Hierarchical NiMn-LDH Hollow Spheres as a Promising Pseudocapacitive Electrode for Supercapacitor Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
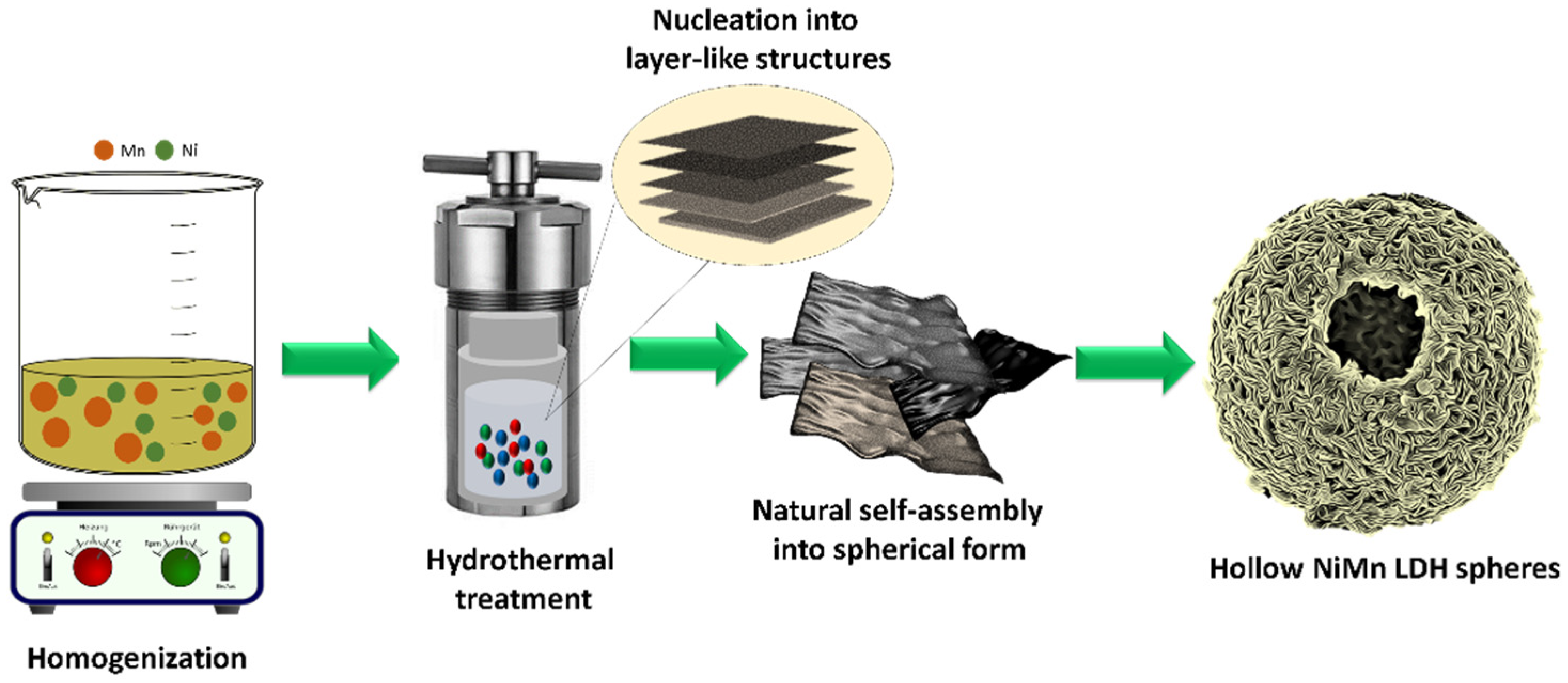
2.2. Synthesis of Hierarchical Hollow-NiMn LDHs
2.3. Characterizations
Electrochemical Analysis
3. Results and Discussion
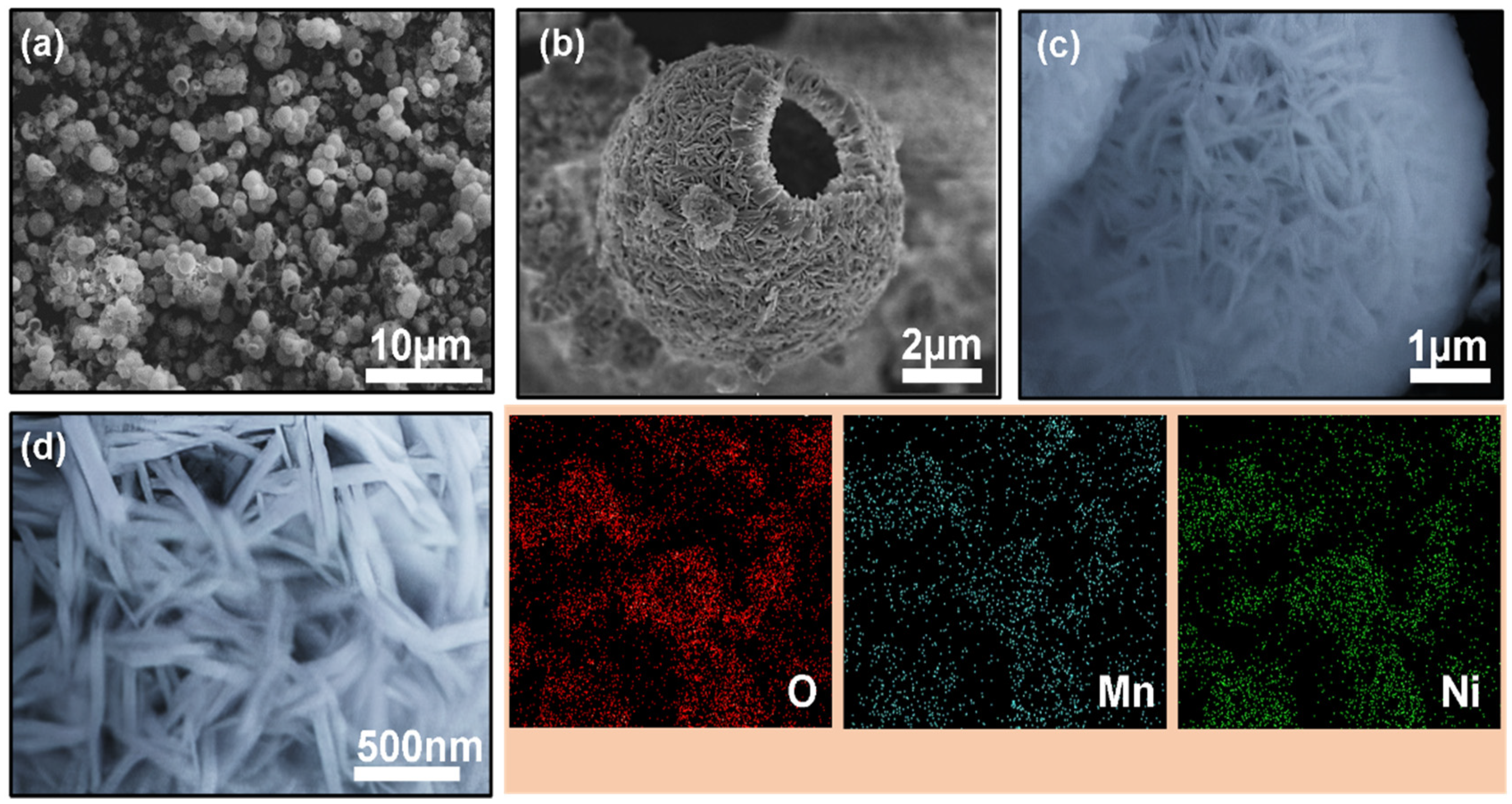
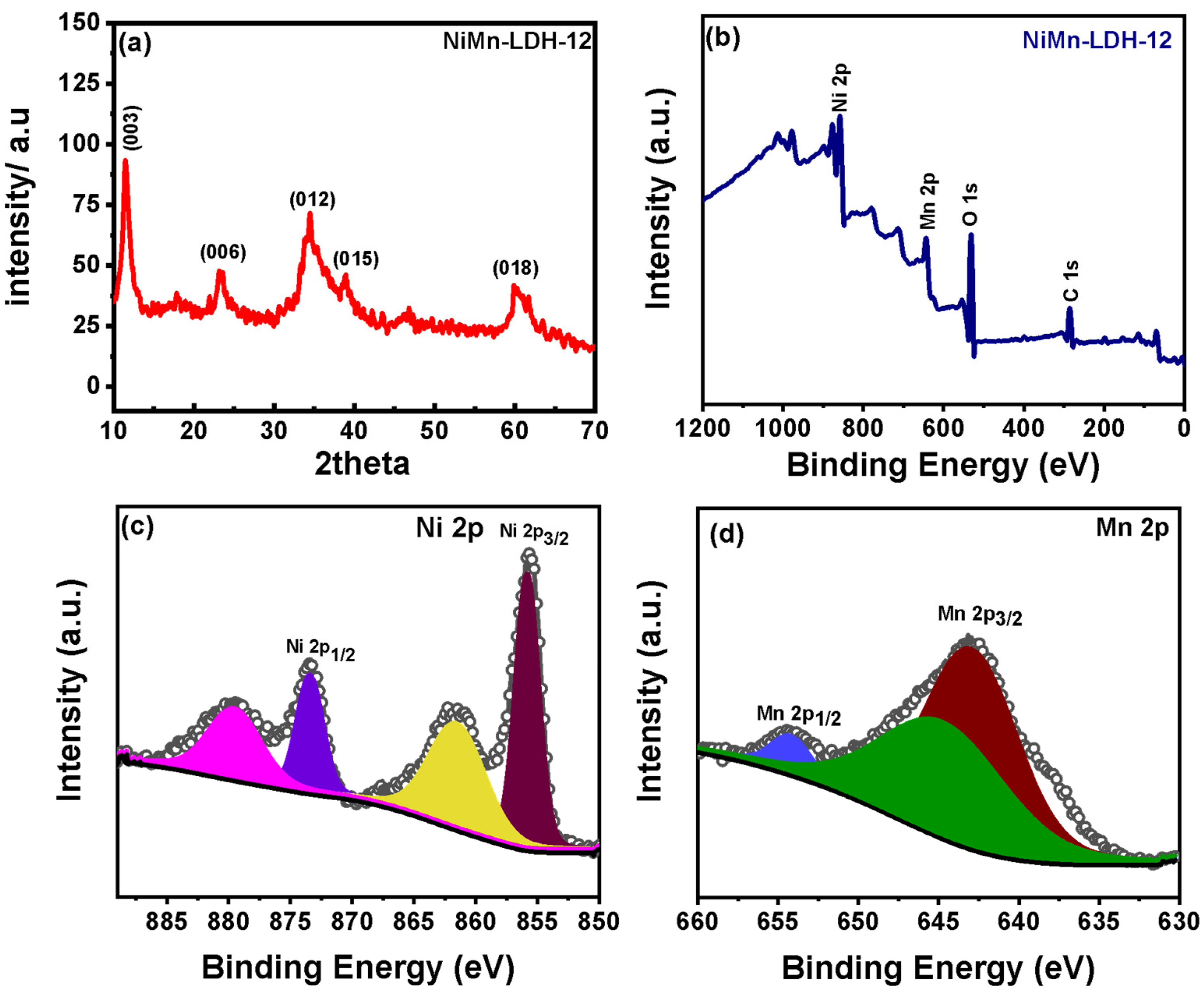
3.1. Synthesis and Characterization of Hollow Sphere NiMn-LDH
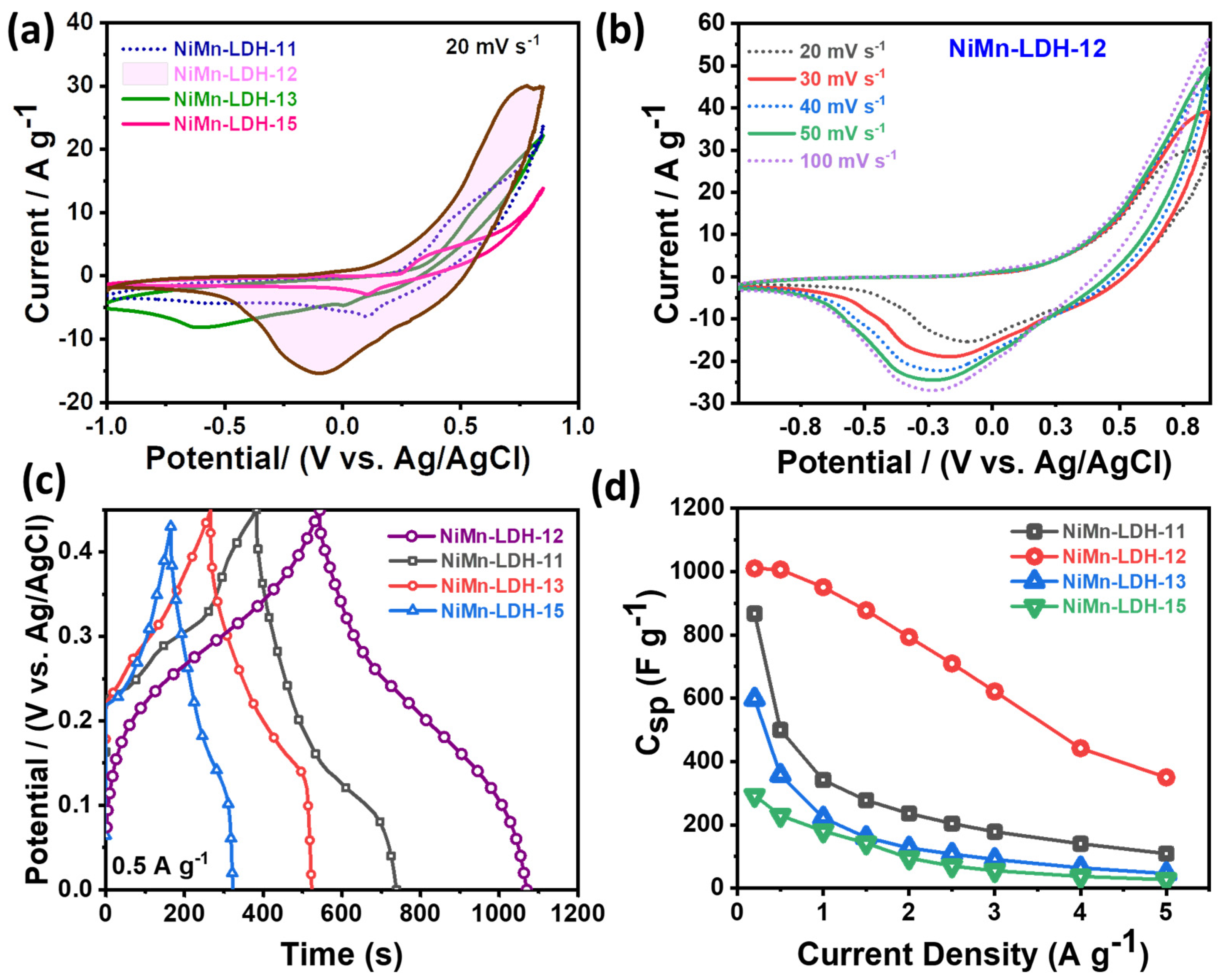
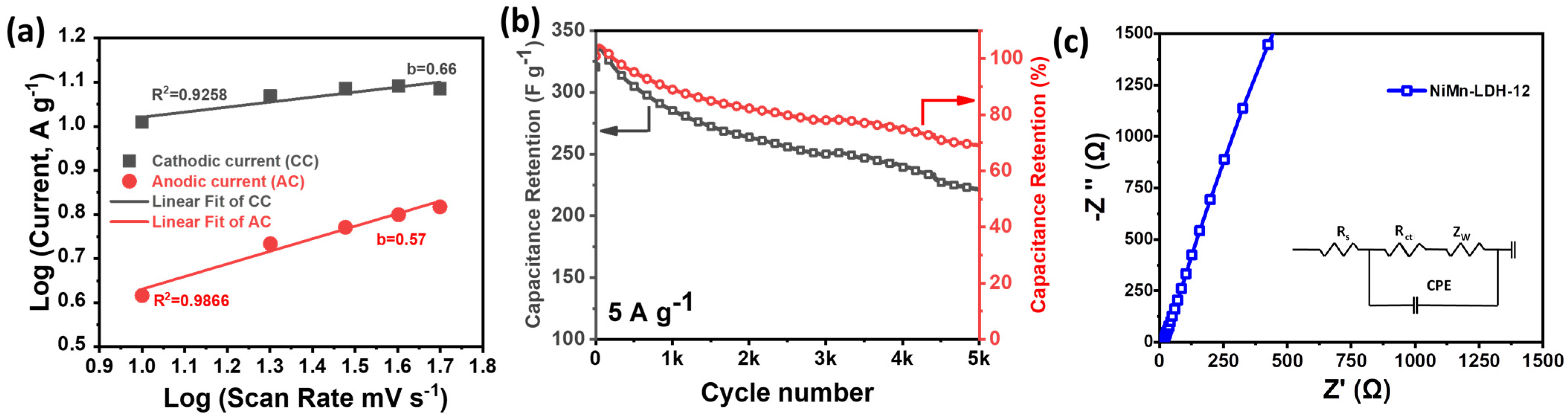
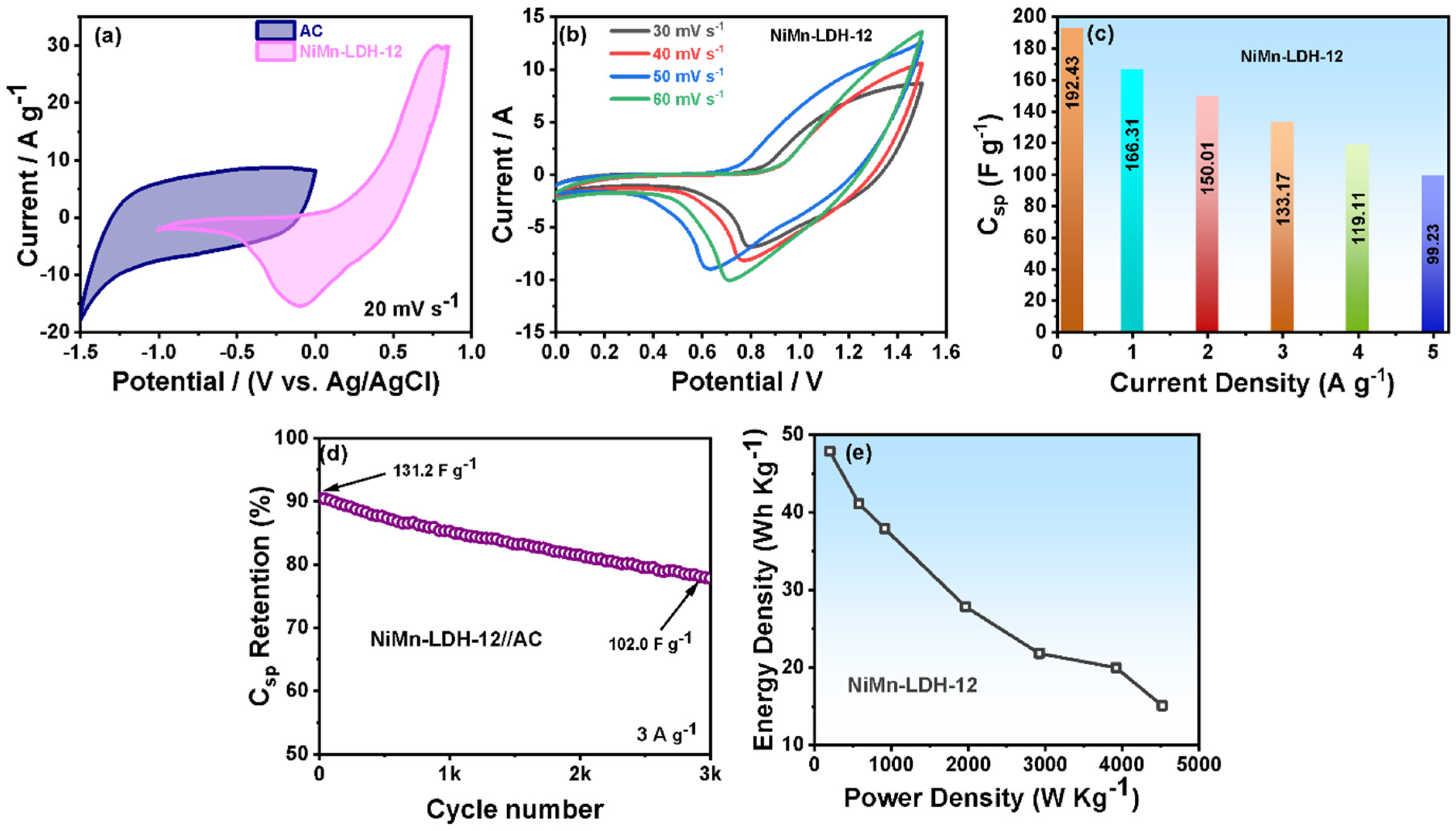
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Xie, K.; Wei, B. Materials and Structures for Stretchable Energy Storage and Conversion Devices. Adv. Mater. 2014, 26, 3592–3617. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible solid-state supercapacitors: Design, fabrication and applications. Energy Environ. Sci. 2014, 7, 2160–2181. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like chitosan porous carbon Spheres/MXene composite with high specific capacitance and rate performance for supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, B.; Feng, L.; Zhang, Q.; Zhang, C.; Yang, W.; Han, J.; Jiang, S.; He, S. Potassium citrate assisted synthesis of hierarchical porous carbon materials for high performance supercapacitors. Diam. Relat. Mater. 2022, 128, 109247. [Google Scholar] [CrossRef]
- Mondal, A.; Lee, C.-Y.; Chang, H.; Hasin, P.; Yang, C.-R.; Lin, J.-Y. Electrodeposited Co0.85Se thin films as free-standing cathode materials for high-performance hybrid supercapacitors. J. Taiwan Inst. Chem. Eng. 2021, 121, 205–216. [Google Scholar] [CrossRef]
- Mondal, A.; Maiti, S.; Mahanty, S.; Baran Panda, A. Large-scale synthesis of porous NiCo2O4 and rGO–NiCo2O4 hollow-spheres with superior electrochemical performance as a faradaic electrode. J. Mater. Chem. A 2017, 5, 16854–16864. [Google Scholar] [CrossRef]
- Feng, L.; Yan, B.; Zheng, J.; Chen, J.; Wei, R.; Jiang, S.; Yang, W.; Zhang, Q.; He, S. Soybean protein-derived N, O co-doped porous carbon sheets for supercapacitor applications. New J. Chem. 2022, 46, 10844–10853. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Jiang, S.; Zhang, C.; Ding, Y.; Han, J.; Chen, W.; He, S. High performance supercapacitors based on wood-derived thick carbon electrodes synthesized via green activation process. Inorg. Chem. Front. 2022, 9, 6108–6123. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Cheng, H.-M. Hierarchical porous nickel oxide and carbon as electrode materials for asymmetric supercapacitor. J. Power Sources 2008, 185, 1563–1568. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, Y.; Zhang, M.-L.; Yan, Y.-D. A new asymmetric supercapacitor based on λ-MnO2 and activated carbon electrodes. Mater. Lett. 2008, 62, 3884–3886. [Google Scholar] [CrossRef]
- Qu, Q.T.; Shi, Y.; Li, L.L.; Guo, W.L.; Wu, Y.P.; Zhang, H.P.; Guan, S.Y.; Holze, R. V2O5·0.6H2O nanoribbons as cathode material for asymmetric supercapacitor in K2SO4 solution. Electrochem. Commun. 2009, 11, 1325–1328. [Google Scholar] [CrossRef]
- Jing, C.; Liu, X.; Yao, H.; Yan, P.; Zhao, G.; Bai, X.; Dong, B.; Dong, F.; Li, S.; Zhang, Y. Phase and morphology evolution of CoAl LDH nanosheets towards advanced supercapacitor applications. CrystEngComm 2019, 21, 4934–4942. [Google Scholar] [CrossRef]
- Hua, X.; Mao, C.-J.; Chen, J.-S.; Chen, P.-P.; Zhang, C.-F. Facile synthesis of new-type MnOOH/NiAl-layered double hydroxide nanocomposite for high-performance supercapacitor. J. Alloys Compd. 2019, 777, 749–758. [Google Scholar] [CrossRef]
- Baig, M.M.; Gul, I.H.; Ahmad, R.; Baig, S.M.; Khan, M.Z.; Iqbal, N. One-step sonochemical synthesis of NiMn-LDH for supercapacitors and overall water splitting. J. Mater. Sci. 2021, 56, 18636–18649. [Google Scholar] [CrossRef]
- Li, S.; Cheng, P.; Luo, J.; Zhou, D.; Xu, W.; Li, J.; Li, R.; Yuan, D. High-Performance Flexible Asymmetric Supercapacitor Based on CoAl-LDH and rGO Electrodes. Nano-Micro Lett. 2017, 9, 31. [Google Scholar] [CrossRef]
- Yang, P.; Jing, C.; Liu, J.C.; Chen, K.; Zhang, Y.X. Controllable crystal growth of a NiCo-LDH nanostructure anchored onto KCu7S4 nanowires via a facile solvothermal method for supercapacitor application. CrystEngComm 2020, 22, 1602–1609. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.P.; Wang, J.; Liu, F.; Zhang, X.B. The growth of nickel-manganese and cobalt-manganese layered double hydroxides on reduced graphene oxide for supercapacitor. Electrochim. Acta 2016, 206, 108–115. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Ma, J.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical NiMn Layered Double Hydroxide/Carbon Nanotubes Architecture with Superb Energy Density for Flexible Supercapacitors. Adv. Funct. Mater. 2014, 24, 2938–2946. [Google Scholar] [CrossRef]
- Sim, H.; Jo, C.; Yu, T.; Lim, E.; Yoon, S.; Lee, J.H.; Yoo, J.; Lee, J.; Lim, B. Reverse Micelle Synthesis of Colloidal Nickel–Manganese Layered Double Hydroxide Nanosheets and Their Pseudocapacitive Properties. Chem.—A Eur. J. 2014, 20, 14880–14884. [Google Scholar] [CrossRef]
- Wan, H.; Liu, J.; Ruan, Y.; Lv, L.; Peng, L.; Ji, X.; Miao, L.; Jiang, J. Hierarchical Configuration of NiCo2S4 Nanotube@Ni–Mn Layered Double Hydroxide Arrays/Three-Dimensional Graphene Sponge as Electrode Materials for High-Capacitance Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 15840–15847. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, W.; Tian, Y.; Yang, S.; Zhang, Q.; Lei, D.; Zhao, Y. Nanosheet-assembled NiCo-LDH hollow spheres as high-performance electrodes for supercapacitors. J. Colloid Interface Sci. 2022, 606, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, D.; Yue, L.; Zheng, K.; Zhang, A.; Jia, Q.; Liu, J. In Situ Fabrication of a Uniform Co-MOF Shell Coordinated with CoNiO2 to Enhance the Energy Storage Capability of NiCo-LDH via Vapor-Phase Growth. ACS Appl. Mater. Interfaces 2020, 12, 47526–47538. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wu, H.B.; Lou, X.W.D. Self-Templated Formation of Hollow Structures for Electrochemical Energy Applications. Acc. Chem. Res. 2017, 50, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yuan, P.; Guo, S.; Liu, F.; Cheng, J.P. Design and synthesis of Ni-Co and Ni-Mn layered double hydroxides hollow microspheres for supercapacitor. Int. J. Hydrogen Energy 2017, 42, 28797–28806. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Z.; Qin, Z.; Sun, H.; Jiao, X.; Chen, D. LDH nanocages synthesized with MOF templates and their high performance as supercapacitors. Nanoscale 2013, 5, 11770–11775. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, Q.; Li, W.; Li, Y.; Yang, C.; Tao, K.; Han, L. Construction of 2D ZIF-derived hierarchical and hollow NiCo-LDH “nanosheet-on-nanosheet” arrays on reduced graphene oxide/Ni foam for boosted electrochemical energy storage. J. Alloys Compd. 2021, 850, 156864. [Google Scholar] [CrossRef]
- Luo, H.; Wang, B.; Liu, T.; Jin, F.; Liu, R.; Xu, C.; Wang, C.; Ji, K.; Zhou, Y.; Wang, D.; et al. Hierarchical design of hollow Co-Ni LDH nanocages strung by MnO2 nanowire with enhanced pseudocapacitive properties. Energy Storage Mater. 2019, 19, 370–378. [Google Scholar] [CrossRef]
- Anitha, T.; Reddy, A.E.; Durga, I.K.; Rao, S.S.; Nam, H.W.; Kim, H.-J. Facile synthesis of ZnWO4@ WS2 cauliflower-like structures for supercapacitors with enhanced electrochemical performance. J. Electroanal. Chem. 2019, 841, 86–93. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Peng, C.-K.; Bu, L.; Chiang, C.-L.; Tian, K.; Zhao, Y.; Zhao, J.; Lin, Y.-G.; Lee, J.-M.; et al. Co-Induced Electronic Optimization of Hierarchical NiFe LDH for Oxygen Evolution. Small 2020, 16, 2002426. [Google Scholar] [CrossRef]
- Shi, L.; Sun, P.; Du, L.; Xu, R.; He, H.; Tan, S.; Zhao, C.; Huang, L.; Mai, W. Flexible honeycomb-like NiMn layered double hydroxide/carbon cloth architecture for electrochemical energy storage. Mater. Lett. 2016, 175, 275–278. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.P.; Liu, F.; Zhang, X.B. In situ growth of nickel-cobalt oxyhydroxide/oxide on carbon nanotubes for high performance supercapacitors. Electrochim. Acta 2015, 178, 439–446. [Google Scholar] [CrossRef]
- Quan, W.; Tang, Z.L.; Wang, S.T.; Hong, Y.; Zhang, Z.T. Facile preparation of free-standing rGO paper-based Ni–Mn LDH/graphene superlattice composites as a pseudocapacitive electrode. Chem. Commun. 2016, 52, 3694–3696. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.C.; Liu, X.L.; Yuan, Y.S.; Li, N.; Lan, T.; Liu, X.Y.; Zhang, Y.X. Facile synthesis of manganese cobalt oxide/nickel cobalt oxide composites for high-performance supercapacitors. Front. Chem. 2019, 6, 661. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, K.; Balaji, D.; Madhavan, J.; Theerthagiri, J.; Lee, S.J.; Kwon, K.-Y.; Choi, M.Y. Cost-effective synthesis of efficient CoWO4/Ni nanocomposite electrode material for supercapacitor applications. Nanomaterials 2020, 10, 2195. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.-Q.; Yang, F.; Zhao, Y.-L.; Xu, X.; Xu, L.; Luo, Y.-Z. Hierarchical MnMoO4/CoMoO4 heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011, 2, 381. [Google Scholar] [CrossRef]
- Neiber, R.R.; Kumar, J.; Soomro, R.A.; Karkus, S.; Abo-Dief, H.M.; Alanazi, A.K.; Altalhia, A.A.; El-Bahy, Z.M. NiZnCoO4/CoWO4 hybrid composite with improved electrochemical performance for supercapacitor application. J. Energy Storage 2022, 52, 104900. [Google Scholar] [CrossRef]
- Chandrasekaran, N.I.; Kumari, M.; Muthukumar, H.; Matheswaran, M. Strategy for Multifunctional Hollow Shelled Triple Oxide Mn–Cu–Al Nanocomposite Synthesis via Microwave-Assisted Technique. ACS Sustain. Chem. Eng. 2018, 6, 1009–1021. [Google Scholar] [CrossRef]
- Wang , Q.; Xu, J.; Wang, X.; Liu, B.; Hou, X.; Yu, G.; Wang, P.; Chen, D.; Shen, G. Core–Shell CuCo2O4@MnO2 Nanowires on Carbon Fabrics as High-Performance Materials for Flexible, All-Solid-State, Electrochemical Capacitors. ChemElectroChem 2014, 1, 559–564. [Google Scholar] [CrossRef]
- Hu, X.-W.; Liu, S.; Qu, B.-T.; You, X.-Z. Starfish-shaped Co3O4/ZnFe2O4 Hollow Nanocomposite: Synthesis, Supercapacity, and Magnetic Properties. ACS Appl. Mater. Interfaces 2015, 7, 9972–9981. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Shen, L.; Gao, B.; Hao, L.; Lu, X.; Zhang, F.; Ding, B.; Yuan, C. Enhanced high-current capacitive behavior of graphene/CoAl-layered double hydroxide composites as electrode material for supercapacitors. J. Power Sources 2012, 199, 395–401. [Google Scholar] [CrossRef]
- Cheng, J.P.; Fang, J.H.; Li, M.; Zhang, W.F.; Liu, F.; Zhang, X.B. Enhanced electrochemical performance of CoAl-layered double hydroxide nanosheet arrays coated by platinum films. Electrochim. Acta 2013, 114, 68–75. [Google Scholar] [CrossRef]
- Fang, J.; Li, M.; Li, Q.; Zhang, W.; Shou, Q.; Liu, F.; Zhang, X.; Cheng, J. Microwave-assisted synthesis of CoAl-layered double hydroxide/graphene oxide composite and its application in supercapacitors. Electrochim. Acta 2012, 85, 248–255. [Google Scholar] [CrossRef]
- Han, J.; Dou, Y.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Flexible CoAl LDH@PEDOT Core/Shell Nanoplatelet Array for High-Performance Energy Storage. Small 2013, 9, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Q.; Qian, Z.; Zhang, X.; Wang, J.; Li, Z.; Yan, H.; Gao, Z.; Zhao, F.; Liu, L. Two steps in situ structure fabrication of Ni–Al layered double hydroxide on Ni foam and its electrochemical performance for supercapacitors. J. Power Sources 2014, 246, 747–753. [Google Scholar] [CrossRef]
- Shao, M.; Ning, F.; Zhao, Y.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Core–Shell Layered Double Hydroxide Microspheres with Tunable Interior Architecture for Supercapacitors. Chem. Mater. 2012, 24, 1192–1197. [Google Scholar] [CrossRef]
- Zhang, L.; Hui, K.N.; Hui, K.S.; Lee, H. Facile synthesis of porous CoAl-layered double hydroxide/graphene composite with enhanced capacitive performance for supercapacitors. Electrochim. Acta 2015, 186, 522–529. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Purushothaman, K.K.; Muralidharan, G. Fabrication of two-dimensional reduced graphene oxide supported V2O5 networks and their application in supercapacitors. Mater. Chem. Phys. 2016, 170, 266–275. [Google Scholar] [CrossRef]
- Javed, M.S.; Shah, S.S.A.; Najam, T.; Siyal, S.H.; Hussain, S.; Saleem, M.; Zhao, Z.; Mai, W. Achieving high-energy density and superior cyclic stability in flexible and lightweight pseudocapacitor through synergic effects of binder-free CoGa2O4 2D-hexagonal nanoplates. Nano Energy 2020, 77, 105276. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Lin, L.-Y.; Huang, Y.-Y.; Hong, W.-L. Nickel precursor-free synthesis of nickel cobalt-based ternary metal oxides for asymmetric supercapacitors. Electrochim. Acta 2018, 281, 692–699. [Google Scholar] [CrossRef]
| Composite Material | Specific Capacitance (F g−1) | Current Density (A g−1) | Electrolyte | Ref. |
|---|---|---|---|---|
| MnCo2O4.5/NiCo2O4 | 325 | 1 | 3 M KOH | [34] |
| CoWO4–Ni3 | 271 | 1 | 6 M KOH | [35] |
| MnMoO4/CoMoO4 | 187 | 1 | 2 M NaOH | [36] |
| ZnNnCO4/CWO4 | 309 | 1 | 2 M KOH | [37] |
| Hollow shelled Mn–Cu–Al–oxide | 319 | 1 | 1 M Na2SO4 | [38] |
| Core-shell CuCo2O4/MnO2 nanowires | 327 | 1.25 | 3 M KOH | [39] |
| Starfish-shaped Co3O4/ZnFe2O4 nanocomposite | 326 | 1 | 6 M KOH | [40] |
| Co–Al LDH/graphene | 712 | 1 | 6 M KOH | [41] |
| Co–Al LDH/Pt | 734 | 3 | 2 M KOH | [42] |
| Co–Al LDH/graphene | 772 | 1 | 6 M KOH | [43] |
| CoAl-LDH/PEDOT | 672 | 1 | 6 M KOH | [44] |
| NiAl LDH | 795 | 0.5 | 1 M KOH | [45] |
| NiAl LDH | 735 | 2 | 1 M KOH | [46] |
| CoAl LDH/graphene | 479 | 1 | 6 M KOH | [47] |
| Hollow sphere NiMn-LDH-12 | 951 | 1 | 3 M KOH | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, J.; Neiber, R.R.; Abbas, Z.; Soomro, R.A.; BaQais, A.; Amin, M.A.; El-Bahy, Z.M. Hierarchical NiMn-LDH Hollow Spheres as a Promising Pseudocapacitive Electrode for Supercapacitor Application. Micromachines 2023, 14, 487. https://doi.org/10.3390/mi14020487
Kumar J, Neiber RR, Abbas Z, Soomro RA, BaQais A, Amin MA, El-Bahy ZM. Hierarchical NiMn-LDH Hollow Spheres as a Promising Pseudocapacitive Electrode for Supercapacitor Application. Micromachines. 2023; 14(2):487. https://doi.org/10.3390/mi14020487
Chicago/Turabian StyleKumar, Jai, Rana R. Neiber, Zaheer Abbas, Razium Ali Soomro, Amal BaQais, Mohammed A. Amin, and Zeinhom M. El-Bahy. 2023. "Hierarchical NiMn-LDH Hollow Spheres as a Promising Pseudocapacitive Electrode for Supercapacitor Application" Micromachines 14, no. 2: 487. https://doi.org/10.3390/mi14020487







