A Scalable, Modular Degasser for Passive In-Line Removal of Bubbles from Biomicrofluidic Devices
Abstract
:1. Introduction
2. Materials and Methods
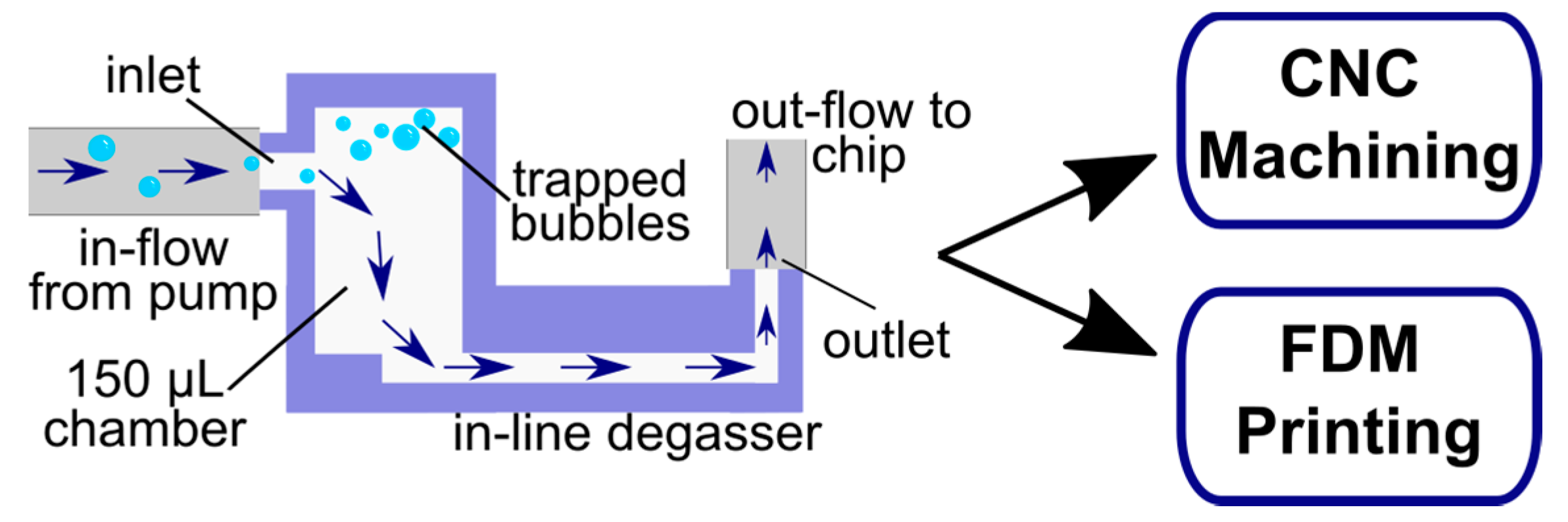
2.1. Chamber Design Adaptation
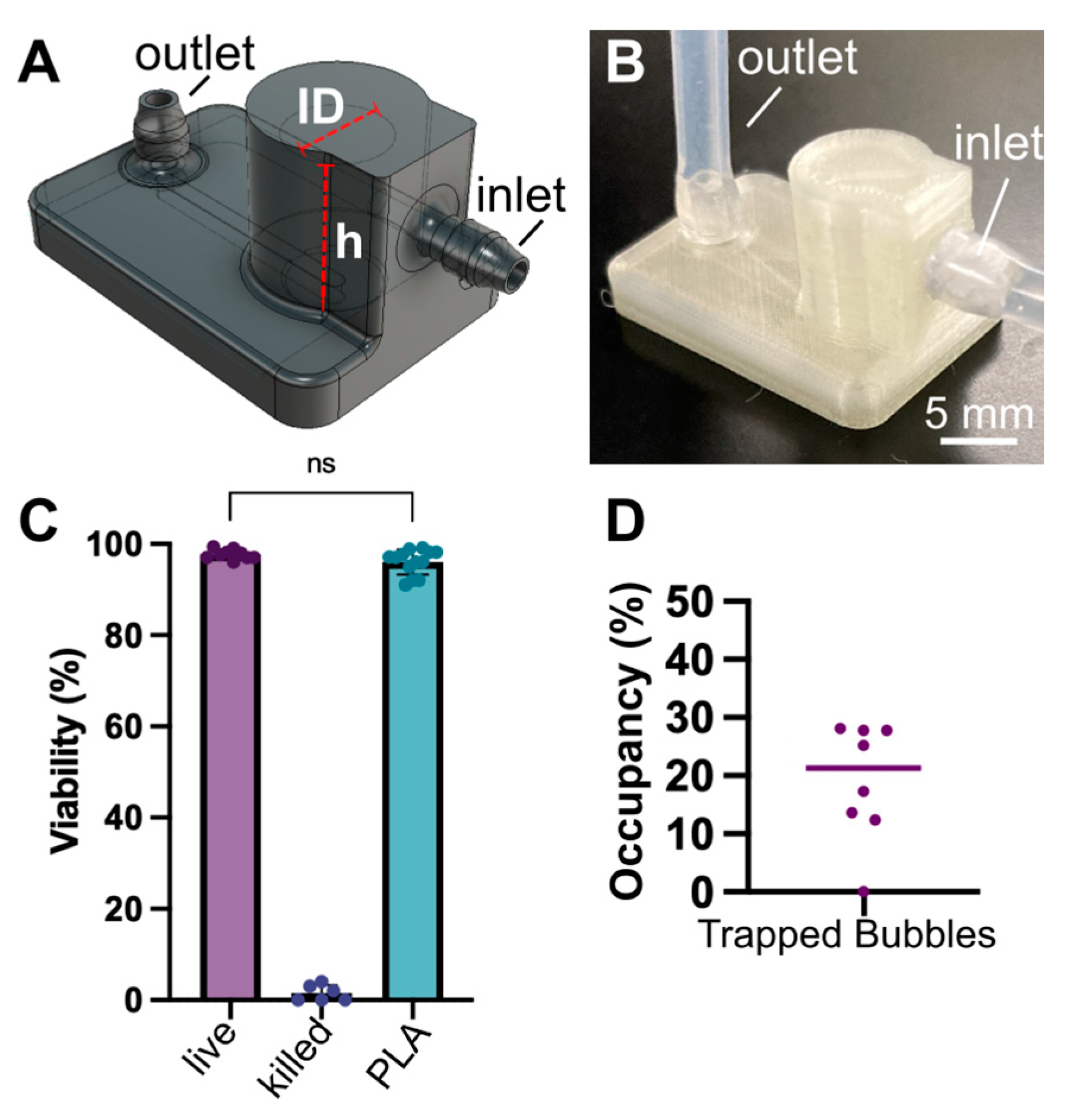
2.2. Device Fabrication by Micromachining
2.3. Device Fabrication by Fused Deposition 3D Printing
2.4. Sterilization and Collection of Effluent
2.5. Cell Sourcing and Determination of Biocompatibility
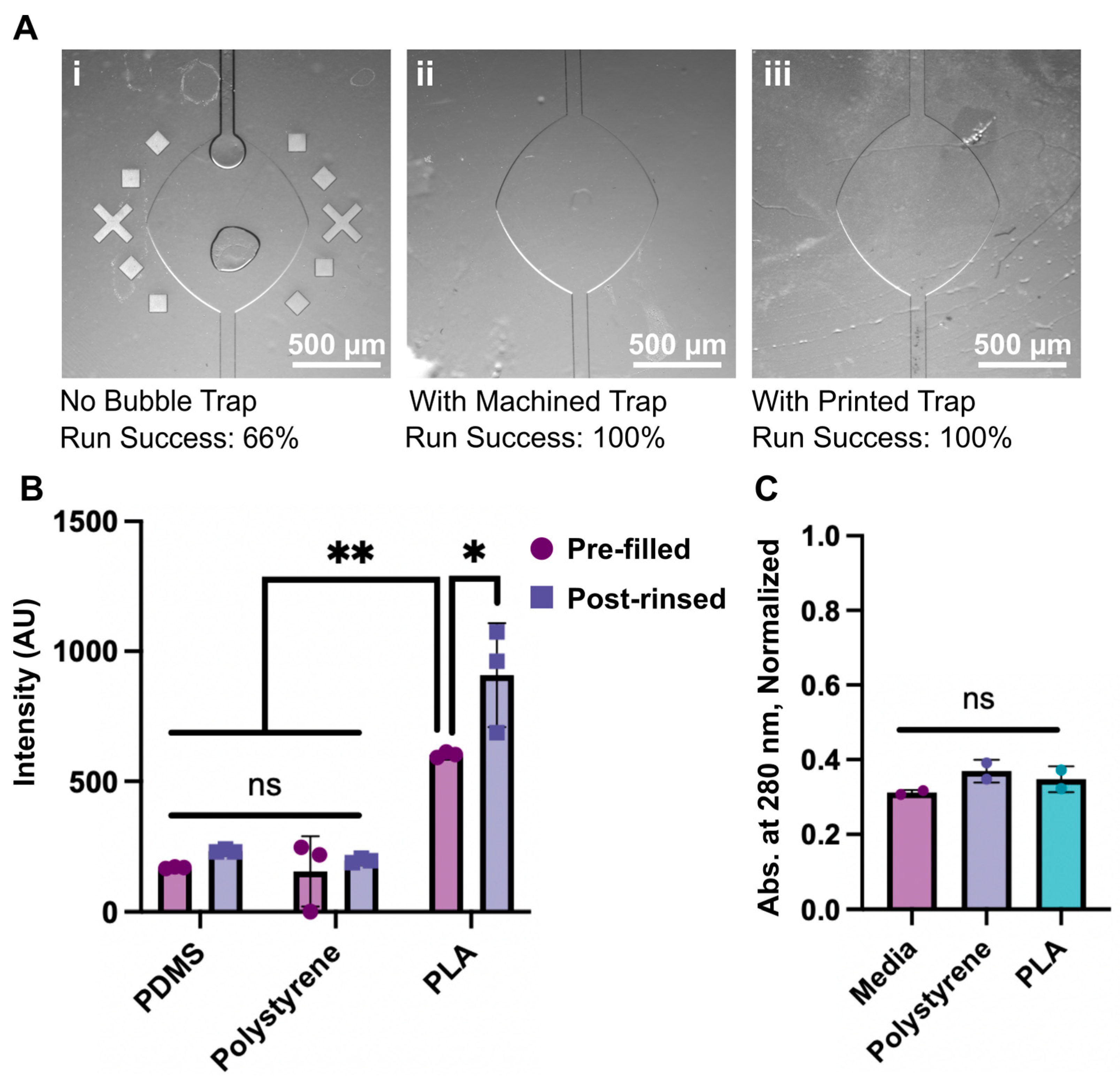
2.6. Determination of Bubble Trapping Efficacy and Fluidic Integrity
2.7. Determination of Absorption
2.8. Determination of Leaching
2.9. Determination of Mechanical Integrity under Varied Pressure Drops
2.10. In-Line Device Application
3. Results and Discussion
3.1. Micromachined Degassers
3.2. Fused Deposition 3D Printed Degassers
3.3. Proof of Concept Application and Fabrication Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereiro, I.; Khartchenko, A.F.; Petrini, L.; Kaigala, G.V. Nip the Bubble in the Bud: A Guide to Avoid Gas Nucleation in Microfluidics. Lab Chip 2019, 19, 2296–2314. [Google Scholar] [CrossRef]
- Qi, Y.; Klausner, J.F. Heterogeneous Nucleation With Artificial Cavities. J. Heat Transf. 2005, 127, 1189–1196. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Trapping and Control of Bubbles in Various Microfluidic Applications. Lab Chip 2020, 20, 4512–4527. [Google Scholar] [CrossRef]
- Karlsson, J.M.; Gazin, M.; Laakso, S.; Haraldsson, T.; Malhotra-Kumar, S.; Mäki, M.; Goossens, H.; van der Wijngaart, W. Active Liquid Degassing in Microfluidic Systems. Lab Chip 2013, 13, 4366. [Google Scholar] [CrossRef]
- Liu, C.; Thompson, J.A.; Bau, H.H. A Membrane-Based, High-Efficiency, Microfluidic Debubbler. Lab Chip 2011, 11, 1688. [Google Scholar] [CrossRef]
- Yang, Z.; Matsumoto, S.; Maeda, R. A Prototype of Ultrasonic Micro-Degassing Device for Portable Dialysis System. Sens. Actuators A Phys. 2002, 95, 274–280. [Google Scholar] [CrossRef]
- Meng, D.D.; Kim, J.; Kim, C.-J. A Degassing Plate with Hydrophobic Bubble Capture and Distributed Venting for Microfluidic Devices. J. Micromech. Microeng. 2006, 16, 419–424. [Google Scholar] [CrossRef]
- Skelley, A.M.; Voldman, J. An Active Bubble Trap and Debubbler for Microfluidic Systems. Lab Chip 2008, 8, 1733. [Google Scholar] [CrossRef]
- Wu, H.-W.; Lin, X.-Z.; Hwang, S.-M.; Lee, G.-B. The Culture and Differentiation of Amniotic Stem Cells Using a Microfluidic System. Biomed. Microdevices 2009, 11, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.J.; Lee, N.K.; Mylott, J.A.; Mazzola, N.; Ahmed, A.; Abhyankar, V.V. A Low-Cost, Rapidly Integrated Debubbler (RID) Module for Microfluidic Cell Culture Applications. Micromachines 2019, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, S.; Edahiro, J.; Kikuchi, K.; Sumaru, K.; Kanamori, T. Pressure-Driven Perfusion Culture Microchamber Array for a Parallel Drug Cytotoxicity Assay. Biotechnol. Bioeng. 2008, 100, 1156–1165. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Z.; Zhang, W.; Jiang, X. A Simple PDMS-Based Microfluidic Channel Design That Removes Bubbles for Long-Term on-Chip Culture of Mammalian Cells. Lab Chip 2010, 10, 2906. [Google Scholar] [CrossRef] [PubMed]
- Markoski, A.; Wong, I.Y.; Borenstein, J.T. 3D Printed Monolithic Device for the Microfluidic Capture, Perfusion, and Analysis of Multicellular Spheroids. Front. Med. Technol. 2021, 3, 646441. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Cárdenas, J.E.; Zatorski, J.M.; Arneja, A.; Montalbine, A.N.; Munson, J.M.; Luckey, C.J.; Pompano, R.R. Towards Spatially-Organized Organs-on-Chip: Photopatterning Cell-Laden Thiol-Ene and Methacryloyl Hydrogels in a Microfluidic Device. Organs Chip 2022, 4, 100018. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Lunardelli, F.; Gagliardi, M.; Miranda, A.; Lamanna, L.; Luminare, A.G.; Gambineri, F.; Lai, M.; Pistello, M.; Cecchini, M. Surface-Acoustic-Wave (SAW) Induced Mixing Enhances the Detection of Viruses: Application to Measles Sensing in Whole Human Saliva with a SAW Lab-On-a-Chip. Adv. Funct. Mater. 2022, 32, 2201958. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Kim, C.; Jin, H.; Bae, J.; Kim, G. Enhancement of Virus Infection Using Dynamic Cell Culture in a Microchannel. Micromachines 2018, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.V.; Ledwig, V.; Bendas, S.; Reichl, S.; Dietzel, A. Tissue Barrier-on-Chip: A Technology for Reproducible Practice in Drug Testing. Pharmaceutics 2022, 14, 1451. [Google Scholar] [CrossRef]
- Kreß, S.; Schaller-Ammann, R.; Feiel, J.; Priedl, J.; Kasper, C.; Egger, D. 3D Printing of Cell Culture Devices: Assessment and Prevention of the Cytotoxicity of Photopolymers for Stereolithography. Materials 2020, 13, 3011. [Google Scholar] [CrossRef]
- Knight, E.; Przyborski, S. Advances in 3D Cell Culture Technologies Enabling Tissue-like Structures to Be Created in Vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Musgrove, H.B.; Catterton, M.A.; Pompano, R.R. Applied Tutorial for the Design and Fabrication of Biomicrofluidic Devices by Resin 3D Printing. Anal. Chim. Acta 2022, 1209, 339842. [Google Scholar] [CrossRef]
- Musgrove, H.; Pompano, R. Threadless Chip-to-World Connections on Resin 3D Printed Microscale Devices. 2022. Available online: https://blogs.rsc.org/chipsandtips/2022/11/02/threadless-chip-to-world-connections-on-resin-3d-printed-microscale-devices (accessed on 8 February 2023).
- Cook, S.R.; Musgrove, H.B.; Throckmorton, A.L.; Pompano, R.R. Microscale Impeller Pump for Recirculating Flow in Organs-on-Chip and Microreactors. Lab Chip 2022, 22, 605–620. [Google Scholar] [CrossRef]
- O’Grady, B.J.; Geuy, M.D.; Kim, H.; Balotin, K.M.; Allchin, E.R.; Florian, D.C.; Bute, N.N.; Scott, T.E.; Lowen, G.B.; Fricker, C.M.; et al. Rapid Prototyping of Cell Culture Microdevices Using Parylene-Coated 3D Prints. Lab Chip 2021, 21, 4814–4822. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.R.; Hudson, A.L.; Dunn, S.M.J.; You, H.; Baker, G.B.; Whittal, R.M.; Martin, J.W.; Jha, A.; Edmondson, D.E.; Holt, A. Bioactive Contaminants Leach from Disposable Laboratory Plasticware. Science 2008, 322, 917–917. [Google Scholar] [CrossRef]
- Carter, S.-S.D.; Atif, A.-R.; Kadekar, S.; Lanekoff, I.; Engqvist, H.; Varghese, O.P.; Tenje, M.; Mestres, G. PDMS Leaching and Its Implications for On-Chip Studies Focusing on Bone Regeneration Applications. Organs Chip 2020, 2, 100004. [Google Scholar] [CrossRef]
- Venzac, B.; Deng, S.; Mahmoud, Z.; Lenferink, A.; Costa, A.; Bray, F.; Otto, C.; Rolando, C.; Le Gac, S. PDMS Curing Inhibition on 3D-Printed Molds: Why? Also, How to Avoid It? Anal. Chem. 2021, 93, 7180–7187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, M.; Yang, M.; Zhu, W.; Wu, J.; Gong, X.; Wen, W. Polydimethylsiloxane-Integratable Micropressure Sensor for Microfluidic Chips. Biomicrofluidics 2009, 3, 034105. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-C.; Mansouri, M.; Ahamed, N.N.N.; Larson, S.M.; Joshi, I.M.; Ahmed, A.; Borkholder, D.A.; Abhyankar, V.V. A Miniaturized 3D Printed Pressure Regulator (ΜPR) for Microfluidic Cell Culture Applications. Sci. Rep. 2022, 12, 10769. [Google Scholar] [CrossRef]

| CNC Machining | FDM 3D Printing | Resin 3D Printing | Soft Lithography | |
|---|---|---|---|---|
| Est. cost (USD, per degasser) | ~$1’s (at 1000s scale)–~$100’s (at 10s scale) 1 | $0.02 | $1.25 | $2.50 |
| Est. fabrication time (25 chips) | 2–3 weeks 1 | 12–25 h 2 | 40 min | 3–5 days 3 |
| Material | Polystyrene | Polylactic acid | Photocurable acrylate resins | Polydimethyl-siloxane |
| Material elasticity | Low | Low | Moderate | High |
| Tubing connections | Inserted adapters, barbed fittings | Integrated barbs | Direct raised ports | Direct ports or fittings |
| Optical transparency | High | Moderate | Moderate—High | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musgrove, H.B.; Saleheen, A.; Zatorski, J.M.; Arneja, A.; Luckey, C.J.; Pompano, R.R. A Scalable, Modular Degasser for Passive In-Line Removal of Bubbles from Biomicrofluidic Devices. Micromachines 2023, 14, 435. https://doi.org/10.3390/mi14020435
Musgrove HB, Saleheen A, Zatorski JM, Arneja A, Luckey CJ, Pompano RR. A Scalable, Modular Degasser for Passive In-Line Removal of Bubbles from Biomicrofluidic Devices. Micromachines. 2023; 14(2):435. https://doi.org/10.3390/mi14020435
Chicago/Turabian StyleMusgrove, Hannah B., Amirus Saleheen, Jonathan M. Zatorski, Abhinav Arneja, Chance John Luckey, and Rebecca R. Pompano. 2023. "A Scalable, Modular Degasser for Passive In-Line Removal of Bubbles from Biomicrofluidic Devices" Micromachines 14, no. 2: 435. https://doi.org/10.3390/mi14020435
APA StyleMusgrove, H. B., Saleheen, A., Zatorski, J. M., Arneja, A., Luckey, C. J., & Pompano, R. R. (2023). A Scalable, Modular Degasser for Passive In-Line Removal of Bubbles from Biomicrofluidic Devices. Micromachines, 14(2), 435. https://doi.org/10.3390/mi14020435






