The Effect of Acetylene Carbon Black (ACB) Loaded on Polyacrylonitrile (PAN) Nanofiber Membrane Electrolyte for DSSC Applications
Abstract
:1. Introduction
2. Materials and Methods
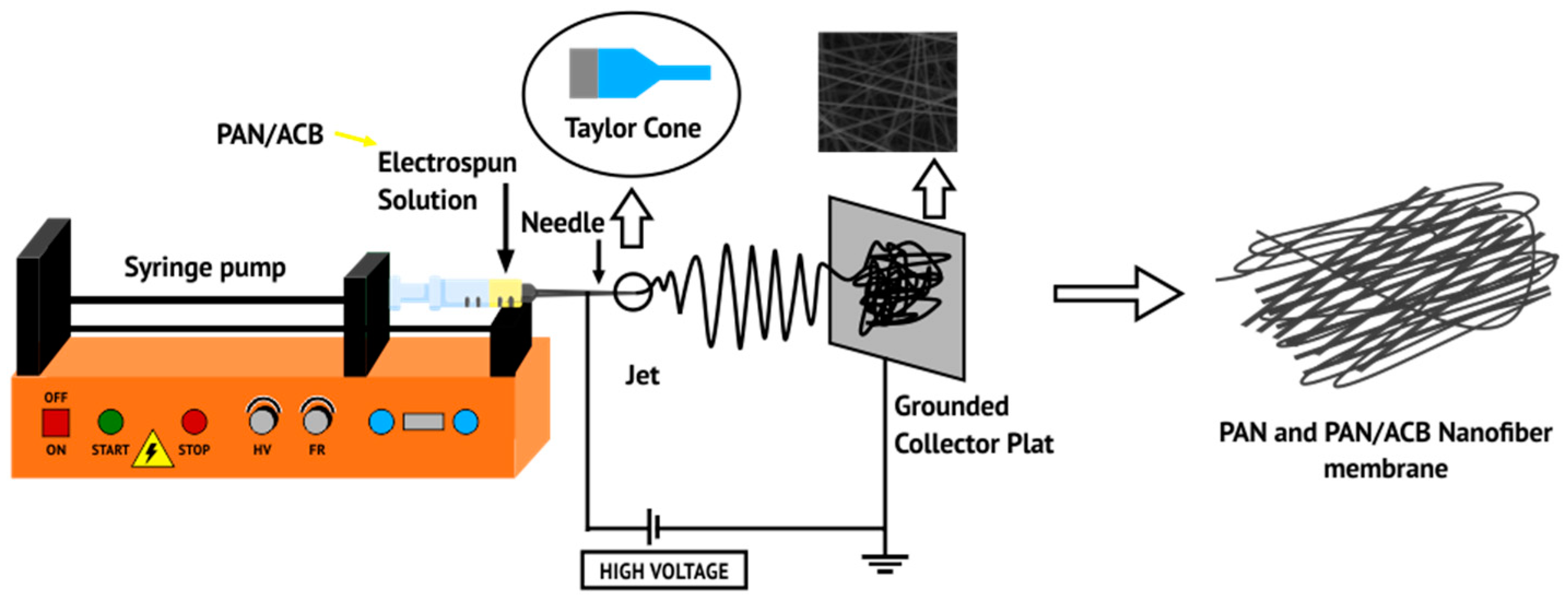
2.1. Synthesis of PAN Membranes and PAN/ACB Nanofiber Composite
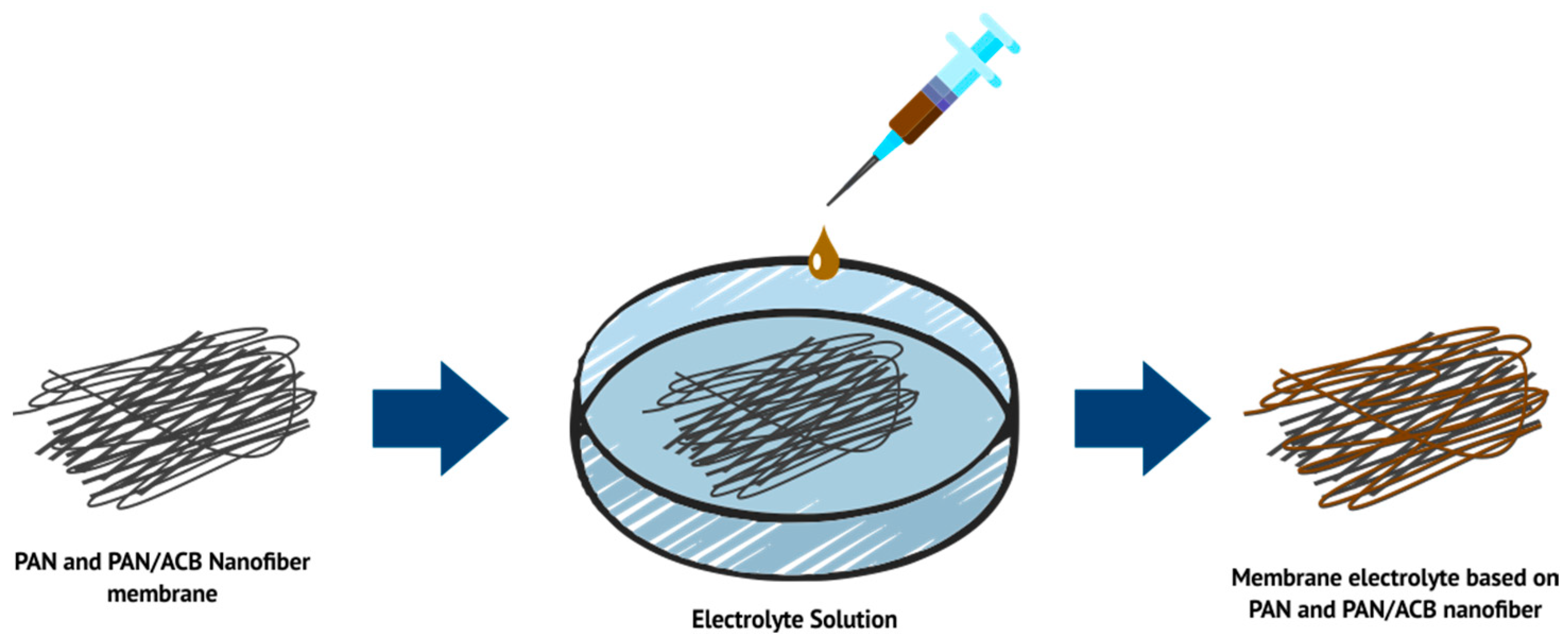
2.2. Preparation of PAN-Based Electrolyte Membranes and PAN/ACB Nanofiber Composites
2.3. DSSC Synthesis and Fabrication
2.4. Characterization
3. Results
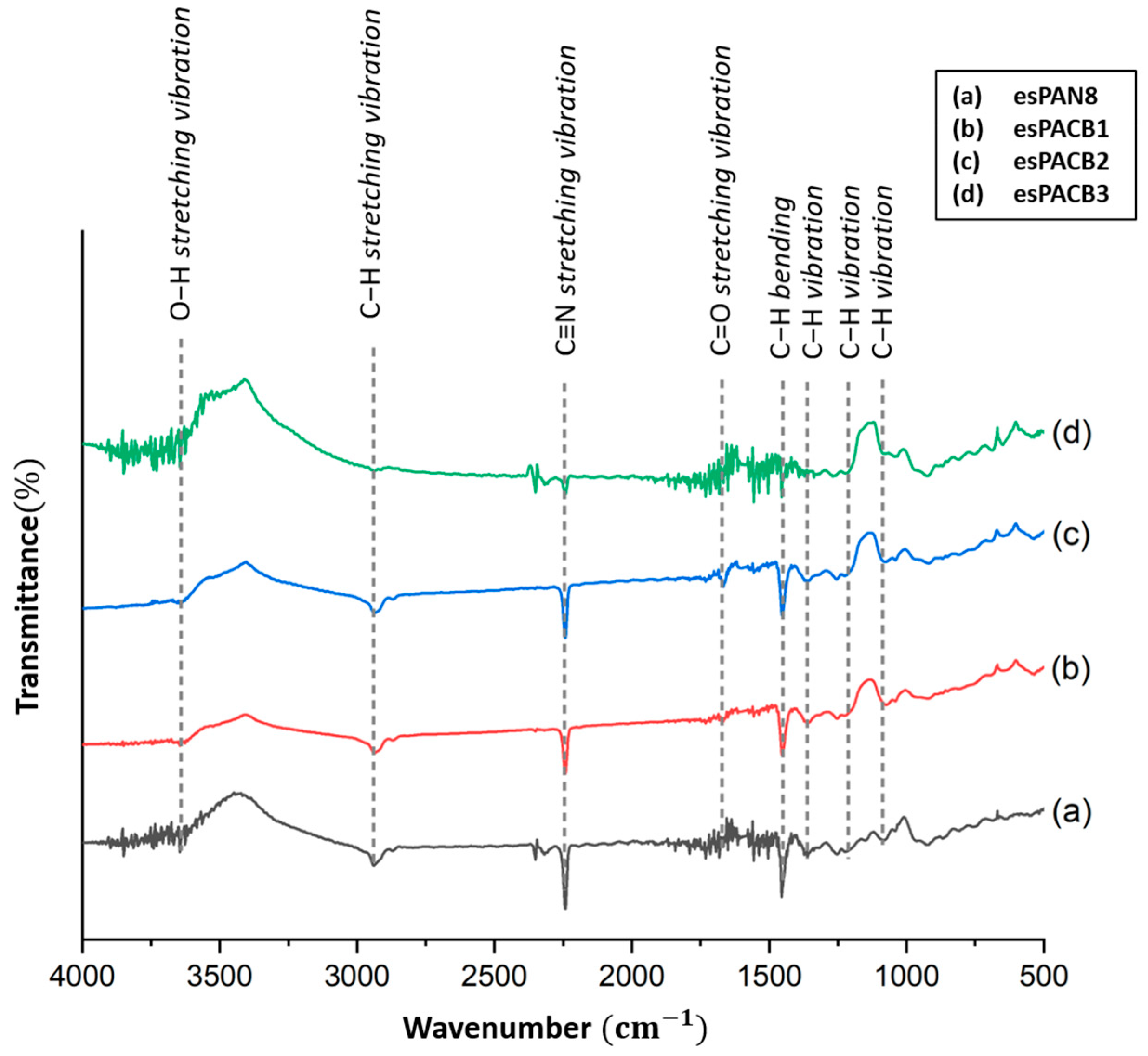
3.1. FT-IR (Fourier Transform Infra-Red)
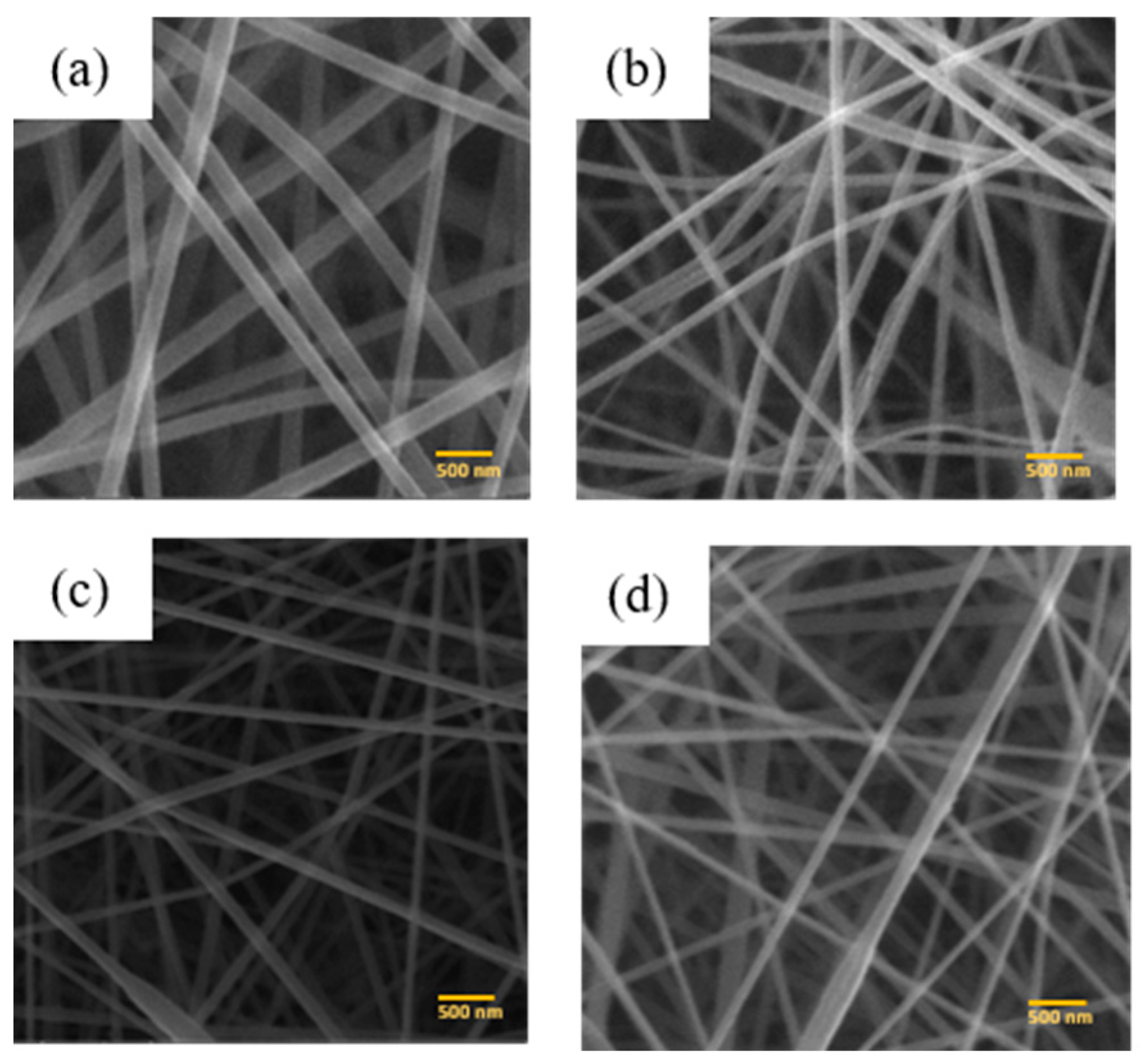
3.2. SEM Image
3.3. Electrolyte Absorption
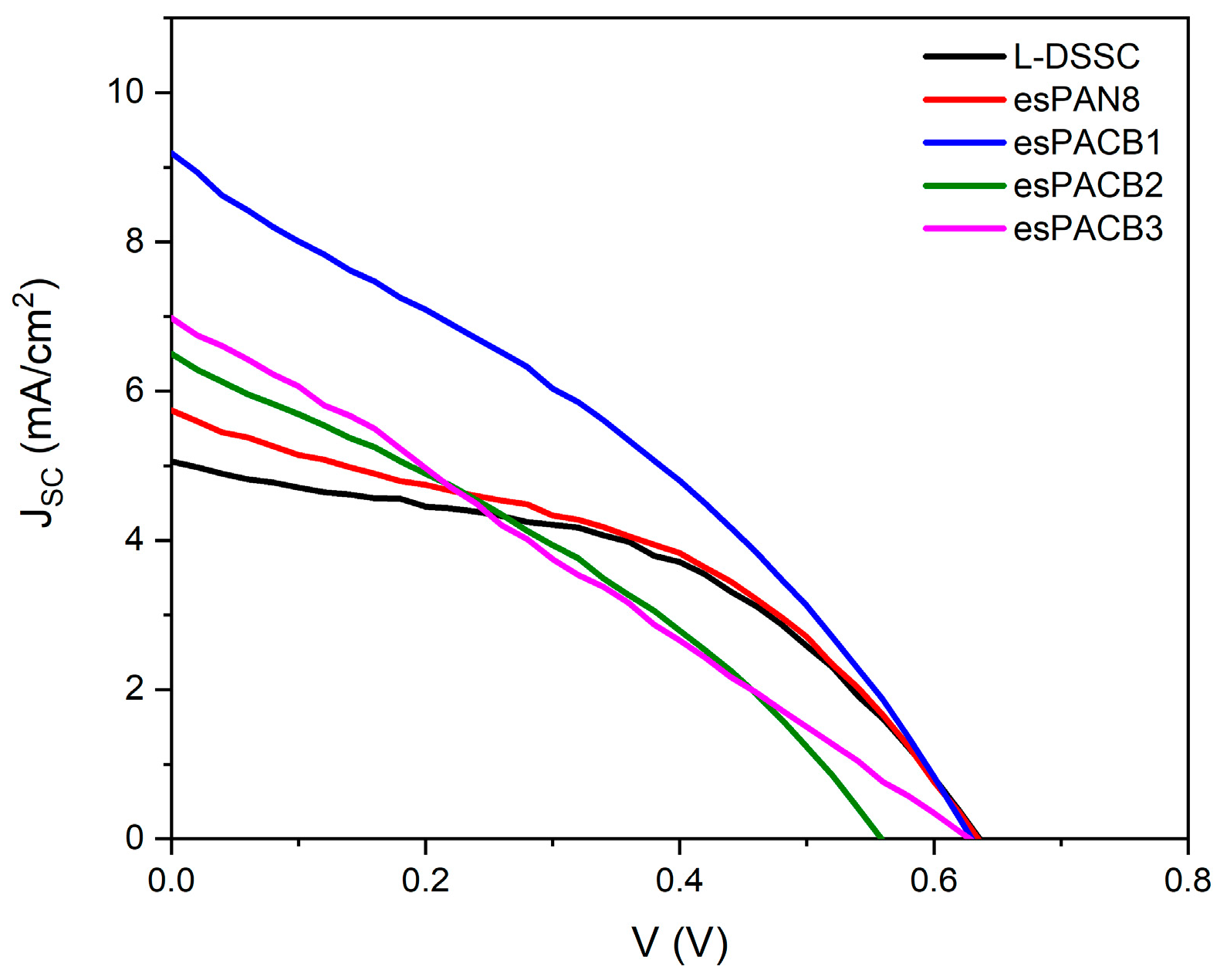
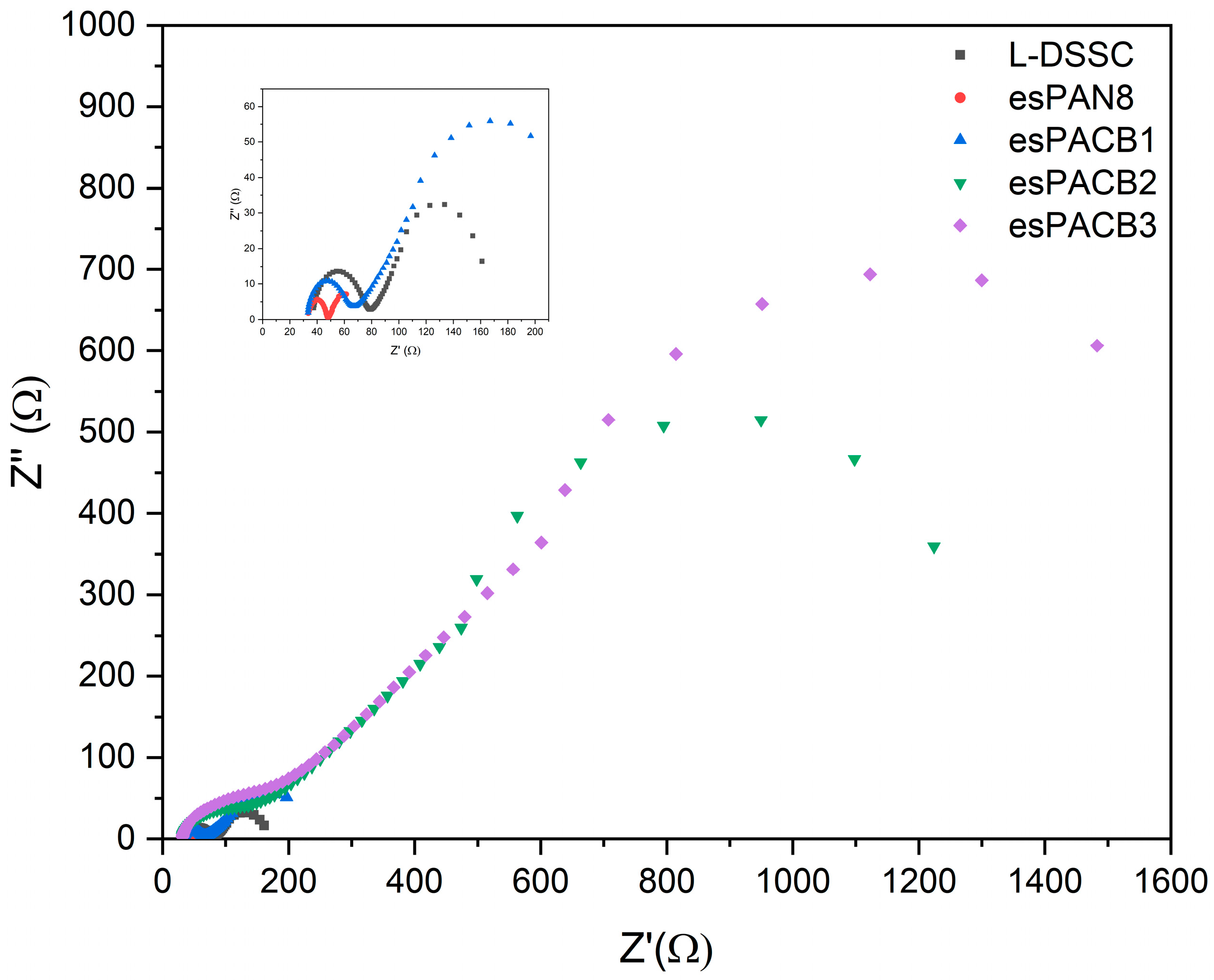
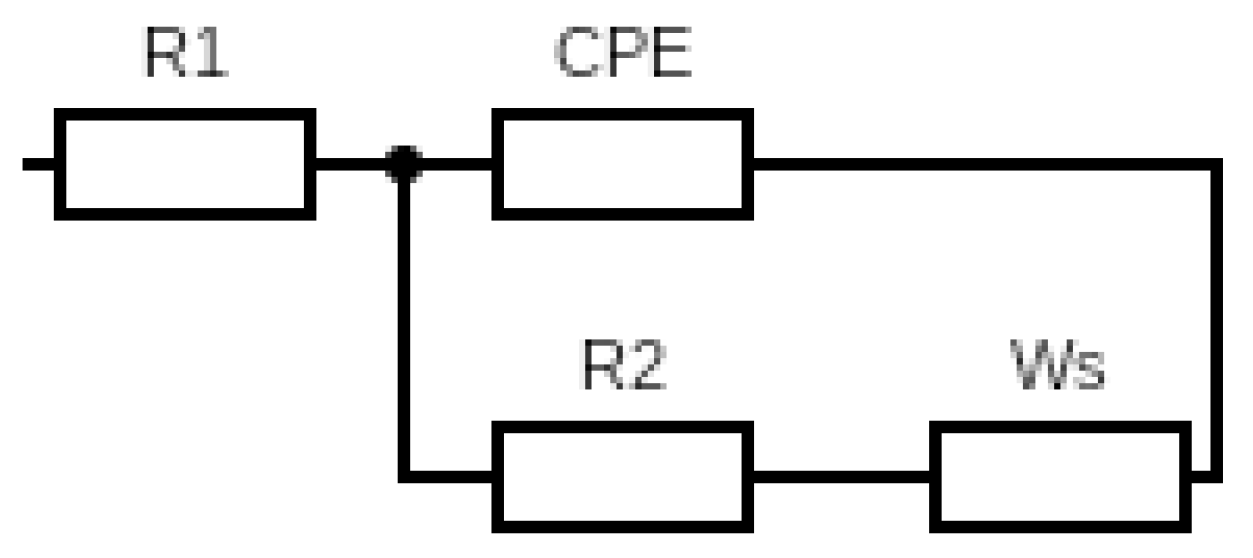
3.4. J–V Curve and Electrochemical Impedance Specctroscopy
4. Discussion
4.1. PAN Membrane Function Groups and PAN/ACB Nanofiber Composite
4.2. Diameter and Porosity of PAN Membranes and PAN/ACB Nanofiber Composite
4.3. Electrolyte Absorption of PAN Membranes and PAN/ACB Nanofiber Composite
4.4. DSSC Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murugadoss, V.; Arunachalam, S.; Elayappan, V.; Angaiah, S. Development of Electrospun PAN/CoS Nanocomposite Membrane Electrolyte for High-Performance DSSC. Ionics 2018, 24, 4071–4080. [Google Scholar] [CrossRef]
- Alhamed, M.; Issa, A.; Doubal, A. Studying of Natural Dyes Properties as Photo-Sensitizer for Dye Sensitized Solar Cells (DSSC). J. Electron. Devices 2012, 16, 1370–1383. [Google Scholar]
- Angaiah, S.; Murugadoss, V.; Arunachalam, S.; Panneerselvam, P.; Krishnan, S. Influence of Various Ionic Liquids Embedded Electrospun Polymer Membrane Electrolytes on the Photovoltaic Performance of DSSC. Eng. Sci. 2018, 4, 44–51. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.M.; Ludin, N.A.; Mohamad, A.B.; Kadhum, A.A.H.; Sopian, K. Extraction, Preparation and Application of Pigments from Cordyline Fruticosa and Hylocereus Polyrhizus as Sensitizers for Dye-Sensitized Solar Cells. Spectrochim. Acta—Part Mol. Biomol. Spectrosc. 2017, 179, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Di Pascasio, F.; Latini, A.; Bonomo, M.; Dini, D. Nanostructured Semiconductor Materials for Dye-Sensitized Solar Cells. J. Nanomater. 2017, 2017, 1–31. [Google Scholar] [CrossRef]
- Jena, A.; Mohanty, S.P.; Kumar, P.; Naduvath, J.; Lekha, P.; Das, J.; Narula, H.K.; Mallick, S.; Bhargava, P.; Gondane, V. Zn2SnO4 as an Alternative Photoanode for Dye Sensitized Solar Cells: Current Status and Future Scope. Trans. Indian Ceram. Soc. 2016, 75, 147–154. [Google Scholar]
- Kim, M.-R.; Park, S.-H.; Kim, J.-U.; Lee, J.-K. Dye-Sensitized Solar Cells Based on Polymer Electrolytes. In Solar Cells-Dye-Sensitized Devices; IntechOpen: London, UK, 2011; Volume 4, pp. 223–244. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D. Progress on Electrolytes Development in Dye-Sensitized Solar Cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef]
- Mohd Faridz Hilmy, N.I.; Yahya, W.Z.N.; Kurnia, K.A. Eutectic Ionic Liquids as Potential Electrolytes in Dye-Sensitized Solar Cells: Physicochemical and Conductivity Studies. J. Mol. Liq. 2020, 320, 114381. [Google Scholar] [CrossRef]
- Wang, P.; Yang, L.; Wu, H.; Cao, Y.; Zhang, J.; Xu, N.; Chen, S.; Decoppet, J.D.; Zakeeruddin, S.M.; Grätzel, M. Stable and Efficient Organic Dye-Sensitized Solar Cell Based on Ionic Liquid Electrolyte. Joule 2018, 2, 2145–2153. [Google Scholar] [CrossRef]
- Leijtens, T.; Ding, I.; Giovenzana, T.; Bloking, J.T.; Mcgehee, M.D.; Sellinger, A. Hole Transport Materials with Low Glass Transition Temperatures and High Solubility for Application In. ACS Nano 2012, 6, 1455–1462. [Google Scholar] [CrossRef]
- Syairah, A.; Khanmirzaei, M.H.; Saidi, N.M.; Farhana, N.K.; Ramesh, S.; Ramesh, K. Effect of Different Imidazolium-Based Ionic Liquids on Gel Polymer Electrolytes for Dye-Sensitized Solar Cells. Ionics 2019, 25, 2427–2435. [Google Scholar] [CrossRef]
- Mahmood, A. Recent Research Progress on Quasi-Solid-State Electrolytes for Dye-Sensitized Solar Cells. J. Energy Chem. 2015, 24, 686–692. [Google Scholar] [CrossRef]
- Aziz, M.F.; Buraidah, M.H.; Careem, M.A.; Arof, A.K. PVA Based Gel Polymer Electrolytes with Mixed Iodide Salts (K+I- and Bu4N+I-) for Dye-Sensitized Solar Cell Application. Electrochim. Acta 2015, 182, 217–223. [Google Scholar] [CrossRef]
- Cheng, F.; Ou, Y.; Liu, G.; Zhao, L.; Dong, B.; Wang, S.; Wen, S. Novel Quasi-Solid-State Electrolytes Based on Electrospun Poly(Vinylidene Fluoride) Fiber Membranes for Highly Effcient and Stable Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, N.; Fu, J.; Liu, Y.; Qiao, J. High-Performance Binary Cross-Linked Alkaline Anion Polymer Electrolyte Membranes for All-Solid-State Supercapacitors and Flexible Rechargeable Zinc-Air Batteries. J. Mater. Chem. A 2019, 7, 11257–11264. [Google Scholar] [CrossRef]
- Thomas, M.; Rajiv, S. Dye-Sensitized Solar Cells Based on an Electrospun Polymer Nanocomposite Membrane as Electrolyte. New J. Chem. 2019, 43, 4444–4454. [Google Scholar] [CrossRef]
- Dissanayake, S.S.; Dissanayake, M.A.K.L.; Seneviratne, V.A.; Senadeera, G.K.R.; Thotawattage, C.A. Performance of Dye Sensitized Solar Cells Fabricated with Electrospun Polymer Nanofiber Based Electrolyte. Mater. Today Proc. 2016, 3, S104–S111. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D. Review Article Electrospinning of Nanofibers and Their Applications for Electrospinning of Nanofibers and Their Applications For. J. Nanomater. 2015, 2015, 140716. [Google Scholar] [CrossRef]
- Jabbarnia, A.; Khan, W.S.; Ghazinezami, A.; Asmatulu, R. Investigating the Thermal, Mechanical, and Electrochemical Properties of PVdF/PVP Nanofibrous Membranes for Supercapacitor Applications. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Alarifi, I.; Alharbi, A.; Khan, W.; Swindle, A.; Asmatulu, R. Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring. Materials 2015, 8, 7017–7031. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Ali Abdelnaby, M.; Osman, T.A.; Hamawandi, B.; Toprak, M.S.; Muhammed, M.; Uheida, A. Photocatalytic Degradation of Organic Dyes and Enhanced Mechanical Properties of PAN/CNTs Composite Nanofibers. Sep. Purif. Technol. 2017, 182, 219–223. [Google Scholar] [CrossRef]
- Almasian, A.; Jalali, M.L.; Fard, G.C.; Maleknia, L. Surfactant Grafted PDA-PAN Nanofiber: Optimization of Synthesis, Characterization and Oil Absorption Property. Chem. Eng. J. 2017, 326, 1232–1241. [Google Scholar] [CrossRef]
- Phan, D.N.; Dorjjugder, N.; Saito, Y.; Taguchi, G.; Lee, H.; Lee, J.S.; Kim, I.S. The Mechanistic Actions of Different Silver Species at the Surfaces of Polyacrylonitrile Nanofibers Regarding Antibacterial Activities. Mater. Today Commun. 2019, 21, 100622. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.; Mohamed, A.; Karim, S.A.; Osman, T.A.; Khattab, A. Preparation, Characterization, and Mechanical Properties of Polyacrylonitrile (PAN)/Graphene Oxide (GO) Nanofibers. Mech. Adv. Mater. Struct. 2020, 27, 346–351. [Google Scholar] [CrossRef]
- Haddad, M.Y.; Alharbi, H.F. Enhancement of Heavy Metal Ion Adsorption Using Electrospun Polyacrylonitrile Nanofibers Loaded with ZnO Nanoparticles. J. Appl. Polym. Sci. 2019, 136, 47209. [Google Scholar] [CrossRef]
- Karim, S.A.; Mohamed, A.; Abdel-Mottaleb, M.M.; Osman, T.A.; Khattab, A. Visible Light Photocatalytic Activity of PAN-CNTs/ZnO-NH2 Electrospun Nanofibers. J. Alloys Compd. 2019, 772, 650–655. [Google Scholar] [CrossRef]
- Liu, P.; Wu, S.; Zhang, Y.; Zhang, H.; Qin, X. A Fast Response Ammonia Sensor Based on Coaxial PPy-PAN Nanofiber Yarn. Nanomaterials 2016, 6, 121. [Google Scholar] [CrossRef]
- Mohamed, A.; Ghobara, M.M.; Abdelmaksoud, M.K.; Mohamed, G.G. A Novel and Highly Efficient Photocatalytic Degradation of Malachite Green Dye via Surface Modified Polyacrylonitrile Nanofibers/Biogenic Silica Composite Nanofibers. Sep. Purif. Technol. 2019, 210, 935–942. [Google Scholar] [CrossRef]
- Hussain, M.; Salam, A.; Arain, M.F.; Ullah, A.; Dao, A.-T.; Vu-Manh, H.; Phan, D.-N.; Ansari, A.S.; Khan, M.Q.; Javed, Z.; et al. Polyacrylonitrile Nanofibers Containing Viroblock as Promising Material for Protective Clothing. Appl. Sci. 2021, 11, 11469. [Google Scholar] [CrossRef]
- Aydın, H.; Çelik, S.Ü.; Bozkurt, A. Electrolyte Loaded Hexagonal Boron Nitride/Polyacrylonitrile Nanofibers for Lithium Ion Battery Application. Solid State Ion. 2017, 309, 71–76. [Google Scholar] [CrossRef]
- Khan, W.S. Chemical and thermal investigations of electrospun polyacrylonitrile nanofibers incorporated with various nanoscale inclusions. J. Therm. Eng. 2017, 3, 1375–1390. [Google Scholar] [CrossRef]
- Haider, M.K.; Sun, L.; Ullah, A.; Ullah, S.; Suzuki, Y.; Park, S.; Kato, Y.; Tamada, Y.; Kim, I.S. Polyacrylonitrile/Carbon Black Nanoparticle/Nano-Hydroxyapatite (PAN/NCB/HA) Composite Nanofibrous Matrix as a Potential Biomaterial Scaffold for Bone Regenerative Applications. Mater. Today Commun. 2021, 27, 102259. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, J.; Kim, J.H.; Lee, T.; Byun, H. Electrospun PAN–GO Composite Nanofibers as Water Purification Membranes. J. Appl. Polym. Sci. 2018, 135, 45858. [Google Scholar] [CrossRef]
- Sethupathy, M.; Pandey, P.; Manisankar, P. Development of Quasi-Solid-State Dye-Sensitized Solar Cell Based on an Electrospun Polyvinylidene Fluoride-Polyacrylonitrile Membrane Electrolyte. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Fathy, M.; El Nady, J.; Muhammed, M.; Ebrahim, S.; Soliman, M.B.; Kashyout, A.E.H.B. Quasi-Solid-State Electrolyte for Dye Sensitized Solar Cells Based on Nanofiber PMA-PVDF and PMA-PVDF/PEG Membranes. Int. J. Electrochem. Sci. 2016, 11, 6064–6077. [Google Scholar] [CrossRef]
- Abdollahi, S.; Sadadi, H.; Ehsani, M.; Aram, E. Highly Efficient Polymer Electrolyte Based on Electrospun PEO/PAN/Single-Layered Graphene Oxide. Ionics 2021, 27, 3477–3487. [Google Scholar] [CrossRef]
- Magdy, G.; Harb, M.E.; Elshaer, A.M.; Saad, L.; Ebrahim, S.; Soliman, M. Preparation of Electrolytic Quasi-Solid-State Nanofibers for Dye-Sensitized Solar Cells. Jom 2019, 71, 1944–1951. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Weerasinghe, A.M.J.S.; Dissanayake, M.A.K.L.; Senadeera, G.K.R.; Furlani, M.; Albinsson, I.; Mellander, B.E. Characterization of Poly (Vinylidene Fluoride-Co-Hexafluoropropylene) (PVdF-HFP) Nanofiber Membrane Based Quasi Solid Electrolytes and Their Application in a Dye Sensitized Solar Cell. Electrochim. Acta 2018, 266, 276–283. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Jose, R.; Brown, T.M.; Fabregat-Santiago, F.; Bisquert, J. A Perspective on the Production of Dye-Sensitized Solar Modules. Energy Environ. Sci. 2014, 7, 3952–3981. [Google Scholar] [CrossRef]
- De Haro, J.C.; Tatsi, E.; Fagiolari, L.; Bonomo, M.; Barolo, C.; Turri, S.; Bella, F.; Griffini, G. Lignin-Based Polymer Electrolyte Membranes for Sustainable Aqueous Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 8550–8560. [Google Scholar] [CrossRef]

| Sample Code | ACB Addition (wt%) |
|---|---|
| esPAN8 | 0 |
| esPACB1 | 0.050 |
| esPACB2 | 0.065 |
| esPACB3 | 0.075 |
| Sample Code | (g) | (g) |
|---|---|---|
| esPAN8 | 0.0003 | 0.0027 |
| esPACB1 | 0.0002 | 0.0039 |
| esPACB2 | 0.0002 | 0.0043 |
| esPACB3 | 0.0002 | 0.0054 |
| Sample Code | ISC (mA) | Jsc (mA/cm2) | Imax (mA) | Vmax (V) | Voc (V) | FF | η (%) |
|---|---|---|---|---|---|---|---|
| L-DSSC | 1.24 | 5.05 | 0.88 | 0.42 | 0.63 | 0.46 | 1.48 |
| esPAN8 | 1.43 | 5.74 | 0.95 | 0.40 | 0.63 | 0.42 | 1.53 |
| esPACB1 | 2.29 | 9.19 | 1.26 | 0.38 | 0.63 | 0.33 | 1.92 |
| esPACB2 | 1.62 | 6.49 | 0.93 | 0.32 | 0.55 | 0.33 | 1.20 |
| esPACB3 | 1.74 | 6.97 | 0.84 | 0.34 | 0.63 | 0.26 | 1.14 |
| Wavenumber (cm−1) | Bonding Characteristics | ||
|---|---|---|---|
| Nanofiber PAN | Nanofiber PAN-ACB | Reference | |
| 1078.21 | 1080.14 | ~1190–1390 | Methylene (C−H) group [29] |
| 1247.94 | 1255.66 | ~1220–1270 | Aliphatic C−H (CH, CH2, CH3) groups vibration [24] |
| 1367.53 | 1363.67 | ~1350–1380 | Aliphatic C−H (CH, CH2, CH3) groups vibration [24] |
| 1454.33 | 1450.47 | ~1450–1460 | Aliphatic C−H (CH, CH2, CH3) groups vibration [24,26,34] |
| 1730.15 | - | 1736 | C=O stretching vibration [28,29] |
| 2243.21 | 2241.28 | ~2241–2265 | C≡N nitrile group stretching vibration [25,26,27] |
| 2929.87 | 2939.51 | ~2925–2942 | Stretching vibration C−H (CH, CH2, CH3) groups [25,26,30,31] |
| 3626.17 | 3635.81 | 3477 | O−H stretching vibration [25,31] |
| Sample Code | Diameter (nm) |
|---|---|
| esPAN8 | 206.1 ± 3.9 |
| esPACB1 | 172.9 ± 2.2 |
| esPACB2 | 284.0 ± 5.2 |
| esPACB3 | 260.3 ± 0.9 |
| Sample Code | |
|---|---|
| esPAN8 | 65.76 |
| esPACB1 | 73.43 |
| esPACB2 | 75.84 |
| esPACB3 | 76.11 |
| Sample Code | Absorption (%) |
|---|---|
| esPAN8 | 800 |
| esPACB1 | 1850 |
| esPACB2 | 2050 |
| esPACB3 | 2600 |
| Sample Code | R1 (Ω) | R2 (Ω) | CPE (S·sa × 10−5) | Ws (Ω) | σ (S·cm−1) |
|---|---|---|---|---|---|
| L-DSSC | 35.001 | 44.014 | 1.626 | 42.944 | 5.91 × 10−5 |
| esPAN8 | 32.748 | 15.229 | 1.248 | 7.4037 | 6.61 × 10−5 |
| esPACB1 | 30.587 | 39.313 | 4.544 | 66.935 | 1.71 × 10−4 |
| esPACB2 | 28.65 | 143.95 | 5.231 | 557.66 | 1.81 × 10−5 |
| esPACB3 | 28.422 | 183.2 | 4.673 | 726.04 | 1.42 × 10−5 |
| Sample Code | Frequency (Hz) | |
|---|---|---|
| L-DSSC | 3981.07 | 0.039 |
| esPAN8 | 7943.28 | 0.020 |
| esPACB1 | 39810.7 | 0.003 |
| esPACB2 | 631.00 | 0.252 |
| esPACB3 | 398.10 | 0.399 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujiarti, H.; Pangestu, Z.A.; Sholeha, N.; Nasikhudin, N.; Diantoro, M.; Utomo, J.; Aziz, M.S.A. The Effect of Acetylene Carbon Black (ACB) Loaded on Polyacrylonitrile (PAN) Nanofiber Membrane Electrolyte for DSSC Applications. Micromachines 2023, 14, 394. https://doi.org/10.3390/mi14020394
Pujiarti H, Pangestu ZA, Sholeha N, Nasikhudin N, Diantoro M, Utomo J, Aziz MSA. The Effect of Acetylene Carbon Black (ACB) Loaded on Polyacrylonitrile (PAN) Nanofiber Membrane Electrolyte for DSSC Applications. Micromachines. 2023; 14(2):394. https://doi.org/10.3390/mi14020394
Chicago/Turabian StylePujiarti, Herlin, Zahrotul Ayu Pangestu, Nabella Sholeha, Nasikhudin Nasikhudin, Markus Diantoro, Joko Utomo, and Muhammad Safwan Abd Aziz. 2023. "The Effect of Acetylene Carbon Black (ACB) Loaded on Polyacrylonitrile (PAN) Nanofiber Membrane Electrolyte for DSSC Applications" Micromachines 14, no. 2: 394. https://doi.org/10.3390/mi14020394





