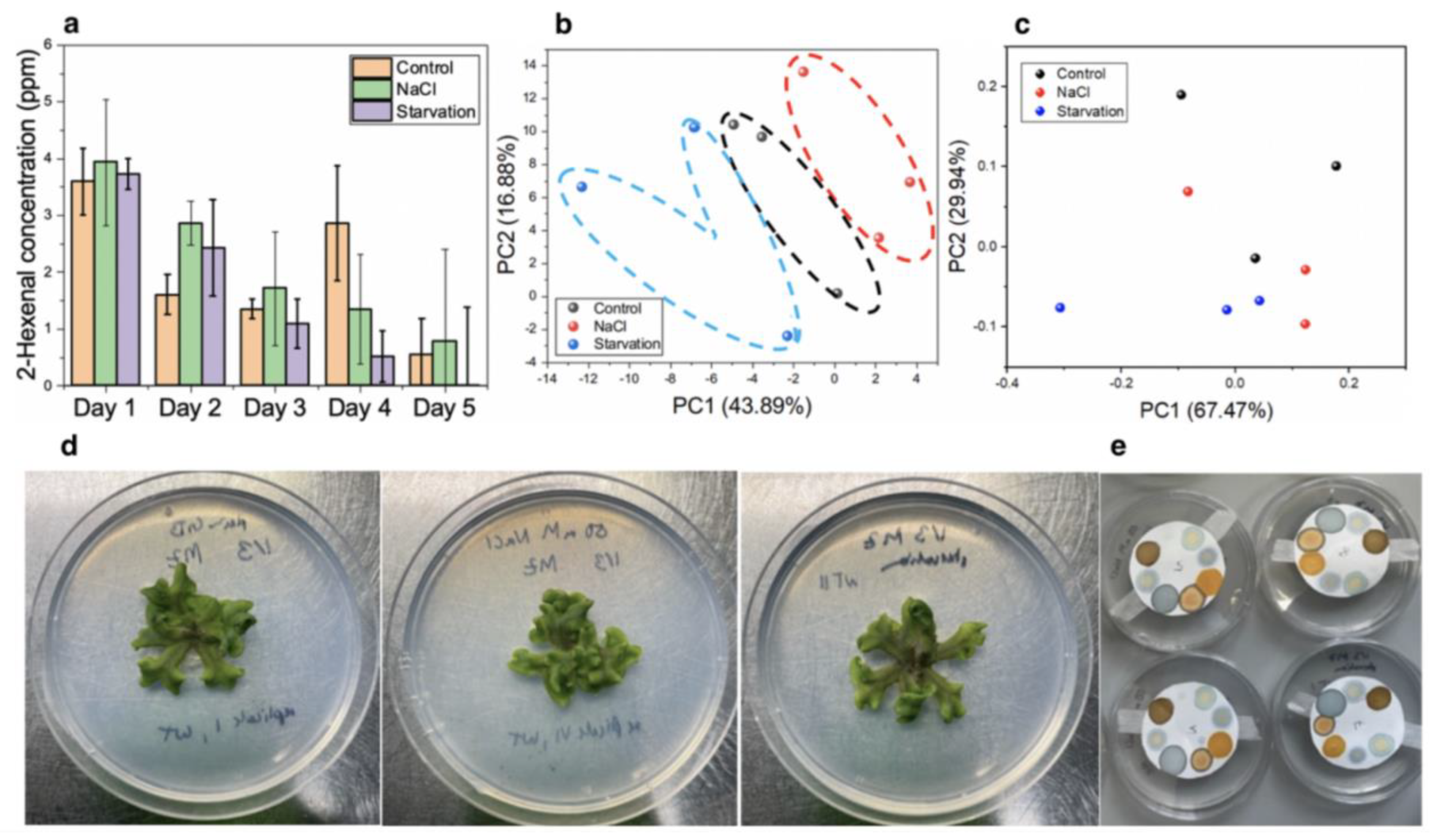
Wild-type gemmae, with an age of about two weeks, were used in each case. The plants were tested in three different growth conditions: control, high salinity, and nutrient starvation. For the control measurements, Gamborg’s medium was used, which is a standard medium for in vitro culture of plants and consists of a nutrient blend of inorganic salts, vitamins, and carbohydrates. To induce salt stress, 50 mM NaCl was added to the standard GB medium. This concentration is high enough to stress the plant but is not lethal [
20]. For the starvation medium, the standard GB medium was diluted to 1/100 of its original concentration in RO water. For each condition, triplicates of the plants and the respective growth medium were made. For test measurements of volatiles produced in plants, sensing strips were attached to the inner side of Petri dish lids and closed for at least 1 h. The sensors were then removed and the colorimetric changes induced by their exposure to the plant volatiles were measured using the TCS34725-based spectrometer. The experiment was run over the course of 5 days to study the dynamical changes in volatile profiles. Further, pictures of the plants were also taken each day to compare the visual differences between the growth conditions.
2.6. Measuring Stress in Plants Using Electronic Noses for Comparison
For direct comparison of our results with electronic noses, in vivo sensing experiments were conducted using a combination of commercially available gas sensors with the
Marchantia polymorpha model. A custom-made e-nose was developed containing MQ4, MQ7, and MQ131 (metal oxide sensors); Bosch BME680 (which contains multiple sensors to measure humidity, temperature, pressure, and total VOCs); and Sensiron SCD30 (a near infrared spectrometer-based sensor) to measure CO
2. Concentrations of the target gases were calculated according to the manufacturer’s calibration. Sensors were pre-conditioned for 1 h prior the experiments in open air, or until a stable signal was reached. The sensors were connected to an Arduino UNO microcontroller board and assembled inside a plastic box, where tubes were connected through a plastic septum to avoid gas leaks. Moreover, to allow a direct quantification in Petri dishes, as conducted in the case of optoelectronic noses, the Petri dishes were modified to allow the connection of an air pump to drive the gas towards the electronic nose (
Figure 3). A tube was also incorporated to connect the Petri dish with the electronic nose. Finally, Petri dishes containing growing Marchantia plants were sealed, and measurements were taken for at least 1 h, as in the case of the optoelectronic noses.
2.7. Development of Low-Cost Spectrometer for Standardised Measurements
To facilitate regular measurements of the colorimetric sensing paper, a portable spectrometer was fabricated, comprising a 3D-printed case with sliding lids, a rotor to move the dye samples, and a display to show live colour results. The case enabled the establishment of standardised conditions for measurement, including background light intensity, since the sample needs to be in darkness during each measurement. Moreover, sliding lids were designed to allow for an easy exchange of samples (
Figure 4).
The 3D model of the case was obtained using the free modelling software SketchUp, and printed using an Ultimaker S3 printer with PLA filament. The case contained a holder for a rotor (Small Reduction Stepper Motor - 5VDC 32- Step 1/16 Gearing, from The Pi Hut) and a circular LED (16-pixel RGB LED ring Rainbow FC-102 for Arduino) to indicate the measurement status. A rotating disc plate to support the sensor papers was also printed. The dimensions of the disc fit the size of the measurement sensor paper, with a diameter of 5.5 cm. The rotor was programmed such that the disc rotated to one of the 8 evenly spread samples, 10 measurements were taken by the colour sensor, and then this process was repeated until all 8 samples were measured. Then, another paper sensor can be placed into the device for further measurements. A ring-shaped display of multiple RGB LEDs was used to indicate the measurement status of the spectrometer. For better user-friendliness and a live analysis of results, a Wio terminal with a 320x240 pixels LCD display was programmed to show sensor data in real time. The Wio terminal shows RGB and HEX values from the colour sensor as numerical values as well as RGB values in a graph. This device can potentially send data wirelessly to an online server for IoT applications.
4. Conclusions
In this study, the selection of multiple chemo-responsive dyes and their application in an optoelectronic nose for non-invasive analysis of plant responses to stress has been demonstrated. Multiple dyes were screened to select those with optimal colorimetric responses to volatile biomarkers for abiotic stress, such as 2-hexen-1-al. Up to eight different dyes were examined for selective responses towards simple volatiles, such as acetic acid, acetone, and ethanol; indole molecules involved in the growth and fungal infections of plants, including auxins and tryptophol; and hexenal. A strong advantage of this approach is the flexibility of the devices, since the sensing dyes can be easily modified to change the selectivity towards different gases. Moreover, the optoelectronic nose developed as part of this work was non-invasive, it could be incorporated directly into Petri dishes without requiring modification, and it achieved a resolution high enough to monitor individual plants. This approach led to a significant improvement in cost compared to previously reported work, with an estimated cost of GBP 1 per strip, which is orders of magnitude lower than commercial e-noses. The simplicity of our device included ease of operability, only requiring a TCS34725 device for the data analysis. Moreover, some of the gases could be qualitatively evaluated by the naked eye given the changes in colour of the paper strips. By processing these measurements with a pattern classification algorithm such as PCA, the device enabled the identification of multiple volatiles, such as indoles and trans-2-hexen-1-al, showing a limit of detection on the sub-ppm level, one of the lowest values ever reported for an optoelectronic nose.
Finally, our sensors were tested in vivo using Marchantia polymorpha as a model plant for abiotic stress response. Our optoelectronic nose was able to differentiate between plants undergoing abiotic stress from healthy plants as early as two days after exposure to the stressors, even prior to the manifestations of symptoms on the plant morphology or colouration.
One limitation of our measurement process is that the RGB colour sensor is only able to detect a homogeneous colour sample, not allowing the study of absorption gradients with the sensors. Moreover, the sensor papers cannot be reused, so for every measurement new sensors have to be manufactured. Further developments could include the optimisation of the sensor paper pore size and an automated classification of gases, for which a machine learning algorithm can be implemented. A simple algorithm such as the K-nearest neighbour (KNN) classification algorithm would be sufficient to achieve this goal. As such, this work reports for the first time a low-cost and highly sensitive paper-based optoelectronic nose for the early detection and monitoring of plant stress. This device has a plethora of applications in environmental analysis and agriculture, among others, and in particular, this approach could contribute to tackle food losses from wheat, corn, and rice, which are at high risk due to climate change.