Patient-Specific 3D-Printed Low-Cost Models in Medical Education and Clinical Practice
Abstract
:1. Introduction
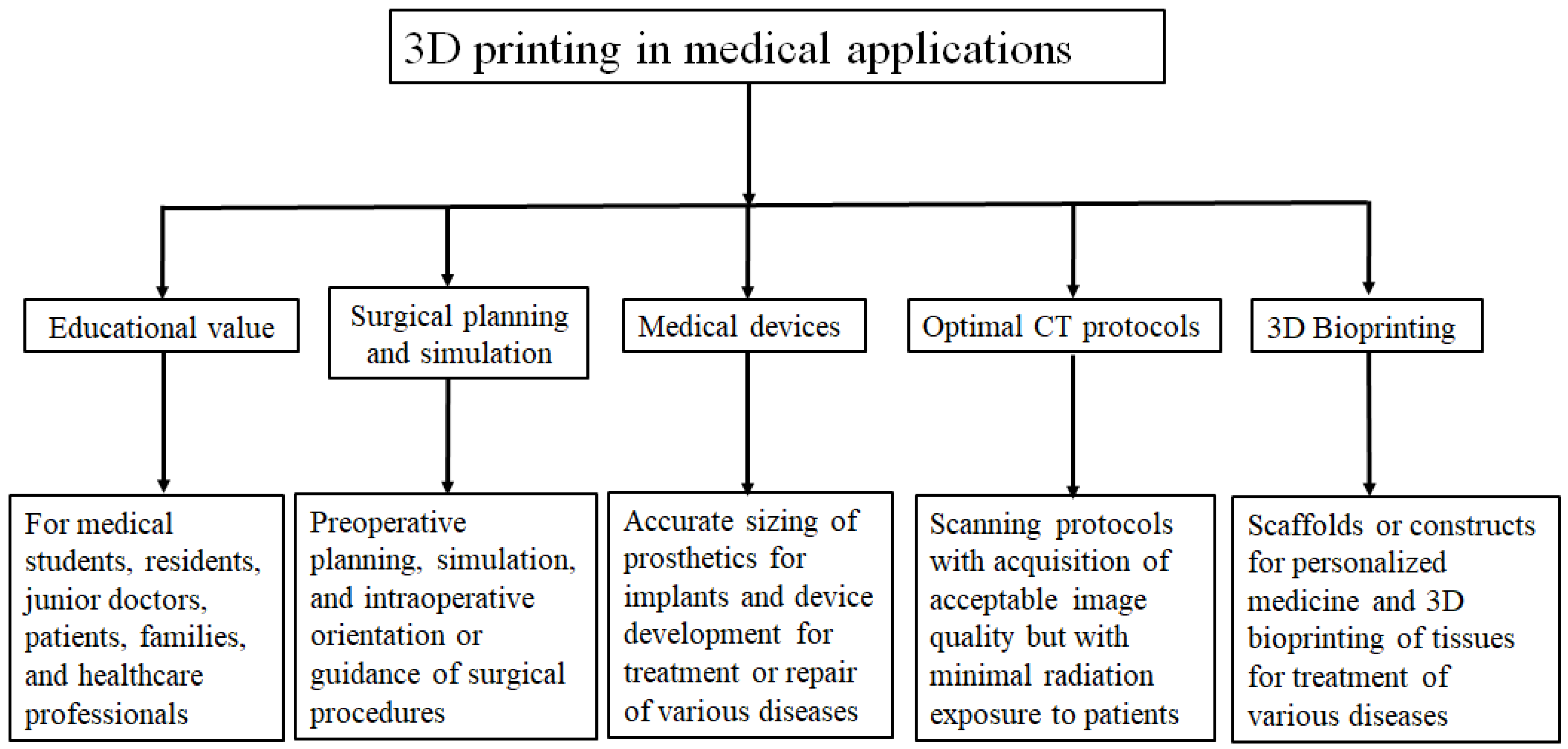
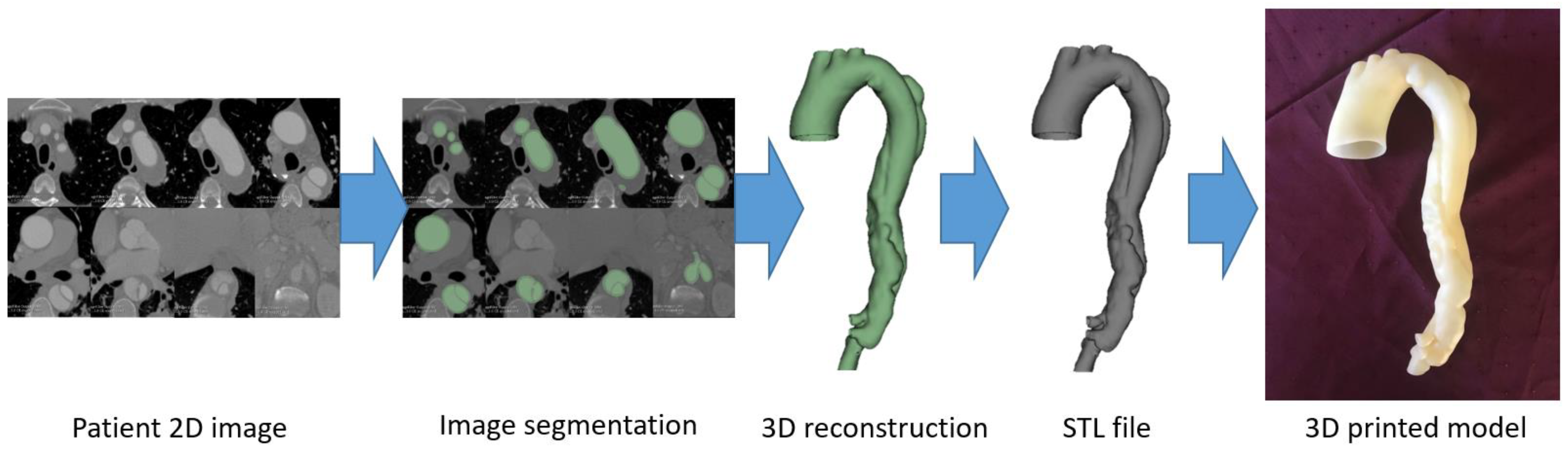
2. 3D Printing Preparation: Image Post-Processing and Segmentation
3. 3D Printing Facility: 3D Printers and Printing Materials
4. Usefulness of 3D-Printed Models in Cardiovascular Disease
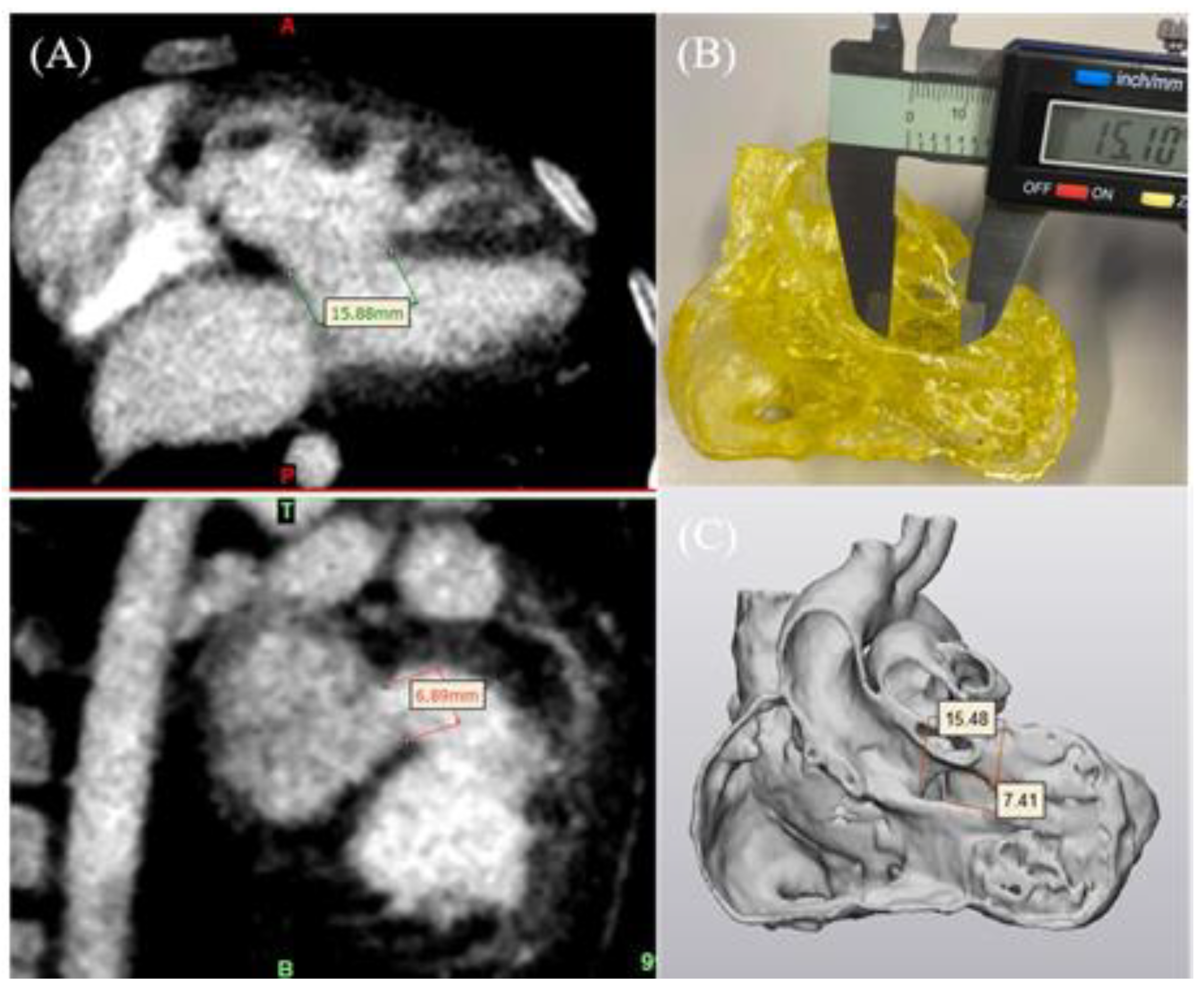
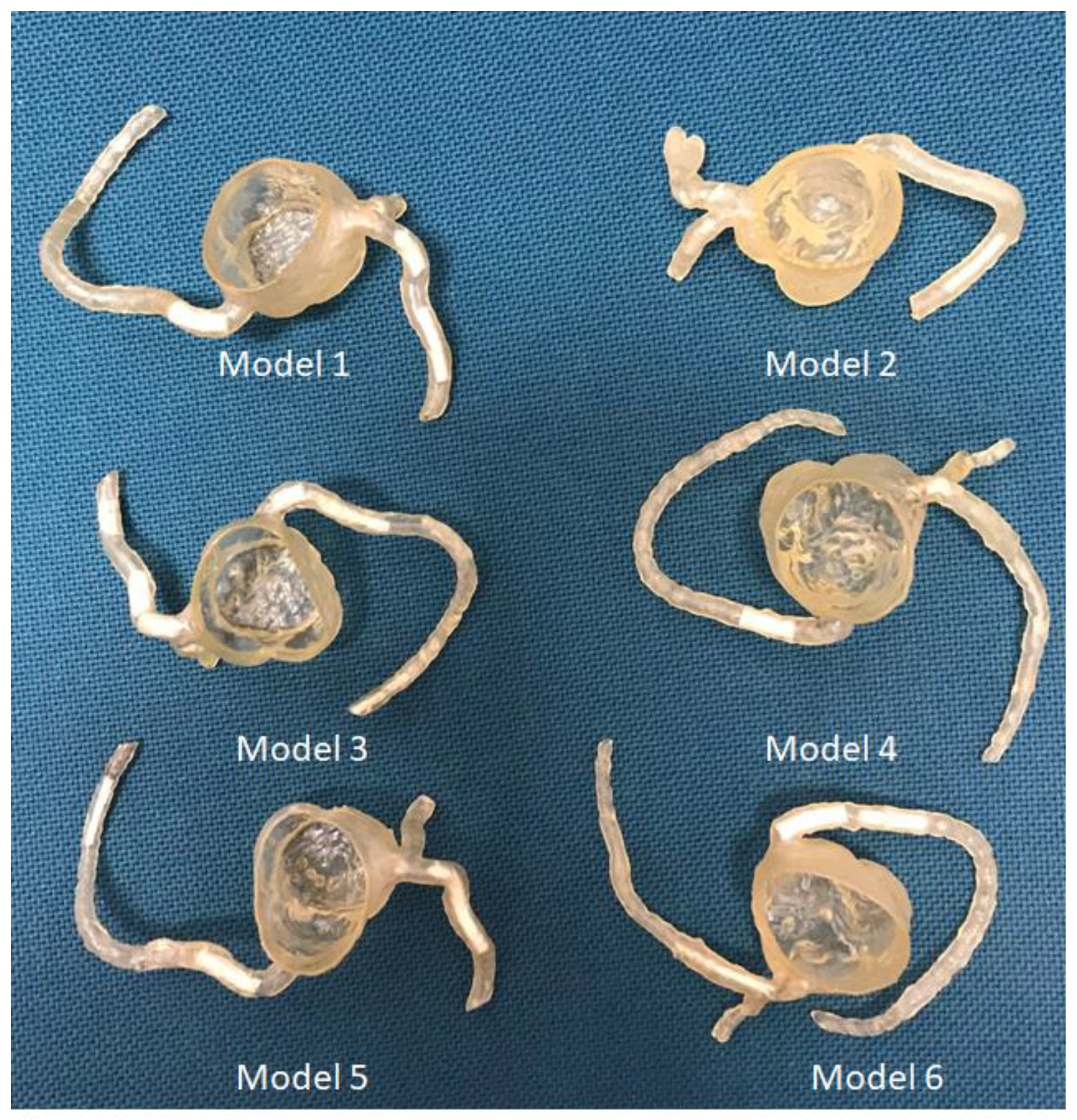
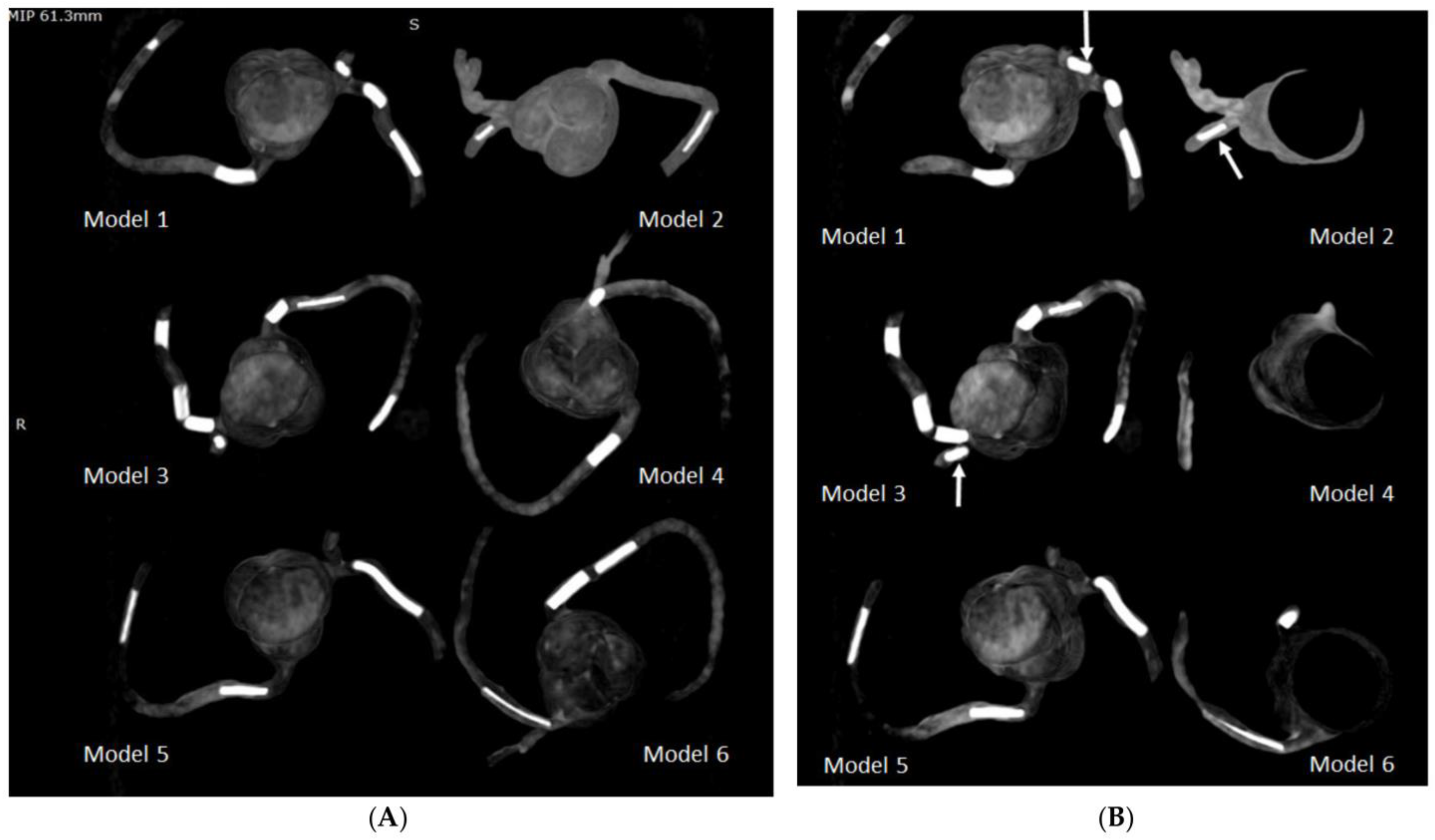
4.1. 3D-Printed CHD Model Accuracy
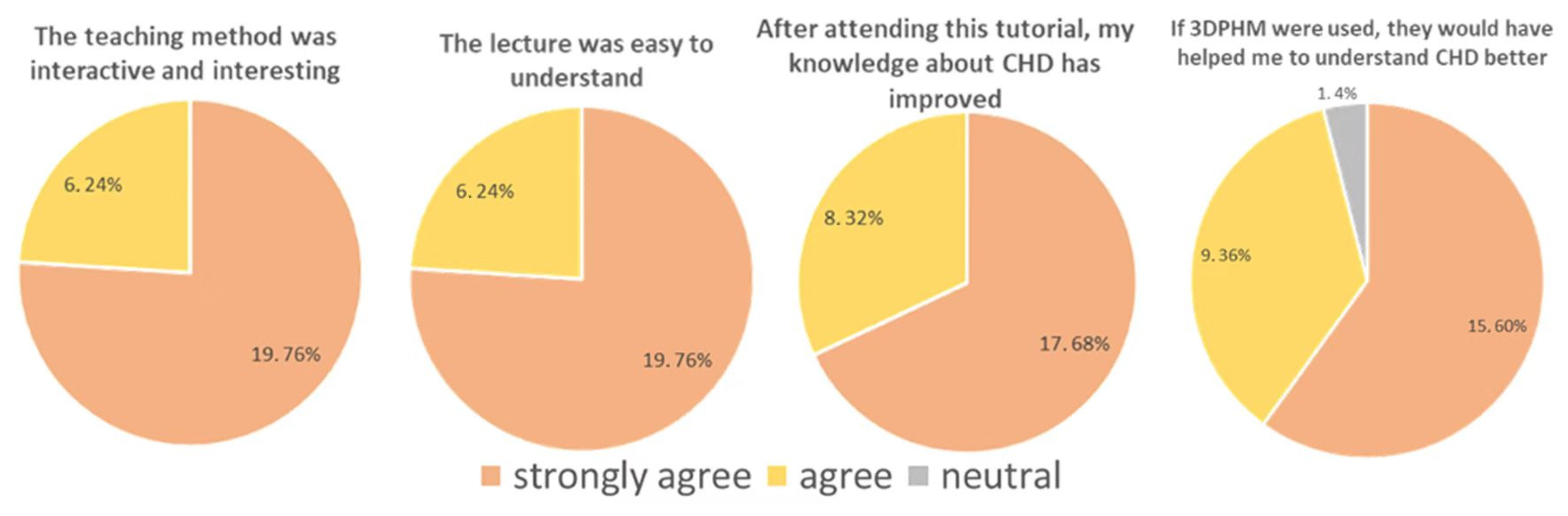
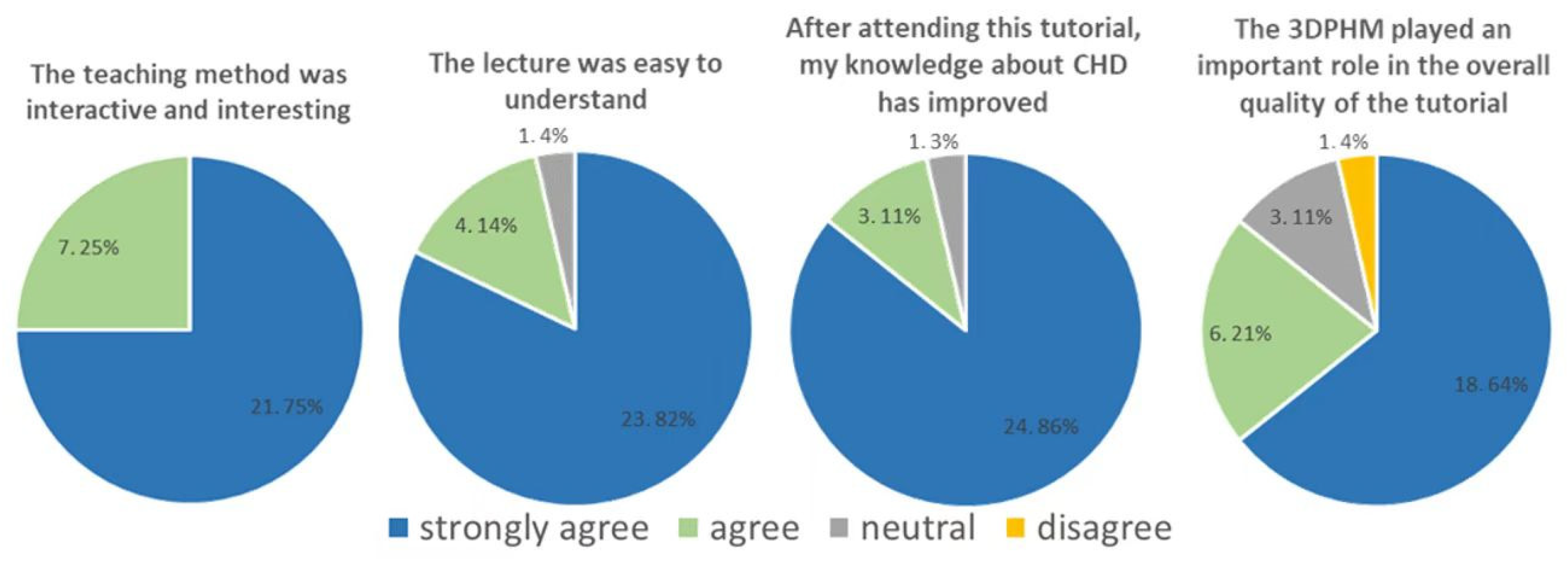
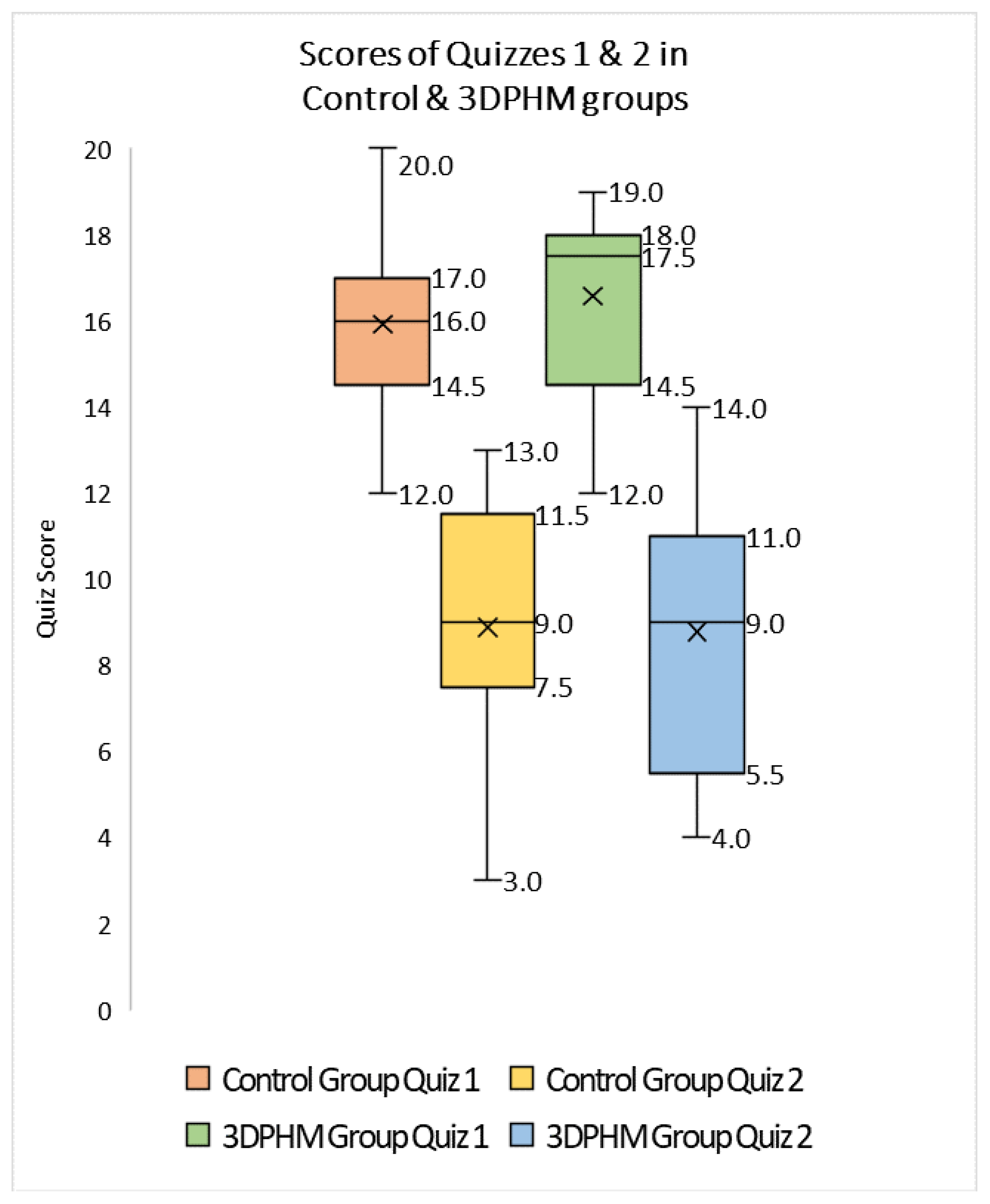
4.2. 3D-Printed CHD Models in Medical Education
4.3. 3D-Printed CHD Models in Preoperative planning
4.4. 3D-Printed Coronary Artery Models
4.5. 3D-Printed Aorta Models
5. 3D-Printed Tumour Models
5.1. 3D-Printed Breast Cancer Model
5.2. 3D-Printed Lung Cancer Model
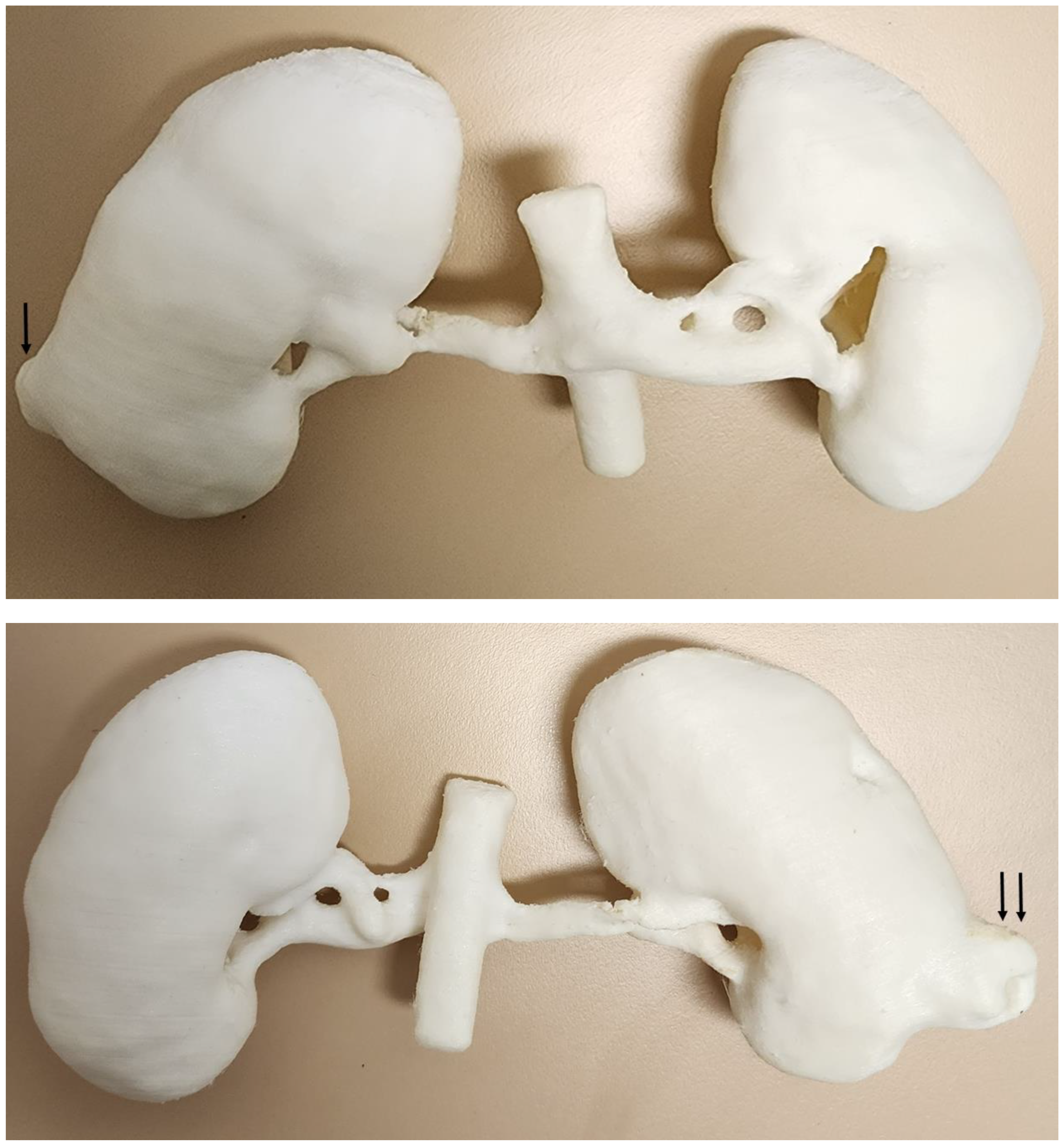
5.3. 3D-Printed Renal Cell Carcinoma Model
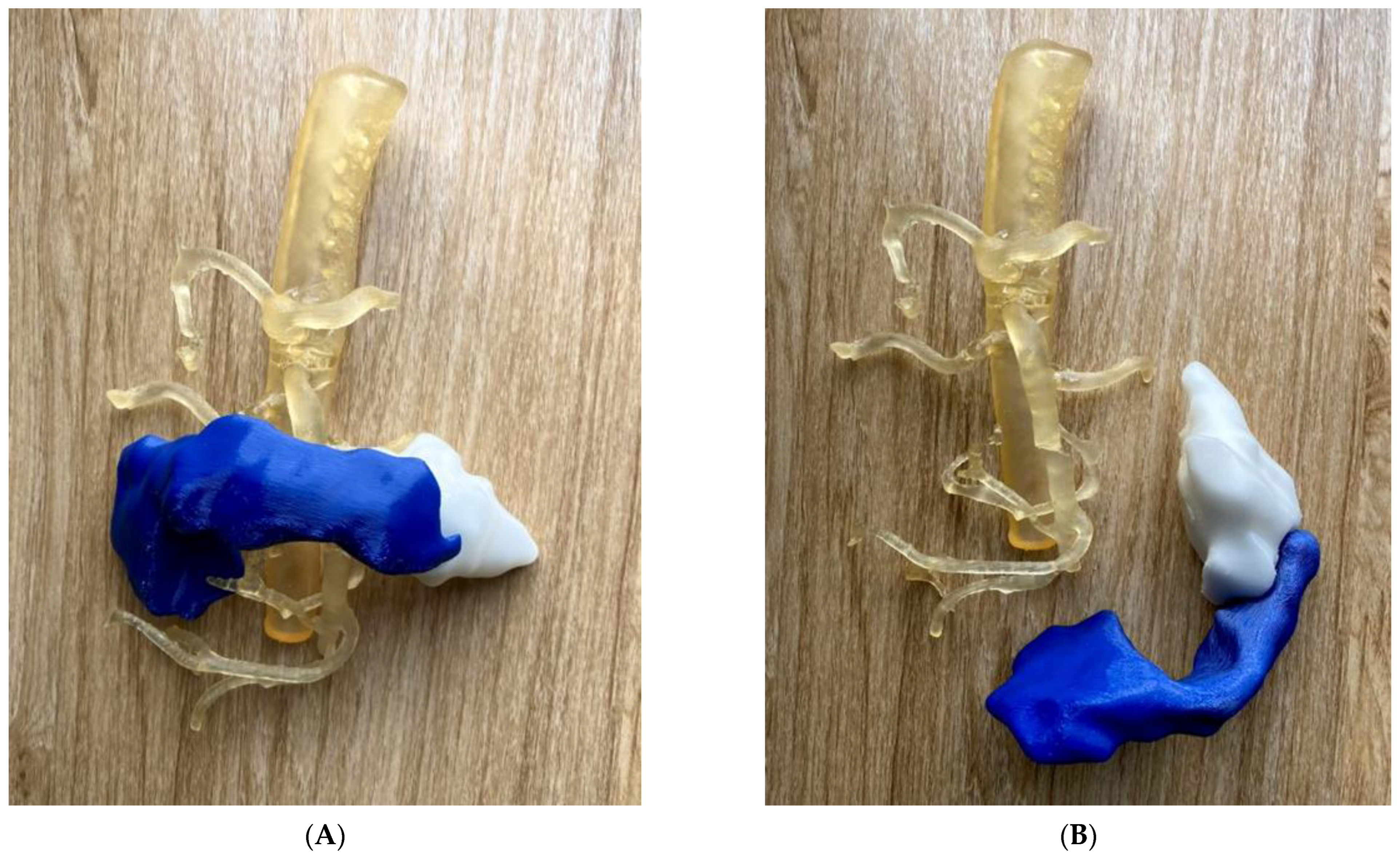
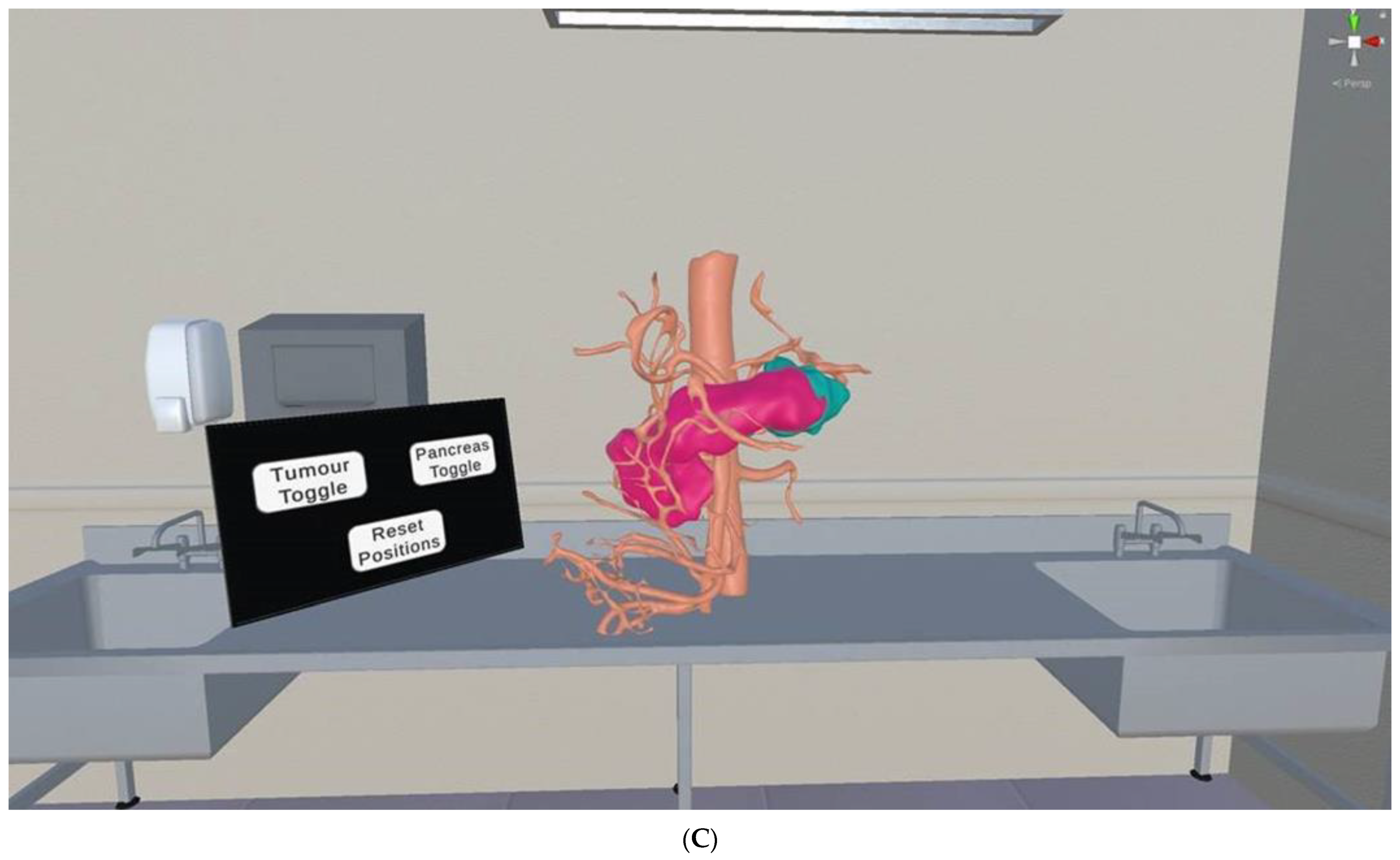
5.4. 3D-Printed Pancreatic Cancer Model
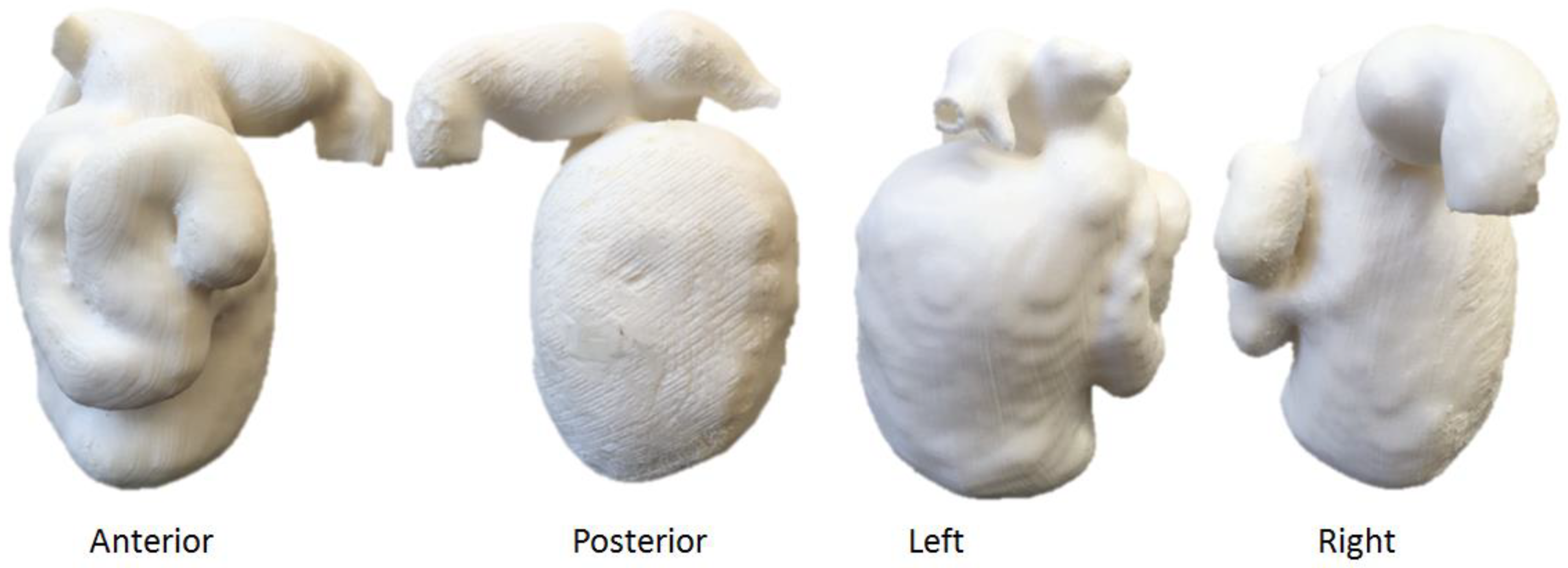
5.5. 3D-Printed Biliary Cyst Model
5.6. 3D-Printed Chest Models
5.7. 3D-Printed Models of Abdominal and Pelvic Organs
6. Limitations and Future Research Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannopoulos, A.A.; Steigner, M.L.; George, E.; Barile, M.; Hunsaker, A.R.; Rybicki, F.J.; Mitsouras, D. Cardiothoracic applications of 3-dimensional printing. J. Thorac. Imaging 2016, 31, 253–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witowski, J.; Wake, N.; Grochowska, A.; Sun, Z.; Budzynski, A.; Major, P.; Popiela, T.J.; Pedziwiatr, M. Investigating accuracy of 3d printed liver models with computed tomography. Quant. Imaging Med. Surg. 2019, 9, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.; Olivieri, L.; Krieger, A.; Thabit, O.; Marshall, M.B.; Yoo, S.J.; Kim, P.C.; Jonas, R.A.; Nath, D.S. Utilizing three-dimensional printing technology to assess the feasibility of high-fidelity synthetic ventricular septal defect models for simulation in medical education. World J. Pediatr. Congenit. Heart Surg. 2014, 5, 421–426. [Google Scholar] [CrossRef]
- Costello, J.P.; Olivieri, L.J.; Su, L.; Krieger, A.; Alfares, F.; Thabit, O.; Marshall, M.B.; Yoo, S.J.; Kim, P.C.; Jonas, R.A.; et al. Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians. Congenit. Heart Dis. 2015, 10, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Javan, R.; Zeman, M. A prototype educational model for hepatobiliary interventions: Unveiling the role of graphic designers in medical 3D printing. J. Digit. Imaging 2018, 31, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lupulescu, C.; Sun, Z. A systematic review of the clinical value and applications of three-dimensional printing in renal surgery. J. Clin. Med. 2019, 8, 990. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Liu, D. A systematic review of clinical value of three-dimensional printing in renal disease. Quant. Imaging Med. Surg. 2018, 8, 311–325. [Google Scholar] [CrossRef] [Green Version]
- Perica, E.; Sun, Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant. Imaging Med. Surg. 2017, 7, 668–677. [Google Scholar] [CrossRef] [Green Version]
- Perica, E.R.; Sun, Z. A systematic review of three-dimensional printing in liver disease. J. Digit. Imaging 2018, 31, 692–701. [Google Scholar] [CrossRef]
- Bhatla, P.; Tretter, J.T.; Chikkabyrappa, S.; Chakravarti, S.; Mosca, R.S. Surgical planning for a complex double-outlet right ventricle using 3D printing. Echocardiography 2017, 34, 802–804. [Google Scholar] [CrossRef]
- Biglino, G.; Milano, E.G.; Capelli, C.; Wray, J.; Shearn, A.I.; Caputo, M.; Bucciarelli-Ducci, C.; Taylor, A.M.; Schievano, S. Three-dimensional printing in congenital heart disease: Considerations on training and clinical implementation from a teaching session. Int. J. Artif. Organs. 2019, 42, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Squelch, A.; Sun, Z. Modelling of aortic aneurysm and aortic dissection through 3D printing. J. Med. Radiat. Sci. 2017, 64, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Godnell, J.; Pietila, T.; Samuel, B.P.; Kurup, H.K.N.; Haw, M.P.; Vettukattil, J.J. Integration of computed tomography and three-dimensional echocardiography for hybrid three-dimensional printing in congenital heart disease. J. Digit. Imaging 2016, 29, 665–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripley, B.; Kelil, T.; Cheezum, M.K.; Goncalves, A.; Di Carli, M.F.; Rybicki, F.J.; Steigner, M.; Mitsouras, D.; Blankstein, R. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomor. 2016, 10, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garas, M.; Vacccarezza, M.; Newland, G.; McVay-Doonbusch, K.; Hasani, J. 3D-printed speciamens as a valuable tool in anatomy education: A pilot study. Ann. Anat. 2018, 219, 57–64. [Google Scholar] [CrossRef]
- Loke, Y.H.; Harahsheh, A.S.; Krieger, A.; Olivier, L.J. Usage of 3D models of tetralogy of Fallot for medical education: Impact on learning congenital heart disease. BMC Med. Educ. 2017, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Shan, Q.; Huang, W.; Shang, M.; Wang, Z.; Xia, N.; Xue, Q.; Wu, Z.; Ding, X.; Mao, A.; Wang, Z. Customization of stent design for treating malignant airway stenosis with the aid of three-dimensional printing. Quant. Imaging Med. Surg. 2021, 11, 1437–1446. [Google Scholar] [CrossRef]
- Charnonnier, G.; Primikiris, P.; Billottet, B.; Louvrier, A.; Vancheri, S.; Ferhat, S.; Biondi, A. Pre-interventional 3D-printing-assisted planning of flow disrupter implantation for the treatment of an intracranial aneurysm. J. Clin. Med. 2022, 11, 2950. [Google Scholar] [CrossRef]
- Qiu, B.; Ji, Y.; Zhao, J.; Xue, Q.; Gao, S. Three-dimensional reconstruction/personalized three-dimensional printed model for thoracoscopic anatomical partial-lobectomy in stage I lung cancer: A retrospective study. Transl. Lung Cancer. Res. 2020, 9, 1235–1246. [Google Scholar] [CrossRef]
- Templin, R.; Tabriz, N.; Hoffmann, M.; Uslar, V.N.; Luck, T.; Schenk, A.; Malaka, R.; Zachmann, G.; Kluge, A.; Weyle, D. Case report: Virtual and interactive 3D vascular reconstruction before planned pancreatic head resection and complex vascular anatomy: A bench-to-bedside transfer of new visualization techniques in pancreatic surgery. Front. Surg. 2020, 7, 38. [Google Scholar] [CrossRef]
- Lau, I.; Sun, Z. Dimensional accuracy and clinical value of 3D printed models in congenital heart disease: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1483. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Squelch, A.; Sun, Z. Quantitative assessment of 3D printed model accuracy in delineating congenital heart disease. Biomolecules 2021, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.; Gomez-Ciriza, G.; Hussain, T.; Suarez-Mejias, C.; Velasco-Forte, M.N.; Byrne, N.; Ordonex, A.; Gonzalez-Calle, A.; Anderson, D.; Hazekamp, M.G.; et al. Three dimensional printed models for surgical planning of complex congenital heart defects: An international multicenter study. Eur. J. Cardiothorac. Surg. 2017, 52, 1139–1148. [Google Scholar] [CrossRef] [Green Version]
- Olejník, P.; Nosal, M.; Havran, T.; Furdova, A.; Cizmar, M.; Slabej, M.; Thurzo, A.; Vitovic, P.; Klvac, M.; Acel, T. Utilisation of three-dimensional printed heart models for operative planning of complex congenital heart defects. Kardiol. Pol. 2017, 75, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, L.J.; Krieger, A.; Loke, Y.-H.; Nath, D.S.; Kim, P.C.; Sable, C.A. Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: Feasibility and relative accuracy. J. Am. Soc. Echocardiogr. 2015, 28, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Lau, I.W.W.; Liu, D.; Xu, L.; Fan, Z.; Sun, Z. Clinical value of patient-specific three-dimensional printing of congenital heart disease: Quantitative and qualitative assessments. PLoS ONE 2018, 13, e0194333. [Google Scholar] [CrossRef] [PubMed]
- Mowers, K.L.; Fullerton, J.B.; Hicks, D.; Singh, G.K.; Johnson, M.C.; Anwar, S. 3D echocardiography provides highly accurate 3D printed models in congenital heart disease. Pediatr. Cardiol. 2021, 42, 131–141. [Google Scholar] [CrossRef]
- Illmann, C.F.; Hosking, M.; Harris, K.C. Utility and access to 3-dimensional printing in the context of congenital heart disease: An International physician survey study. CJC Open 2020, 2, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Hoashi, T.; Ichikawa, H.; Nakata, T.; Shimada, M.; Ozawa, H.; Higashida, A.; Kurosaki, K.; Kanzaki, S.; Shiraishi, I. Utility of a super-flexible three-dimensional printed heart model in congenital heart surgery. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 749–755. [Google Scholar] [CrossRef]
- Hussein, N.; Honji, O.; Barron, D.J.; Haller, C.; Coles, J.G.; Yoo, S.J. The incorporation of hands-on surgical training in a congenital heart surgery training curriculum. Ann. Thorac. Surg. 2021, 112, 1672–1680. [Google Scholar] [CrossRef]
- Sun, Z.; Wee, C. 3D printed models in cardiovascular disease: An exciting future to deliver personalized medicine. Micromachines 2022, 13, 1575. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. Clinical applications of patient-specific 3D printed models in cardiovascular disease: Current status and future directions. Biomolecules 2020, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lau, I.; Wong, Y.H.; Yeong, C.H. Personalized three-dimensional printed models in congenital heart disease. J. Clin. Med. 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.A.; Squelch, A.; Jansen, S.; Sun, Z. Optimization of computed tomography angiography protocols for follow-up type B aortic dissection patients by using 3D printed model. Appl. Sci. 2021, 11, 6844. [Google Scholar] [CrossRef]
- Moore, R.A.; Riggs, K.W.; Kourtidou, S.; Schneider, K.; Szugye, N.; Troja, W.; D’Souza, G.; Rattan, M.; Bryant, R., 3rd; Taylor, M.D.; et al. Three-dimensional printing and virtual surgery for congenital heart procedural planning. Birth Defects Res. 2018, 110, 1082–1090. [Google Scholar] [CrossRef]
- Parimi, M.; Buelter, J.; Thanugundla, V.; Condoor, S.; Parkar, N.; Danon, S.; King, W. Feasibility and validity of printing 3D heart models from rotational angiography. Pediatr. Cardiol. 2018, 39, 653–658. [Google Scholar] [CrossRef]
- Lau, I.; Squelch, A.; Wan, Y.L.; Wong, A.M.; Ducke, V.; Sun, Z. Patient-specific 3D printed model in delineating brain glioma and surrounding structures in a pediatric patient. Digit. Med. 2017, 3, 86–92. [Google Scholar]
- Nizam, A.; Gopal, R.N.; Naing, L.; Hakim, A.B.; Samsudin, A.R. Dimensional accuracy of the skull models produced by rapid prototyping technology using stereolithography apparatus. Arch. Orofac. Sci. 2006, 1, 60–66. [Google Scholar]
- Lim, K.H.; Loo, Z.Y.; Goldie, S.; Adams, J.; McMenamin, P. Use of 3D printed models in medical education: A randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat. Sci. Educ. 2016, 9, 213–221. [Google Scholar] [CrossRef]
- Karsenty, C.; Guitarte, A.; Dulac, Y.; Briot, J.; Hascoet, S.; Vincent, R.; Delepaul, B.; Vignaud, P.; Djeddai, C.; Hadeed, K.; et al. The usefulness of 3D printed heart models for medical student education in congenital heart disease. BMC Med. Edc. 2021, 21, 480. [Google Scholar] [CrossRef]
- Su, W.; Xiao, Y.; He, S.; Huang, P.; Deng, X. Three-dimensional printing models in congenital heart disease education for medical students: A controlled comparative study. BMC Med. Educ. 2018, 18, 178. [Google Scholar] [CrossRef]
- Valverde, I.; Gomez, G.; Byrne, N.; Anwar, S.; Silva Cerpa, M.A.; Talavera, M.M.; Pushparajah, K.; Velasco Forte, M.N. Criss-cross heart three-dimensional printed models in medical education: A multicentre study on their value as a supporting tool to conventional imaging. Anat. Sci. Educ. 2022, 15, 719–730. [Google Scholar] [CrossRef] [PubMed]
- White, S.C.; Sedler, J.; Jones, T.W.; Seckeler, M. Utility of three-dimensional models in resident education on simple and complex intracardiac congenital heart defects. Congenit. Heart Dis. 2018, 13, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Smerling, J.; Marboe, C.C.; Lefkowitch, J.H.; Pavilicova, M.; Bacha, E.; Einstein, A.J.; Naka, Y.; Glickstein, J.; Farooqi, K.M. Utility of 3D printed cardiac models for medical student education in congenital heart disease: Across a spectrum of disease severity. Pediatr. Cardiol. 2019, 40, 1258–1265. [Google Scholar] [CrossRef]
- Sun, Z.; Lee, S. A systematic review of 3D printing in cardiovascular and cerebrovascular diseases. Anatol. J. Cardiol. 2017, 17, 423–435. [Google Scholar] [PubMed]
- Lau, I.; Sun, Z. Three-dimensional printing in congenital heart disease: A systematic review. J. Med. Radiat. Sci. 2018, 65, 226–236. [Google Scholar] [CrossRef]
- Biglino, G.; Capelli, C.; Koniordou, D.; Robertshaw, D.; Leaver, L.K.; Schievano, S.; Taylor, A.M.; Wray, J. Use of 3D models of congenital heart disease as an education tool for cardiac nurses. Congenit. Heart Dis. 2017, 12, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Huang, E.; Deng, X.; Ouyang, S. Application of 3D printing technology combined with PBL teaching model in teaching clinical nursing in congenital heart surgery: A case-control study. Medicine 2021, 100, 20. [Google Scholar] [CrossRef]
- Lau, I.; Sun, Z. The role of 3D printed heart models in immediate and long-term knowledge retention in medical education. Rev. Cardiovasc. Med. 2022, 23, 022. [Google Scholar] [CrossRef]
- Lau, I.; Wong, Y.H.; Yeong, C.H.; Abdul Aziz, Y.F.; Md Sari, N.S.; Hasim, S.A.; Sun, Z. Quantitative and qualitative comparison of low- and high-cost 3D-printed heart models. Quant. Imaging Med. Surg. 2019, 9, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z. Patient-specific 3D printed models in pediatric congenital heart disease. Children 2023, 10, 319. [Google Scholar] [CrossRef]
- Lau, I.; Gupta, A.; Sun, Z. Clinical value of virtual reality versus 3D printing in congenital heart disease. Biomolecules 2021, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ng, C.K.C.; Wong, Y.H.; Yeong, C.H. 3D-printed coronary plaques to simulate high calcification in the coronary arteries for investigation of blooming artifacts. Biomolecules 2021, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. 3D printed coronary models offer new opportunities for developing optimal coronary CT angiography protocols in imaging coronary stents. Quant. Imaging Med. Surg. 2019, 9, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jansen, S. Personalized 3D printed coronary models in coronary stenting. Quant. Imaging Med. Surg. 2019, 9, 1356–1367. [Google Scholar] [CrossRef]
- Misra, A.; Walters, H.L.; Kobayashi, D. Utilisation of a three-dimensional printed model for the management of coronary-pulmonary artery fistula from left main coronary artery. Cardiol. Young. 2019, 29, 431–434. [Google Scholar] [CrossRef]
- Velasco Forte, M.N.; Byrne, N.; Valverde Perez, I.; Bell, A.; Gómez-Ciriza, G.; Krasemann, T.; Sievert, H.; Simpson, J.; Pushparajah, K.; Razavi, R.; et al. 3D printed models in patients with coronary artery fistulae: Anatomical assessment and interventional planning. EuroIntervention 2017, 13, e1080–e1083. [Google Scholar] [CrossRef]
- Torres, I.O.; De Luccia, N. A simulator for training in endovascular aneurysm repair: The use of three dimensional printers. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Karkkainen, J.M.; Sandri, G.; Tenorio, E.R.; Alexander, A.; Bjellum, K.; Matsumoto, J.; Morris, J.; Mendes, B.C.; DeMartino, R.R.; Oderich, G.S. Simulation of endovascular aortic repair using 3D printed abdominal aortic aneurysm model and fluid pump. Cardiovasc. Intervent. Radiol. 2019, 42, 1627–1634. [Google Scholar] [CrossRef]
- Kaufmann, R.; Zech, C.J.; Takes, M.; Brantner, P.; Thieringer, F.; Dentschmann, M.; Hergan, K.; Scharinger, B.; Hecht, S.; Rezar, R.; et al. Vascular 3D printing with a novel biological tissue mimicking resin for patient-specific procedure simulations in interventional radiology: A feasibility study. J. Digit. Imaging 2022, 35, 9–20. [Google Scholar] [CrossRef]
- Wu, C.; Squelch, A.; Sun, Z. Investigation of three-dimensional printing materials for printing aorta model replicating type B aortic dissection. Curr. Med. Imaging 2021, 17, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, M.J.; Patel, R.; Powell, J.T.; Greenhalgh, R.; for the EVAR trial investigators. Endovascular repair of abdominal aortic aneurysm in patients physically ineligible for open repair: Very long-term follow-up in the EVAR trial-2 randomized controlled trial. Ann. Surg. 2017, 266, 713–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Khan, S.; Salata, K.; Hussain, M.A.; de Mestral, C.; Greco, E.; Aljarbi, B.A.; Forbes, T.L.; Verma, S.; Al-Omran, M. A systematic review and meta-analysis of the long-term outcomes of endovascular versus open repair of abdominal aortic aneurysm. J. Vasc. Surg. 2019, 70, 954–969. [Google Scholar] [CrossRef]
- Wu, C.; Squelch, A.; Sun, Z. Assessment of optimization of computed tomography angiography protocols for follow-up type B aortic dissection patients by using a 3D-printed model. J. 3D Print. Med. 2022, 6, 117–127. [Google Scholar] [CrossRef]
- Pereira, H.R.; Barzegar, M.; Hamadelseed, O.; Valls Esteve, A.; Munuera, J. 3D surgical planning of pediatric tumours: A review. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Sindi, R.; Wong, Y.H.; Yeong, C.H.; Sun, Z. Development of patient-specific 3D-printed breast phantom using silicone and peanut oils for magnetic resonance imaging. Quant. Imaging Med. Surg. 2020, 10, 1237–1248. [Google Scholar] [CrossRef]
- Sindi, R.; Wong, Y.H.; Yeong, C.H.; Sun, Z. Quantitative measurement of breast density using personalized 3D-printed breast model for magnetic resonance imaging. Diagnostics 2020, 10, 793. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Dyer, B.A.; Boone, J.M.; Liu, S.; Chen, T.; Zheng, F.; Zhu, Y.; Sun, Y.; Rong, Y.; et al. 3D printed breast phantom for multi-purpose and multi-modality imaging. Quant. Imaging Med. Surg. 2019, 9, 63–74. [Google Scholar] [CrossRef]
- He, Y.; Qin, S.; Dyer, B.A.; Zhang, H.; Zhao, L.; Chen, T.; Zheng, F.; Sun, Y.; Shi, L.; Rong, Y.; et al. Characterizing mechanical and medical imaging properties of polyvinyl chloride-based tissue-mimicking materials. J. Appl. Clin. Med. Phys. 2019, 20, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Freed, M.; de Zwart, J.A.; Loud, J.T.; El Khouli, R.H.; Myers, K.J.; Greene, M.H.; Duyn, J.H.; Badano, A. An anthropomorphic phantom for quantitative evaluation of breast MRI. Med. Phys. 2011, 38, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Pitz, C.C.M.; Riviere, A.B.; Swieten, H.A.; Duurkens, V.A.; Lammers, J.J.; Bosch, J.M. Surgical treatment of Pancoast tumours. Eur. J. Cardiothorac. Surg. 2004, 26, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Yek, W.Y.; Wong, Y.H.; Yeong, C.H.; Sun, Z. Clinical application of three-dimensional printed models in preoperative planning of Pancoast tumour resection. Australas. Med. J. 2020, 13, 292–296. [Google Scholar]
- Yoon, S.H.; Park, S.; Kang, C.H.; Park, I.K.; Goo, J.M.; Kim, Y.T. Personalized 3D-printed model for informed consent for stage I lung cancer: A randomized pilot trial. Semin. Thoracic. Surg. 2018, 31, 316–318. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Chen, Q.; Li, T.; Chen, K.; Yu, Q.; Lin, X. Three-dimensional printing technology for localised thoracoscopic segmental resection for lung cancer: A quasi-randomised clinical trial. World J. Surg. Oncol. 2020, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Bertolo, R.; Checcucci, E.; Amparore, D.; Autorino, R.; Dasgupta, P.; Wiklund, P.; Tewari, A.; Liatsikos, E.; Fiori, C. Development and validation of 3D printed virtual models for robot-assisted radical prostatectomy and partial nephrectomy: Urologists’ and patients’ perception. World J. Urol. 2017, 36, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wake, N.; Rosenkrantz, A.B.; Huang, R.; Park, K.U.; Wysock, J.S.; Taneja, S.S.; Huang, W.C.; Sodickson, D.K.; Chandarana, H. Patient-specific 3D printed and augmented reality kidney and prostate cancer models: Impact on patient education. 3D Print Med. 2019, 5, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Golab, A.; Slojewski, M.; Brykczynski, M.; Lukowiak, M.; Boehlke, M.; Matias, D.; Smektala, T. Three-dimensional printing as an interdisciplinary communication tool: Preparing for removal of a giant renal tumor and atrium neoplastic mass. Heart Surg. Forum. 2016, 19, E185–E186. [Google Scholar] [CrossRef] [PubMed]
- Lupulescu, C.; Sun, Z. The 3D printing of patient-specific kidney models to facilitate pre-surgical planning of renal cell carcinoma using CT datasets. Australas. Med. J. 2021, 14, 211–222. [Google Scholar]
- Wake, N.; Nussbaum, J.E.; Elias, M.I.; Nikas, C.V.; Bjulin, M.A. 3D printing, augmented reality, and virtual reality for the assessment and management of kidney and prostate cancer: A systematic review. Urology 2020, 143, 20–32. [Google Scholar] [CrossRef]
- Bati, A.H.; Guler, E.; Ozer, M.A.; Govsa, F.; Erozkan, K.; Vatansever, S.; Ersin, M.S.; Elmas, Z.N.; Harman, M. Surgical planning with patient-specific three-dimensional printed pancreaticobiliary disease models-cross-sectional study. Int. J. Surg. 2020, 80, 175–183. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, D.; Zhao, J.; Wu, Y.; Zheng, B. 3D printout models vs. 3D-rendered images: Which is better for preoperative planning? J. Surg. Educ. 2016, 73, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.; Kealley, C.; Squelch, A.; Wong, Y.H.; Yeong, C.H.; Sun, Z. Patient-specific 3D printed model of biliary ducts with congenital cyst. Quant. Imaging Med. Surg. 2019, 9, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhir, V.; Itoi, T.; Fockens, P.; Perez-Miranda, M.; Khashab, M.A.; Seo, D.W.; Yang, A.M.; Laurence, K.Y.; Maydeo, A. Novel ex vivo model for hands-on teaching of and training in EUS-guided biliary drainage: Creation of “Mumbai EUS” stereolithography/3D printing bile duct prototype (with videos). Gastrointest. Endosc. 2015, 81, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.H.; Wake, N.; Witowski, J.; Rybicki, F.J.; Sheikh, A. RSNA special Interest Group for 3D Printing Abdominal, Hepatobiliary and Gastrointestinal Conditions Voting Group. Radiological Society of North America (RSNA) 3D Printing Special Interest Group (SIG) clinical situations for which 3D printing is considered an appropriate representation or extension of data contained in a medical imaging examination: Abdominal, hepatobiliary, and gastrointestinal conditions. 3D Print. Med. 2020, 6, 13. [Google Scholar]
- Aldosari, S.; Jansen, S.; Sun, Z. Optimization of computed tomography pulmonary angiography protocols using 3D printed model with simulation of pulmonary embolism. Quant. Imaging Med. Surg. 2019, 9, 53–62. [Google Scholar] [CrossRef]
- Aldosari, S.; Jansen, S.; Sun, Z. Patient-specific 3D printed pulmonary artery model with simulation of peripheral pulmonary embolism for developing optimal computed tomography pulmonary angiography protocols. Quant. Imaging Med. Surg. 2019, 9, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Fulcher, A.S.; Turner, M.A. Abdominal manifestations of situs anomalies in adults. Radiographics 2002, 22, 1439–1456. [Google Scholar] [CrossRef]
- Etherton, D.; Tee, L.; Tillett, C.; Wong, Y.H.; Yeong, C.H.; Sun, Z. 3D visualization and 3D printing in abnormal gastrointestinal system manifestations of situs ambiguus. Quant. Imaging Med. Surg. 2020, 10, 1877–1883. [Google Scholar] [CrossRef]
- Guler, E.; Ozer, M.A.; Bati, A.H.; Govsa, F.; Erozkan, K.; Vatansever, S.; Ersin, M.S.; Elmas, N.Z. Patient-centered oncosurgical planning with cancer models in subspecialty education. Surg. Oncol. 2021, 37, 101537. [Google Scholar] [CrossRef]
- Povey, M.; Powell, S.; Howes, N.; Vimalachandran, D.; Sutton, P. Evaluating the potential utility of three-dimensional printed models in preoperative planning and patient consent in gastrointestinal cancer surgery. Ann. R Coll. Surg. Engl. 2021, 103, 615–620. [Google Scholar]
- Oxford, K.; Walsh, G.; Quigley, S.; Dubrowski, A. Development, manufacture and initial assessment of validity of a 3-dimensional-printed bowel anastomosis simulation training model. Can. J. Surg. 2021, 64, E484–E490. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Liang, J.; Zhang, Z.; Cao, T.; Zhang, L. Preliminary application of three-dimensional printing in congenial uterine anomalies based on three-dimensional transvaginal ultrasonographic data. BMC. Womens Health 2022, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ciriza, G.; Gomez-Cia, T.; Rivas-Gonzalez, J.A.; Velasco Forte, M.N.; Valverde, I. Affordable three-dimensional printed heart models. Front. Cardiovasc. Med. 2021, 8, 642011. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Spray, T.; Austin, E.H.; Yun, T.J.; van Arsdell, G.S. Hands-on surgical training of congenital heart surgery suing 3-dimensional print models. J. Thorac. Cardiovasc. Surg. 2017, 153, 15301–15540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, B.S.; Thierij, A.; Jakob, A.; Tengler, A.; Grab, M.; Thierfelder, N.; Leuner, C.J.; Haas, N.A.; Hopfner, C. 3D-printed heart models for hands-on training in pediatric cardiology-the future of modern learning and teaching? GMS J. Med. Educ. 2022, 39, Doc23. [Google Scholar]
- Kaufmann, R.; Deutschmann, M.; Meissnitzer, M.; Scharinger, B.; Hergan, K.; Votsch, A.; Dinges, C.; Hecht, S. Manufacturing flexible vascular models for cardiovascular surgery planning and endovascular procedure simulations: An approach to segmentation and post-processing with open-source software and end-user 3D printers. Int. J. Bioprint. 2023, 9, 669. [Google Scholar] [CrossRef]
- Sommer, K.N.; Lyer, V.; Kumamaru, K.K.; Rava, R.A.; Ionita, C.N. Method to simulate distal flow resistance in coronary arteries in 3D printed patient specific coronary models. 3D Print. Med. 2020, 6, 19. [Google Scholar] [CrossRef]
- Scanlan, A.B.; Nguyen, A.V.; IIina, A.; Lasso, A.; Cripe, L.; Jegatheeswaran, A.; Silvestro, E.; McGowan, F.X.; Mascio, C.E.; Fuller, S.; et al. Comprison of 3D echocardiography-derived 3D printed valve models to molded models for simulated repair of pediatric atrioventricular valves. Pediatr. Cardiol. 2018, 39, 538–547. [Google Scholar] [CrossRef]
- Sutherland, J.; Belek, J.; Sheikh, A.; Chepelev, L.; Althobaity, W.; Chow, B.J.W.; Mitsouris, D.; Christensen, A.; Rybicki, F.J.; La Russa, D.J. Applying modern virtual and augmented reality technologies to medical images and models. J. Digit. Imaging 2019, 32, 38–53. [Google Scholar] [CrossRef]
- Dhar, P.; Rocks, T.; Samarasinghe, R.M.; Stephenson, G.; Smith, C. Augmented reality in medical education: Students’ experience and learning outcomes. Med. Educ. Online 2021, 26, 1953953. [Google Scholar] [CrossRef]
- Patel, N.; Costa, A.; Sanders, S.P.; Ezon, D. Stereoscopic virtual reality does not improve knowledge retention of congenital heart disease. Int. J. Cardiovasc. Imaging 2021, 37, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Maresky, H.S.; Oikonomou, A.; Ali, I.; Ditkofsky, N.; Pakkal, M.; Ballyk, B. Virtual reality and cardiac anatomy: Exploring immersive three-dimensional cardiac imaging, a pilot study in undergraduate medical anatomy education. Clin. Anat. 2019, 32, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Barteit, S.; Lanfermann, L.; Barnighausen, T.; Neuhann, F.; Beiersmann, C. Augmentted reality, mixed and virtual reality-based head-mounted devices for medical education: Systematic review. JMIR. Serious Games 2021, 9, e29080. [Google Scholar] [CrossRef]
- Lau, I.; Gupta, A.; Ihdayhid, A.; Sun, Z. Clinical applications of mixed reality and 3D printing in congenital heart disease. Biomolecules 2022, 12, 1548. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Van De Bruaene, A.; Farooqi, K.; Valverde, I.; Jung, C.; Votta, E.; Sturla, F.; Paul Diller, G.; Brida, M.; Sun, Z.; et al. Three-dimensional printing, holograms, computational modelling, and artificial intelligence for adult congenital heart disease care: An exciting future. Eur. Heart J. 2022, 43, 2672–2684. [Google Scholar]








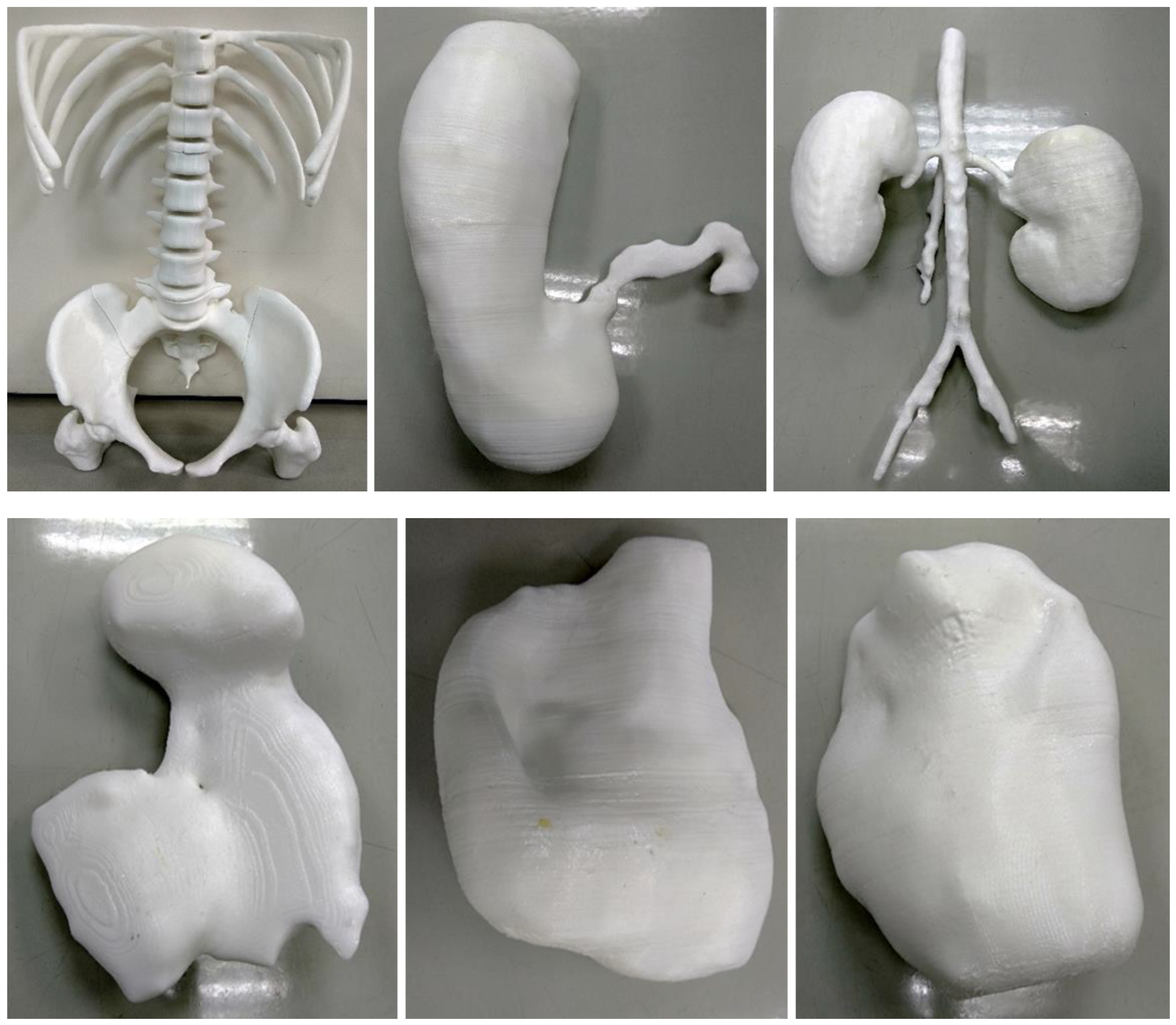
| Anatomical Region | Number of Models | Original Data Source | Applications of 3D Printed Models | 3D Printer/Printing Materials/Costs | 3D Printing Parameters (Resolution, Printing Time) |
|---|---|---|---|---|---|
| Cardiovascular system | |||||
| Heart | 4 | CT | Congenital heart disease for education and preoperative planning | Printer: Anycubic Photon S Material: Polyurethane (PU) 80A Cost: USD 25 per model | Model was printed at a resolution of 47 μm for the x- and y-axis planes; 10 μm for z-axis planes Time: ~10 h per model |
| Coronary artery | 6 | CT | Coronary stenosis for optimal CT protocols | Printer: Anycubic Photon S Material: Polyurethane (PU) 80A Cost: USD 15 per model | Model was printed at a resolution of 47 μm for the x- and y-axis planes. 10 μm for z-axis planes Time: ~6 h per model |
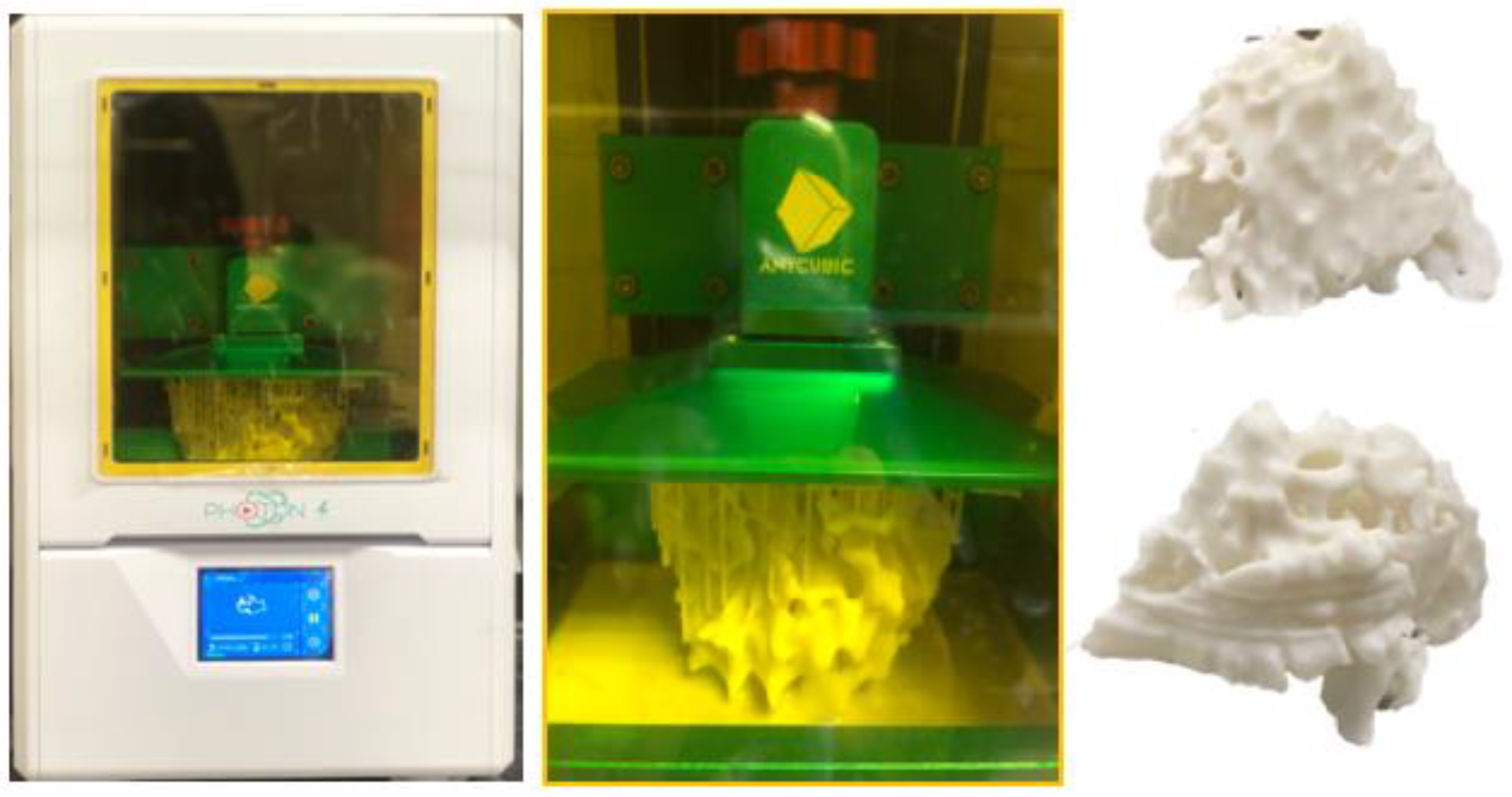
| Calcified plaque | 22 | N/A | For the simulation of calcified plaques | Printer: The mould (circular rod) was printed with polylactic acid (PLA) using Ultimaker 2 + Extended Material: Silicone + 32.8% calcium carbonate Cost: USD 10 for the mould | The mould was printed at a resolution of 12.5 μm for the x, y and z-axis planes. Time: ~3 h for the mould |
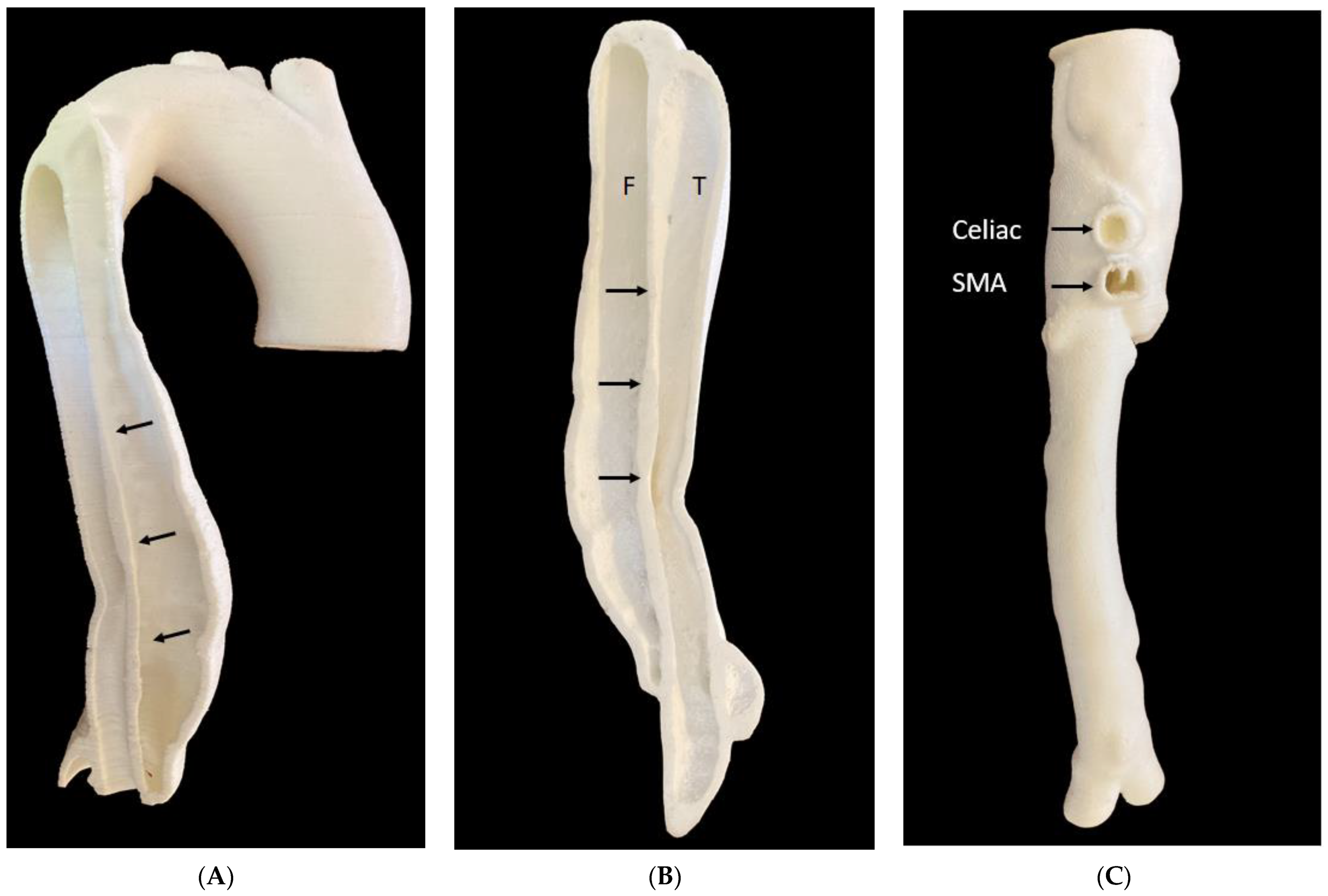
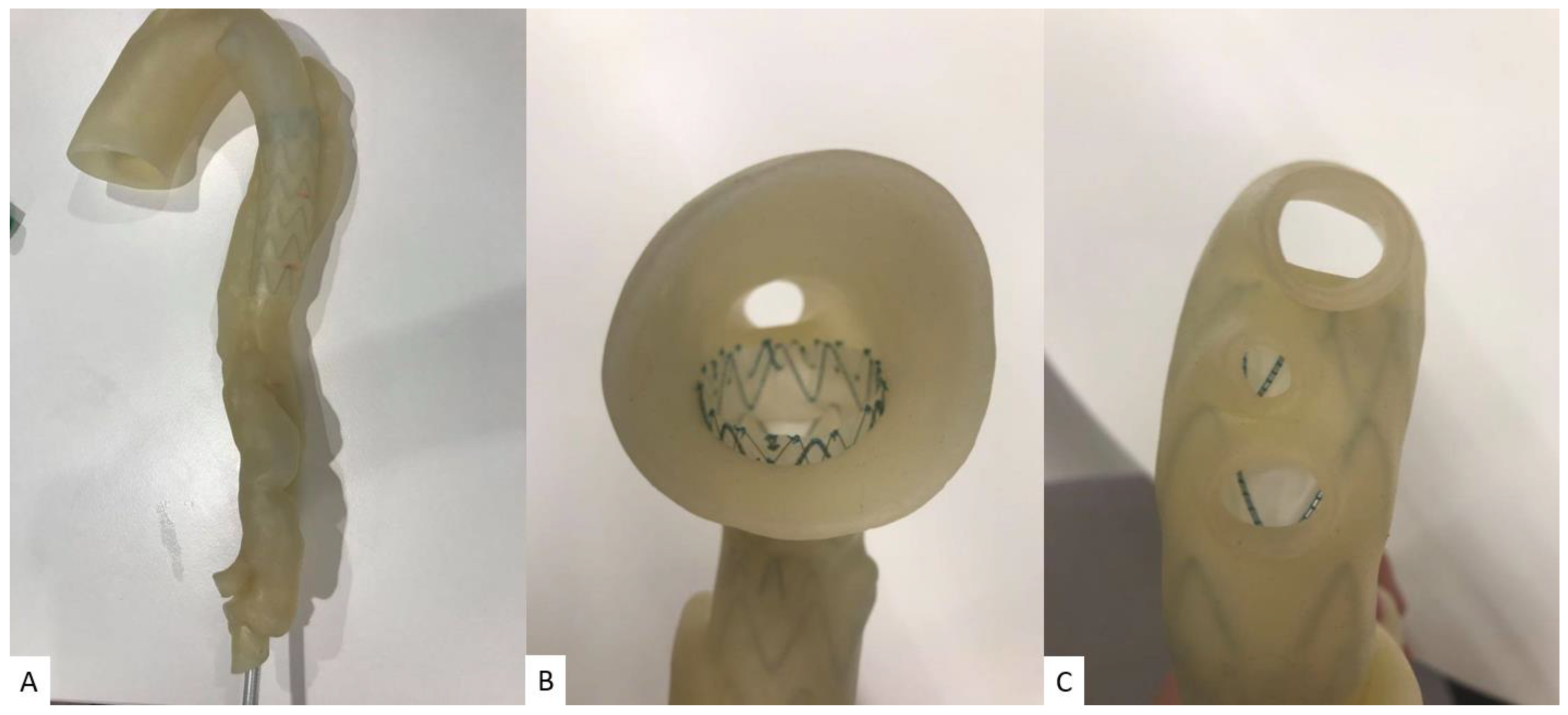
| Aorta | 6: Abdominal aortic aneurysm: 5 Aortic dissection: 1 | CT | Aortic aneurysm and aortic dissection for the simulation of endovascular repair and CT protocols | Printer: Ultimaker 2+ Extended/Raise3D N2 Plus Materials: Thermoplastic polyurethane (TPU) 95A, PLA, polyethylene terephthalate glycol (PETG), polymethacrylate (PMMA) and high impact polystyrene (HIPS) Cost: USD 50 per model | Aorta was printed with PLA, HIPS, PMMA were at a resolution of 12.5 μm for the x, y and z-axis planes. Aorta was printed with TPU95A was at a resolution of 12.5 μm for the x and y-axis planes; 10 μm for z-axis plane Time: ~100 h per model |
| Tumours | |||||
| Breast | 1 | MRI | Breast cancer model for breast MRI protocols | Printer: Breast skin shell was printed using Raise3D N2 Plus; Fibroglandular tissues were printed using Anycubic Photon S Materials: Breast skin shell was printed PLA; Fibroglandular tissues were printed using Magma H LINE Photopolymer Resin Cost: USD 30 for breast skin shell and USD 25 for fibroglandular tissues | Breast skin shell was printed at a resolution of 12.5 μm for the x and y-axis planes; 10 μm for z-axis plane Fibroglandular tissues were printed at a resolution of 47 μm for the x, y and z-axis planes Time: ~40 h for breast skin shell and 50 h for fibroglandular tissues |
| Biliary cyst | 1 | CT | Accuracy and preoperative planning | Ultimaker 2+ Extended Material: TPU 95A Cost: USD 35 | Model was printed at a resolution of 12.5 μm for the x, y and z-axis plane Time: ~70 h |
| Pancreas | 2: Pancreatic tumour: 1 Abdominal aorta and branches: 1 | CT | Pancreatic cancer for preoperative planning and education | Printer: Abdominal aorta and arterial branches were printed using Anycubic Photon S. Pancreatic tumour was printed using Raise3D N2 Plus. Materials: Abdominal aorta and arterial branches were printed with PU80A; Pancreatic tumour was printed with PLA Cost: USD 20 | Abdominal aorta and arterial branches were printed at a resolution of 47 μm x, y and z-axis planes. Pancreatic tumour was printed at resolution of 12.5 μm x, y and z-axis planes Time: ~20 h |
| Kidneys | 1 | CT | Renal cell carcinoma for preoperative planning | Ultimaker 2+ Extended Material: TPU 95A Cost: USD 20 | Model was printed at a resolution of 12.5 μm for the x, y and z-axis planes Time: ~70 h |
| Others (thoracic and abdominal organs) | |||||
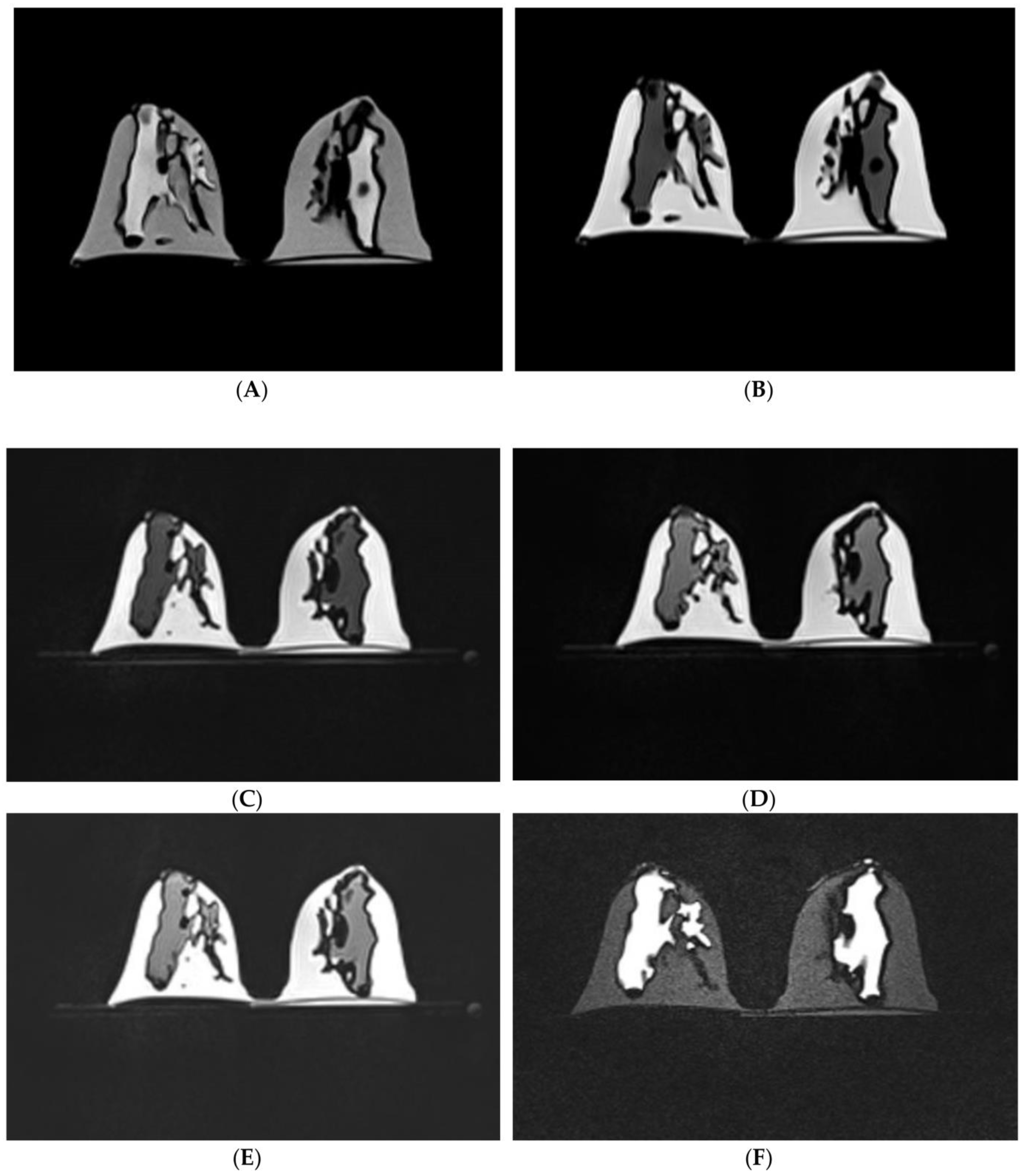
| Chest (lungs, thoracic vertebral column and ribs) | 1 with three compoents: lung shell, thoracic ribs/vertebrae and trachea | CT | Creation of anatomical environment for cardiovascular imaging studies | Printer: Thoracic ribs and lung shell were printed using Raise3D N2 Plus; Trachea was printed using Ultimaker 2+ Extended Materials: Thoracic ribs/vertebrae and lung shell were printed with PLA Trachea was printed with TPU 95A. Cost: USD 75 for thoracic ribs and lung shell; USD 10 for trachea | Thoracic ribs and lung shell were printed at a resolution of 12.5 μm for the x and y-axis planes; 10 μm for z-axis plane Trachea was printed at a resolution of 12.5 μm for the x, y and z-axis planes Time: ~450 h for thoracic ribs and lung shell; ~17 h for trachea |
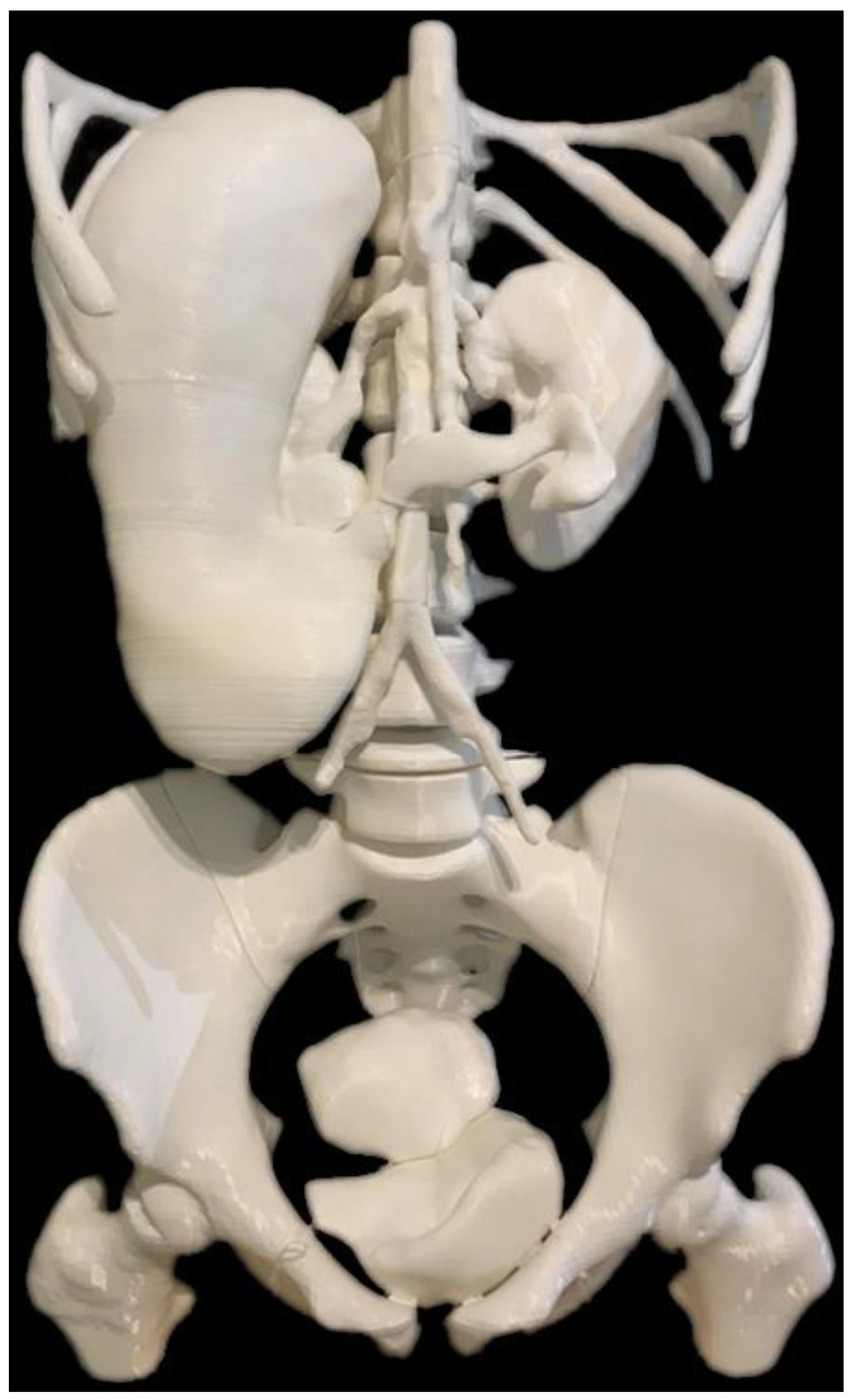
| Abdomen and pelvis | Stomach: 1 Kidneys: 1 Spleen: 1 Bladder: 1 Uterus: 1 Skeleton: 1 | CT | Multiple organs for a case of situs ambiguus | Printer: Skeleton was printed using Raise3D N2 Plus (Raise3D, USA) Other organs were printed using Ultimaker 2+ Extended (Ultimaker BV, Netherland) Material: Skeleton: PLA Other organs: TPU 95A Cost: USD 55 for skeleton and USD 75 for other organs | Skeleton was printed with a resolution of 12.5 μm for the x and y-axis planes and 10 μm for z-axis plane Other organs were printed at a resolution of 12.5 μm for the x, y and z-axis plane Time: Skeleton: ~250 h Other organs: ~250 h |
| Studies Reporting Accuracy Comparison | No. of Models Printed | Comparisons | Mean Difference (mm) | Analysis Method |
|---|---|---|---|---|
| Lee et al. [22] | 3 | 3D model vs. original CT 3D model vs. CT of 3D model 3D model vs. STL files Original CT images vs. STL files | 0.21 ± 0.37 mm −0.11 ± 0.47 mm 0.1 ± 0.28/ 0.17 ± 0.48 mm 0.12 ± 0.23/ 0.12 ± 0.25 mm | Pearson’s correlation/ Bland–Altman plot |
| Valverde et al. [23] | 40 (20 selected for accuracy comparison) | 3D model vs. both CT and MRI 3D model vs. original CT 3D model vs. original MRI | 0.27 ± 0.73 mm −0.16 ± 0.85 mm −0.30 ± 0.67 mm | Bland–Altman plot |
| Olejník et al. [24] | 8 | CT images vs. STL | 0.19 ± 0.38 mm | Bland–Altman plot |
| 3D model vs. in vivo | 0.13 ± 0.26 mm | |||
| Olivieri et al. [25] | 9 | 3D model vs. echocardiography | 0.4 ± 0.9 mm | Pearson’s correlation/ Bland–Altman plot |
| Lau et al. [26] | 1 | 3D model vs. CT | 0.23 mm | Pearson’s correlation |
| Mowers et al. [27] | 5 | 2D echo vs. digital 3D | 0 mm | Pearson’s correlation/ Bland–Altman plot |
| 2D echo vs. 3D model | 0.3 mm | |||
| Parimi et al. [36] | 5 | 3D model vs. rotational angiography | No significant difference between 3D models and biplane angiography measurements (p = 0.14) | Pearson’s correlation/ Bland–Altman plot |
| Question | Doctors’ Group, n = 9 | Non-Doctors’ Group, n = 20 | Mann–Whitney U-Value | p-Value | |
|---|---|---|---|---|---|
| Option | |||||
| Rate the usefulness of VR models in medical education | 4 (11.22) | 4 (16.70) | 56.00 | 0.07 | |
| Rate the usefulness of 3DPHM in medical education | 4 (11.11) | 5 (16.75) | 55.00 | 0.07 | |
| Rate the usefulness of VR models in pre-operative planning | 4 (11.06) | 4 (16.77) | 54.50 | 0.07 | |
| Rate the usefulness of 3DPHM in pre-operative planning | 4 (12.39) | 4.5 (16.18) | 66.50 | 0.23 | |
| 3D Printing Applications | Challenges and Limitations | References |
|---|---|---|
| Pre-surgical planning and simulation (cardiac disease and tumours) |
| [26,31,33,37,50,52,72,78,82] |
| Simulation of interventional cardiac/radiological procedures |
| [33,34,53,54,55,61,64,66,67,85,86] |
| Medical education |
| [49,52,88] |
| Patient/family education/communication |
| [88,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Wong, Y.H.; Yeong, C.H. Patient-Specific 3D-Printed Low-Cost Models in Medical Education and Clinical Practice. Micromachines 2023, 14, 464. https://doi.org/10.3390/mi14020464
Sun Z, Wong YH, Yeong CH. Patient-Specific 3D-Printed Low-Cost Models in Medical Education and Clinical Practice. Micromachines. 2023; 14(2):464. https://doi.org/10.3390/mi14020464
Chicago/Turabian StyleSun, Zhonghua, Yin How Wong, and Chai Hong Yeong. 2023. "Patient-Specific 3D-Printed Low-Cost Models in Medical Education and Clinical Practice" Micromachines 14, no. 2: 464. https://doi.org/10.3390/mi14020464
APA StyleSun, Z., Wong, Y. H., & Yeong, C. H. (2023). Patient-Specific 3D-Printed Low-Cost Models in Medical Education and Clinical Practice. Micromachines, 14(2), 464. https://doi.org/10.3390/mi14020464








