Study of the Effect of Cell Prestress on the Cell Membrane Penetration Behavior by Atomic Force Microscopy
Abstract
:1. Introduction
2. Materials and Methods
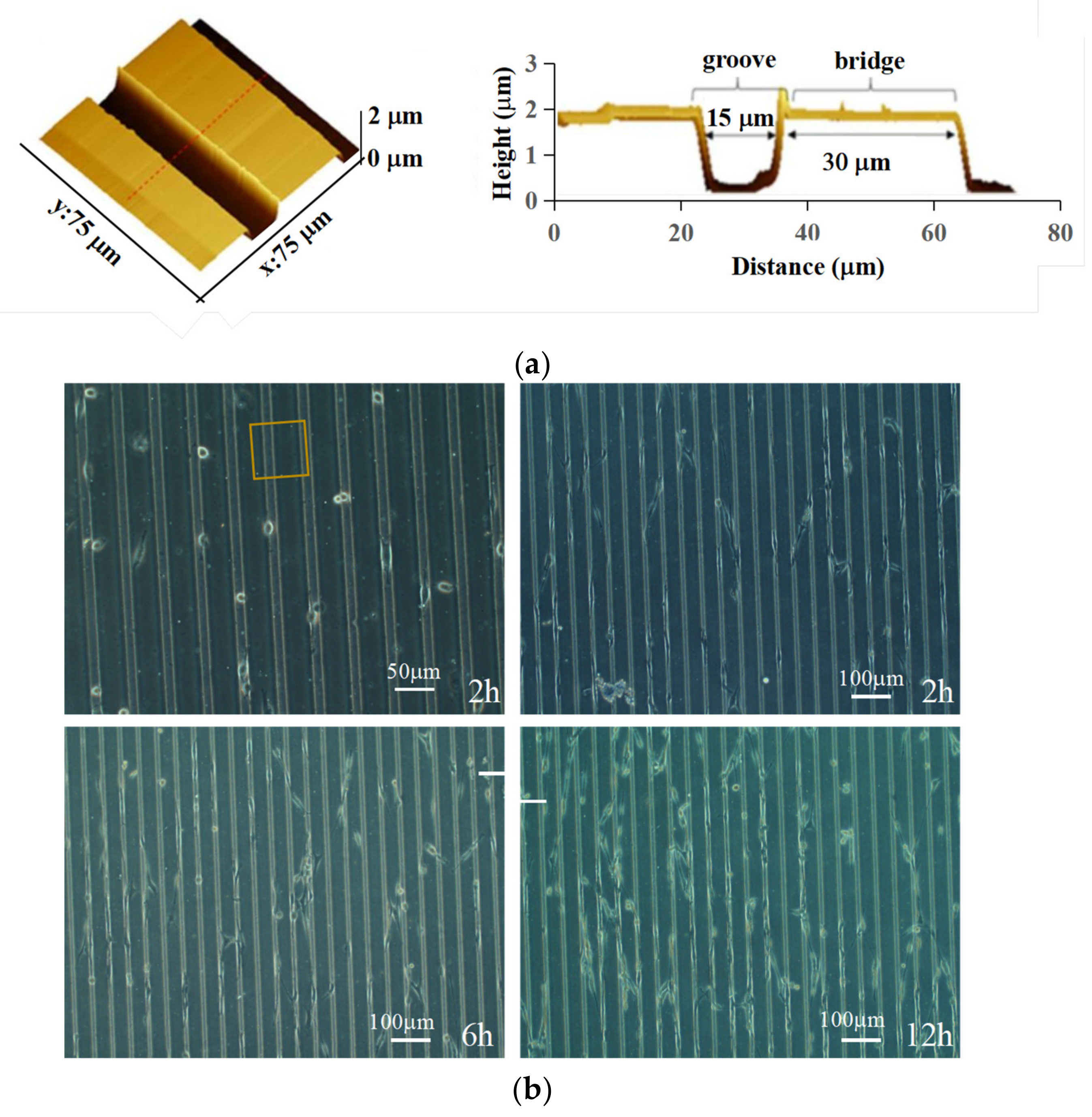
2.1. PDMS Culture Dish Preparation
2.2. Cell Culture
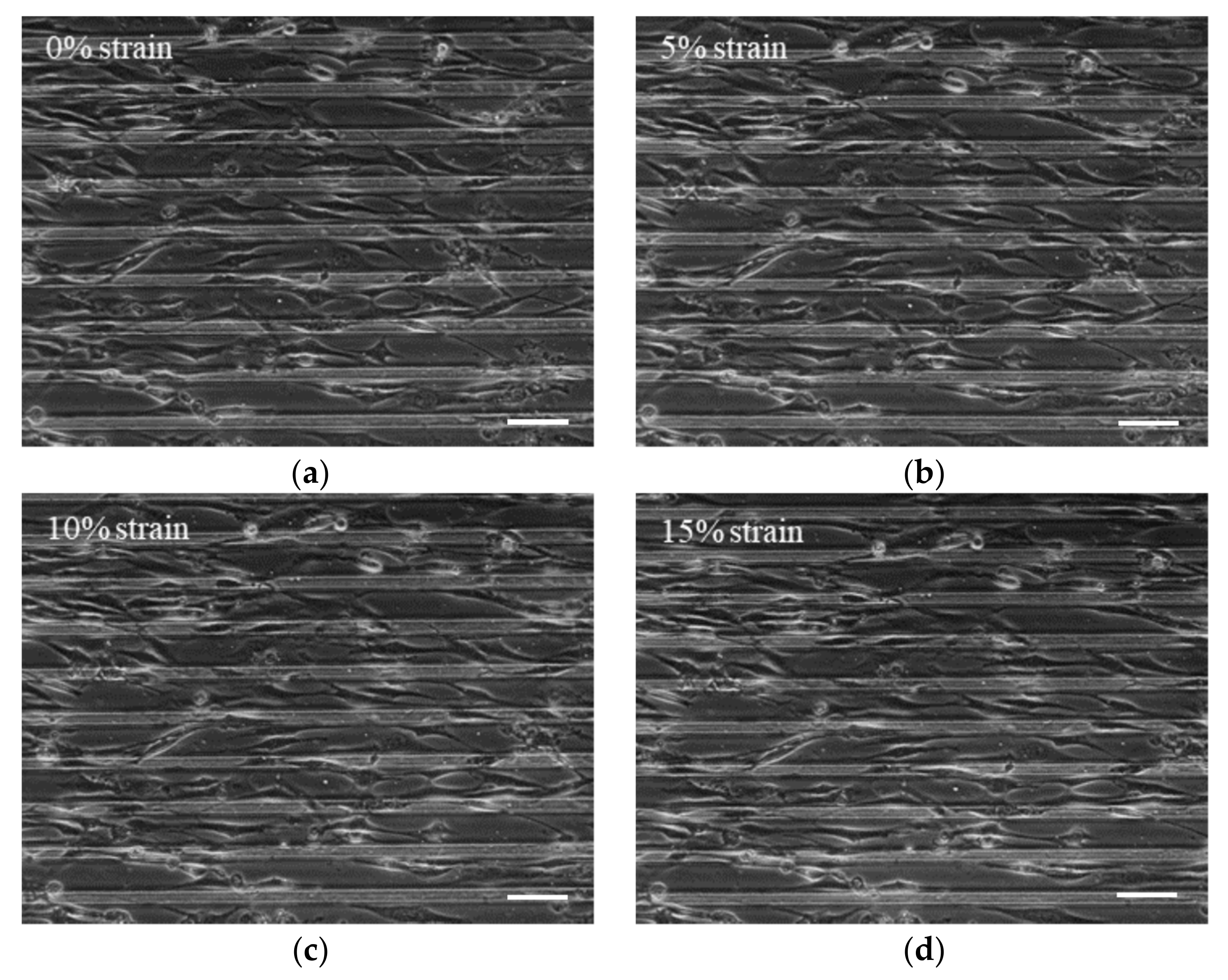
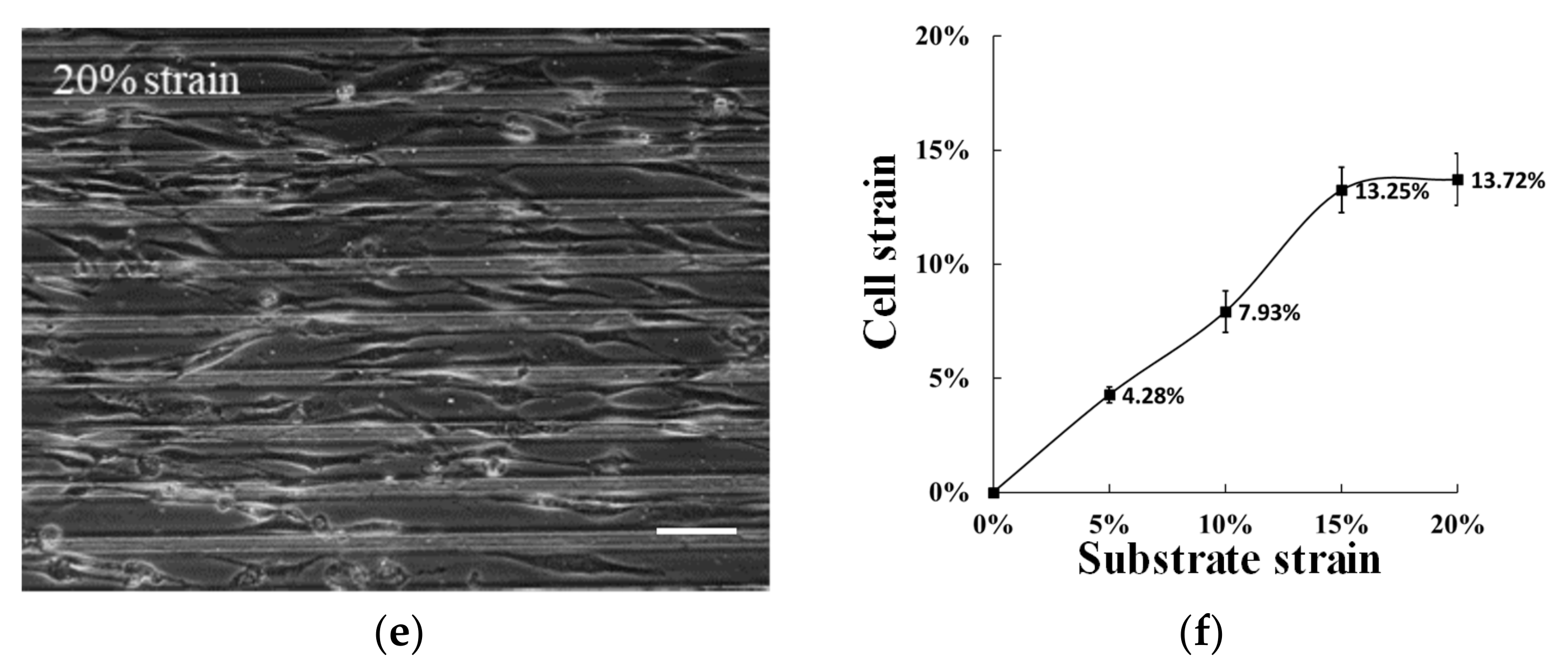
2.3. Cell Stretching
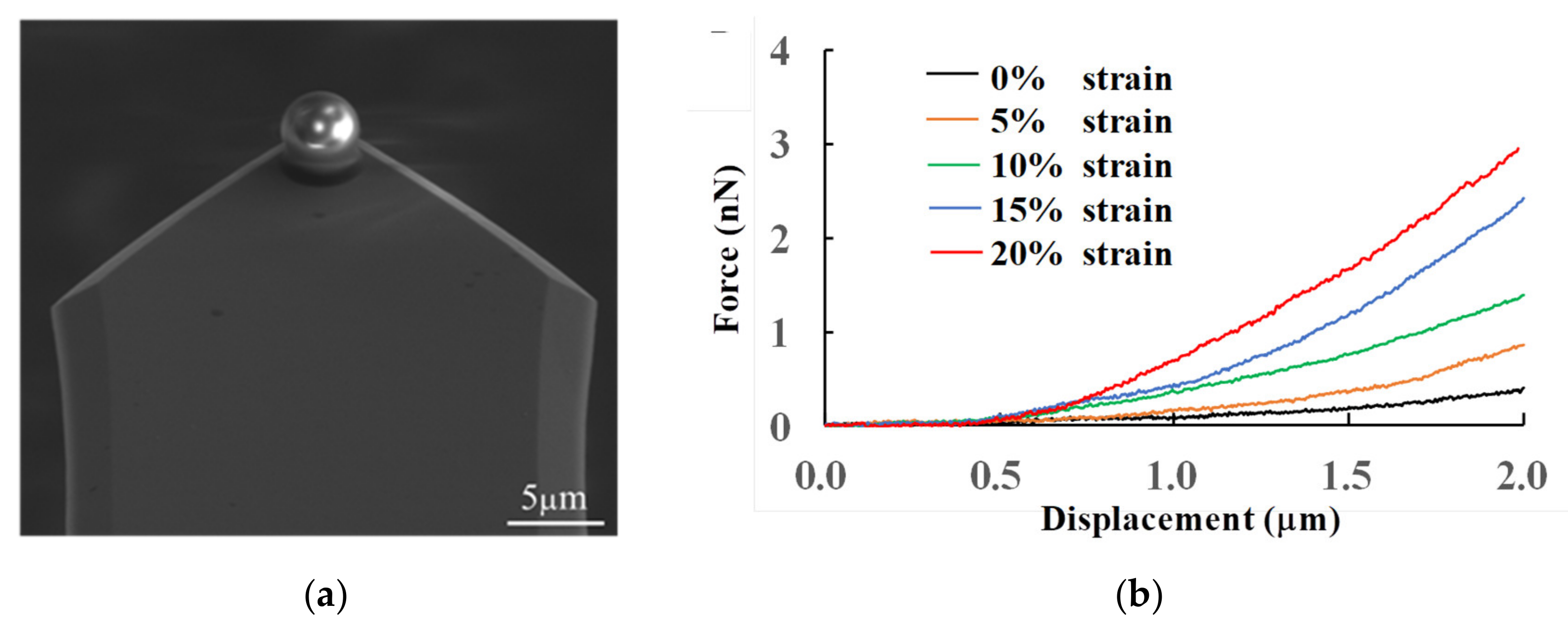
2.4. Cell Stiffness Measurement and Cell Insertion Behavior
2.5. Actin and Nucleic Acid Staining
2.6. Finite Elements Analysis
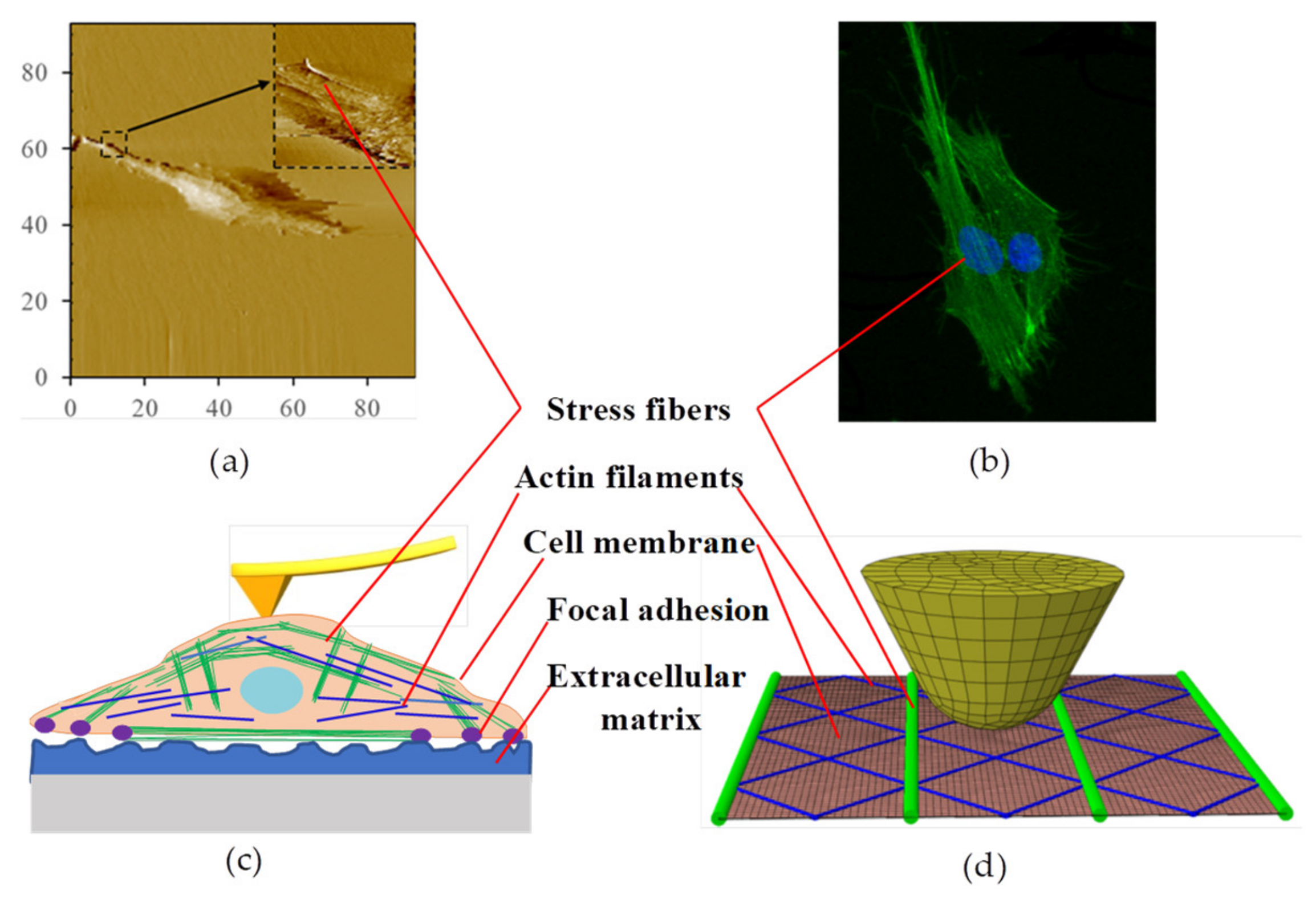
3. Results and Discussion
3.1. Cell Alignment
3.2. Apply Prestress to The Cells
3.3. Cell Stiffness
3.3.1. Measurements of Cell Stiffness
3.3.2. Finite Element Analysis
3.4. Insertion Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, X.; Wang, J.; Zhou, Q.; Zhang, L.; Wang, S.; Zhang, Z.; Yao, C. Advanced physical techniques for gene delivery based on membrane perforation. Drug Deliv. 2018, 25, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Hamann, A.; Nguyen, A.; Pannier, A.K. Nucleic acid delivery to mesenchymal stem cells: A review of nonviral methods and applications. J. Biol. Eng. 2019, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Negro, M.; Barrán-Berdón, A.L.; Aicart-Ramos, C.; Moyá, M.L.; de Ilarduya, C.T.; Aicart, E.; Junquera, E. Transfection of plasmid DNA by nanocarriers containing a gemini cationic lipid with an aromatic spacer or its monomeric counterpart. Colloids Surf. B Biointerfaces 2018, 161, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Y.; Wang, X.; Liu, P.; Chen, X. High-Throughput Platform for Efficient Chemical Transfection, Virus Packaging, and Transduction. Micromachines 2019, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Canoy, R.J.; André, F.; Shmakova, A.; Wiels, J.; Lipinski, M.; Vassetzky, Y.; Germini, D. Easy and robust electrotransfection protocol for efficient ectopic gene expression and genome editing in human B cells. Gene Ther. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Matano, M.; Nanki, K.; Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 2015, 10, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Hardee, C.L.; Arévalo-Soliz, L.M.; Hornstein, B.D.; Zechiedrich, L. Advances in Non-Viral DNA Vectors for Gene Therapy. Genes 2017, 8, 65. [Google Scholar] [CrossRef]
- Zhang, G.; Fan, N.; Lv, X.; Liu, Y.; Guo, J.; Yang, L.; Peng, B.; Jiang, H. Investigation of the Mechanical Properties of the Human Osteosarcoma Cell at Different Cell Cycle Stages. Micromachines 2017, 8, 89. [Google Scholar] [CrossRef]
- Ke, C.; Humeniuk, M.; S-Gracz, H.; Marszalek, P.E. Direct measurements of base stacking interactions in DNA by single-molecule atomic-force spectroscopy. Phys. Rev. Lett. 2007, 99, 18302. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, H.; Fan, N.; Yang, L.; Guo, J.; Peng, B. Molecular dynamics simulation of cell membrane penetration by atomic force microscopy tip. Mod. Phys. Lett. B 2018, 32, 1850198. [Google Scholar] [CrossRef]
- Cuerrier, C.M.; Lebel, R.; Grandbois, M. Single cell transfection using plasmid decorated AFM probes. Biochem. Biophys. Res. Commun. 2007, 355, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Gabi, M.; Behr, P.; Studer, P.; Vörös, J.; Niedermann, P.; Bitterli, J.; Polesel-Maris, J.; Liley, M.; Heinzelmann, H.; et al. FluidFM: Combining Atomic Force Microscopy and Nanofluidics in a Universal Liquid Delivery System for Single Cell Applications and Beyond. Nano Lett. 2009, 9, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Jiang, H.; Ye, Z.; Wu, G.; Kang, Y.; Wang, Q.; Ran, X.; Guo, J.; Zhang, G.; Wang, G.; et al. The Insertion Mechanism of a Living Cell Determined by the Stress Segmentation Effect of the Cell Membrane during the Tip-Cell Interaction. Small 2018, 14, e1703868. [Google Scholar] [CrossRef] [PubMed]
- Guillaume-Gentil, O.; Potthoff, E.; Ossola, D.; Dörig, P.; Zambelli, T.; Vorholt, J.A. Microfluidics: Force-Controlled Fluidic Injection into Single Cell Nuclei (Small 11/2013). Small 2013, 9, 1870. [Google Scholar] [CrossRef]
- Kang, W.; Yavari, F.; Minary-Jolandan, M.; Giraldo-Vela, J.P.; Safi, A.; McNaughton, R.L.; Parpoil, V.; Espinosa, H.D. Nanofountain Probe Electroporation (NFP-E) of Single Cells. Nano Lett. 2013, 13, 2448–2457. [Google Scholar] [CrossRef]
- Coughlin, M.F.; Stamenović, D. A Prestressed Cable Network Model of the Adherent Cell Cytoskeleton. Biophys. J. 2003, 84, 1328–1336. [Google Scholar] [CrossRef]
- Lam, R.H.W.; Weng, S.; Lu, W.; Fu, J. Live-cell subcellular measurement of cell stiffness using a microengineered stretchable micropost array membrane. Integr. Biol. 2012, 4, 1289. [Google Scholar] [CrossRef]
- Harris, A.R.; Charras, G.T. Experimental validation of atomic force microscopy-based cell elasticity measurements. Nanotechnology 2011, 22, 345102. [Google Scholar] [CrossRef]
- Alcaraz, J.; Buscemi, L.; Grabulosa, M.; Trepat, X.; Fabry, B.; Farré, R.; Navajas, D. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys. J. 2003, 84, 2071–2079. [Google Scholar] [CrossRef]
- Kishino, A.; Yanagida, T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature 1988, 334, 74–76. [Google Scholar] [CrossRef]
- Tseng, Y.; Schafer, B.W.; Almo, S.C.; Wirtz, D. Functional Synergy of Actin Filament Cross-linking Proteins*. J. Biol. Chem. 2002, 277, 25609–25616. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; McNaughton, R.L.; Yavari, F.; Minary-Jolandan, M.; Safi, A.; Espinosa, H.D. Microfluidic Parallel Patterning and Cellular Delivery of Molecules with a Nanofountain Probe. SLAS Technol. 2014, 19, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kohl, P.; Camelliti, P.; Gallagher, J.O.; McCulloch, A.D. Micropatterned cell cultures on elastic membranes as an in vitro model of myocardium. Nat. Protoc. 2006, 1, 1379–1391. [Google Scholar] [CrossRef]
- Hayashi, K. Tensile Properties and Local Stiffness of Cells. In Mechanics of Biological Tissue; Springer: Berlin/Heidelberg, Germany, 2006; pp. 137–152. [Google Scholar]
- Roca-Cusachs, P.; Alcaraz, J.; Sunyer, R.; Samitier, J.; Farré, R.; Navajas, D. Micropatterning of Single Endothelial Cell Shape Reveals a Tight Coupling between Nuclear Volume in G1 and Proliferation. Biophys. J. 2008, 94, 4984–4995. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Martinez-Lemus, L.A.; Hill, M.A.; Meininger, G.A. Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites. Am. J. Physiol.-Cell Physiol. 2008, 295, C268–C278. [Google Scholar] [CrossRef]
- Satcher, R.L.; Dewey, C.F. Theoretical estimates of mechanical properties of the endothelial cell cytoskeleton. Biophys. J. 1996, 71, 109–118. [Google Scholar] [CrossRef]
- Bathe, M.; Heussinger, C.; Claessens, M.M.A.E.; Bausch, A.R.; Frey, E. Cytoskeletal Bundle Mechanics. Biophys. J. 2008, 94, 2955–2964. [Google Scholar] [CrossRef] [Green Version]


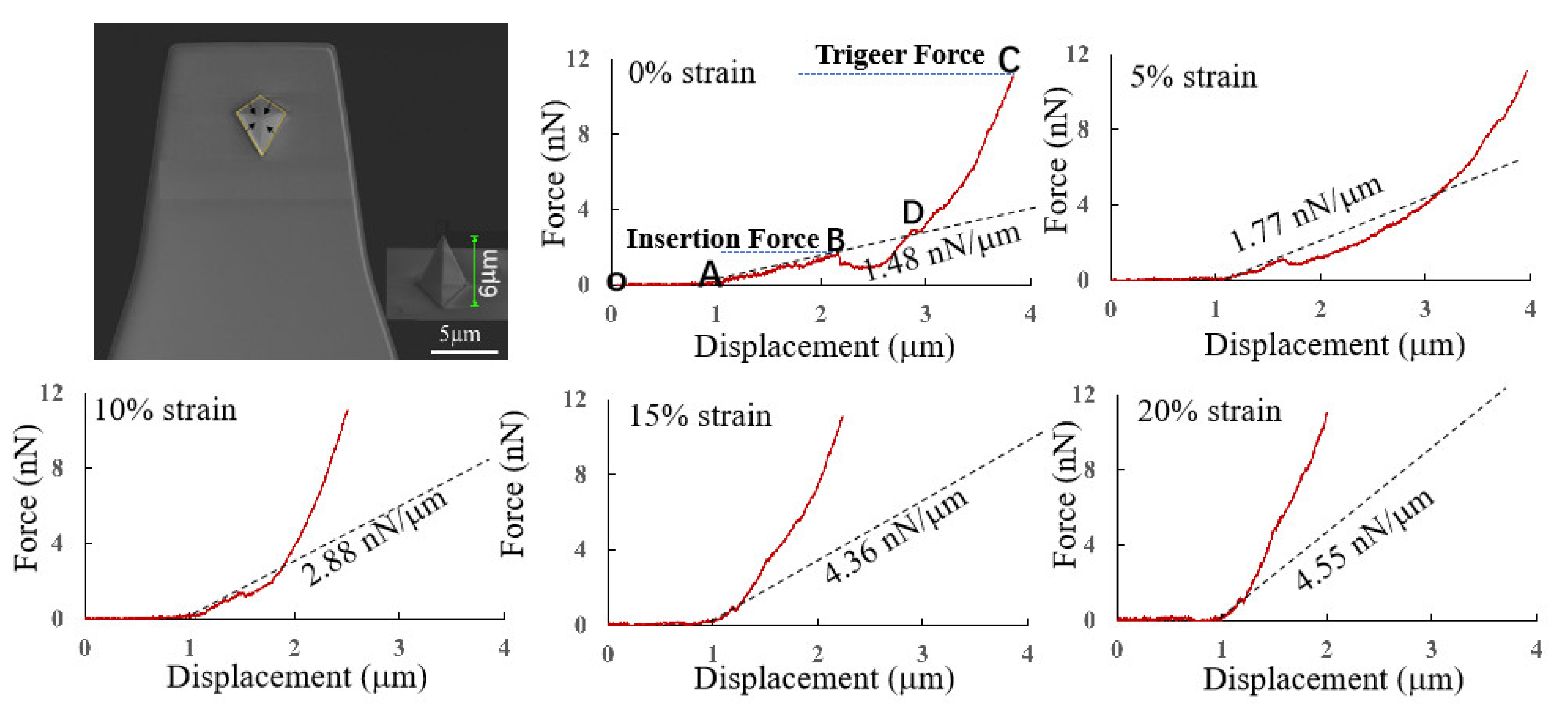
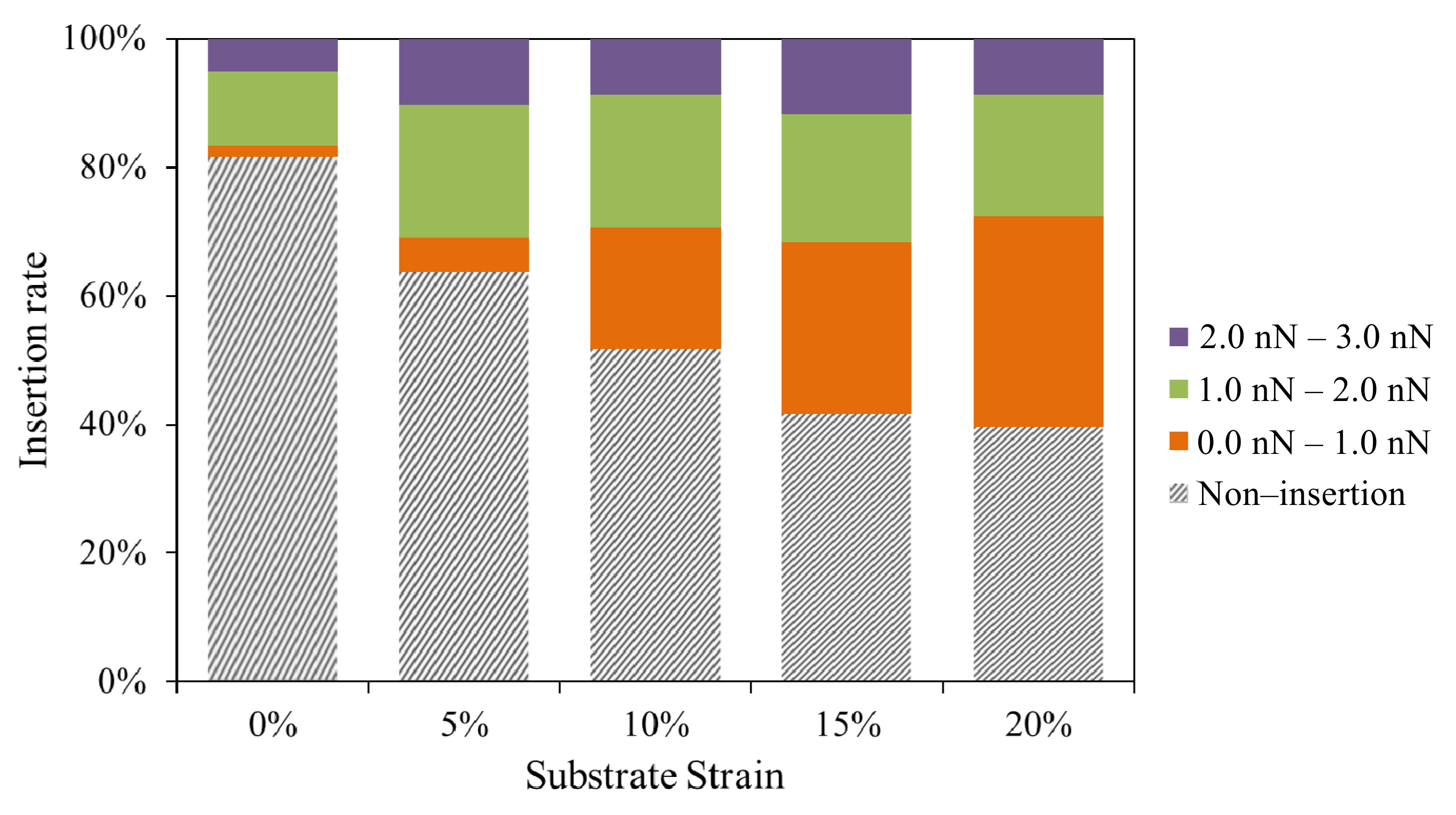
| Trigger Force (nN) | Substrate Strain | Average Insertion Force (nN) | Average Insertion Displacement (μm) | Indentation Speed (μm/s) | Average Insertion Time (s) | Insertion Rate |
|---|---|---|---|---|---|---|
| 12 | 0% | 2.0 ± 0.3 | 1.2 ± 0.5 | 1 | 1.2 ± 0.5 | 19% (11/60) |
| 12 | 5% | 1.8 ± 0.3 | 0.7 ± 0.3 | 1 | 0.7 ± 0.3 | 39% (23/60) |
| 12 | 10% | 1.5 ± 0.2 | 0.5 ± 0.1 | 1 | 0.5 ± 0.1 | 52% (31/60) |
| 12 | 15% | 1.0 ± 0.2 | 0.2 ± 0.1 | 1 | 0.2 ± 0.1 | 60% (36/60) |
| 12 | 20% | 1.0 ± 0.3 | 0.2 ± 0.1 | 1 | 0.2 ± 0.1 | 62% (37/60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Chang, Y.; Fan, N.; Yan, B.; Li, X.; Yang, Z.; Yu, Z. Study of the Effect of Cell Prestress on the Cell Membrane Penetration Behavior by Atomic Force Microscopy. Micromachines 2023, 14, 397. https://doi.org/10.3390/mi14020397
Zhang G, Chang Y, Fan N, Yan B, Li X, Yang Z, Yu Z. Study of the Effect of Cell Prestress on the Cell Membrane Penetration Behavior by Atomic Force Microscopy. Micromachines. 2023; 14(2):397. https://doi.org/10.3390/mi14020397
Chicago/Turabian StyleZhang, Guocheng, Yufang Chang, Na Fan, Bin Yan, Xianmeng Li, Zihan Yang, and Zhenyang Yu. 2023. "Study of the Effect of Cell Prestress on the Cell Membrane Penetration Behavior by Atomic Force Microscopy" Micromachines 14, no. 2: 397. https://doi.org/10.3390/mi14020397




