Development and Characterization of Multi-Alkali Antimonide Photocathodes for High-Brightness RF Photoinjectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photocathode Laboratory
2.2. Photocathode Preparation
3. Results
3.1. Fabrication Recipes
3.1.1. Sb Structure
3.1.2. K-Sb Structure
3.1.3. K-Cs-Sb Structure
3.2. Lifetime Study
4. Density Functional Theory (DFT) Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McNeil, B.W.J.; Thompson, N.R. X-ray free-electron lasers. Nat. Photon. 2010, 4, 814–821. [Google Scholar] [CrossRef]
- Merminga, L. Energy Recovery Linacs. In Synchrotron Light Sourcesand Free-Electron Lasers; Jaeschke, E., Khan, S., Schneider, J., Hastings, J., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Europe turns on bright X-ray source. Nat. Photon. 2017, 11, 609. [CrossRef]
- Hamamatsu Photonics K.K. Photomultiplier Tubes. Electron Tubes Division. 2006. Available online: https://www.hamamatsu.com/content/dam/hamamatsu-photonics/sites/documents/99_SALES_LIBRARY/etd/PMT_handbook_v4E.pdf (accessed on 15 May 2023).
- Wang, E.; Litvinenko, V.N.; Pinayev, I.; Gaowei, M.; Skaritka, J.; Belomestnykh, S.; Ben-Zvi, I.; Brutus, J.C.; Jing, Y.; Biswas, J.; et al. Long lifetime of bialkali photocathodes operating in high gradient superconducting radio frequency gun. Sci. Rep. 2021, 11, 4477. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kuriki, M.; Yokota, A.; Urano, M.; Negishi, K. Substrate dependence of CsK2Sb photo-cathode performance. Prog. Theor. Exp. Phys. 2017, 2017, 033G01. [Google Scholar] [CrossRef]
- Honda, Y.; Matsuba, S.; Jin, X.; Miyajima, T.; Yamamoto, M.; Uchiyama, T.; Kuwahara, M.; Takeda, Y. Temporal Response Measurements of GaAs-Based Photocathodes. Jpn. J. Appl. Phys. 2013, 52, 086401. [Google Scholar] [CrossRef]
- Vecchione, T.; Ben-Zvi, I.; Dowell, D.H.; Feng, J.; Rao, T.; Smedley, J.; Wan, W.; Padmore, H.A. A low emittance and high efficiency visible light photocathode for high brightness accelerator-based X-ray light sources. Appl. Phys. Lett. 2011, 99, 034103. [Google Scholar] [CrossRef]
- Michelato, P.; di Bona, A.; Pagani, C.; Sertore, D.; Valeri, S. R & D activity on high QE alkali photocathodes for RF guns. In Proceedings of the 16th Particle Accelerator Conf. (PAC’95), Dallas, TX, USA, 1–5 May 1995; paper WPC23; pp. 1049–1051. [Google Scholar]
- Cultrera, L.; Maxson, J.; Bazarov, I.; Belomestnykh, S.; Dobbins, J.; Dunham, B.; Karkare, S.; Kaplan, R.; Kostroun, V.; Li, Y.; et al. Photocathode behavior during high current running in the Cornell energy recovery linac photoinjector. Phys. Rev. Spéc. Top. Accel. Beams 2011, 14, 120101. [Google Scholar] [CrossRef]
- Qian, H.; Feng, J.; Filippetto, D.; Li, R.; Nasiatka, J.; Padmore, H.; Sannibale, F.; Schmerge, J.; Zhou, F. Alkali Cathode Testing for LCLS-II at APEX. In Proceedings of the 37th International Free Electron Laser Conference (FEL 2015), Daejeon, Republic of Korea, 23–28 August 2015. [Google Scholar] [CrossRef]
- Stephan, F.; Boulware, C.H.; Krasilnikov, M.; Bähr, J.; Asova, G.; Donat, A.; Gensch, U.; Grabosch, H.J.; Hänel, M.; Hakobyan, L.; et al. Detailed characterization of electron sources yielding first demonstration of European X-ray Free-Electron Laser beam quality. Phys. Rev. Spéc. Top. Accel. Beams 2010, 13, 020704. [Google Scholar] [CrossRef]
- Sommer, A.H.; Marton, L. Photoemissive Materials, 2nd ed.; E. Krieger: New York, NY, USA, 1980. [Google Scholar]
- Mohanty, S.K.; Qian, H.; Aboulbanine, Z.; Adhikari, G.; Aftab, N.; Boonpornprasert, P.; Good, J.; Gross, M.; Hoffmann, A.; Krasilnikov, M.; et al. Development and test results of multi-alkali antimonides photocathodes in the high gradient RF gun at PITZ. In Proceedings of the 40th International Free Electron Laser Conference, FEL2022, Trieste, Italy, 21–26 August 2022. [Google Scholar]
- Sertore, D.; Bertucci, M.; Monaco, L.; Rocco, G.G.; Mohanty, S.K.; Qian, H.; Stephan, F. R&D on high QE photocath-odes at INFN LASA. In Proceedings of the IPAC’22, Bangkok, Thailand, 12–17 June 2022; pp. 2633–2636. [Google Scholar] [CrossRef]
- Sertore, D.; Michelato, P.; Monaco, L.; Pagani, C. R&D activity on alkali-antimonide photocathodes at INFN-Lasa. In Proceedings of the 9th International Particle Accelerator Conference (IPAC’18), Vancouver, BC, Canada, 29 April–4 May 2018; pp. 4284–4286. [Google Scholar] [CrossRef]
- Sertore, D.; Favia, D.; Michelato, P.; Monaco, L.; Pierini, P. Cesium telluride and metals photoelectron thermal emittance measurements using a time-of-flight spectrometer. In Proceedings of the 9th European Particle Accelerator Conference (EPAC’04), Lucerne, Switzland, 5–9 July 2004; paper MOPKF045. pp. 408–410. [Google Scholar]
- Palik, E.D. Handbook of Optical Constants of Solids, 3rd ed.; Academic Press: Orlando, FL, USA, 1985; ISBN 978-0-08-054721-3. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Rocco, G.G.; Michelato, P.; Monaco, L.; Pagani, C.; Sertore, D. Development of a Multialkali Antimoni Photocathode at INFN LASA. In Proceedings of the FEL’19, Hamburg, Germany, 26–30 August 2019; pp. 448–451. [Google Scholar] [CrossRef]
- Sertore, D.; Rocco, G.G.; Michelato, P.; Mohanty, S.K.; Monaco, L.; Pagani, C. Photocathode Activities at INFN LASA. In Proceedings of the IPAC’19, Melbourne, VIC, Australia, 19–24 May 2019; pp. 2203–2206. [Google Scholar] [CrossRef]
- Xie, J.; Demarteau, M.; Wagner, R.; Schubert, S.; Gaowei, M.; Attenkofer, K.; Walsh, J.; Smedley, J.; Wong, J.; Feng, J.; et al. Synchrotron X-ray study of a low roughness and high efficiency K2CsSb photocathode during film growth. J. Phys. D Appl. Phys. 2017, 50, 205303. [Google Scholar] [CrossRef]
- Schubert, S.; Wong, J.; Feng, J.; Karkare, S.; Padmore, H.; Ruiz-Osés, M.; Smedley, J.; Muller, E.; Ding, Z.; Gaowei, M.; et al. Bi-alkali antimonide photocathode growth: An X-ray diffraction study. J. Appl. Phys. 2016, 120, 035303. [Google Scholar] [CrossRef]
- Monaco, L.; Rocco, G.G.; Michelato, P.; Pagani, C.; Sertore, D. Growing and Characterization of Cs2Te Photocatodes with Different Thicknesses at INFN LASA. In Proceedings of the FEL’19, Hamburg, Germany, 26–30 August 2019; pp. 297–300. [Google Scholar] [CrossRef]
- Ghosh, C.; Varma, B.P. Preparation and study of properties of a few alkali antimonide photocathodes. J. Appl. Phys. 1978, 49, 4549–4553. [Google Scholar] [CrossRef]
- Ruiz-Osés, M.; Schubert, S.; Attenkofer, K.; Ben-Zvi, I.; Liang, X.; Muller, E.; Padmore, H.; Rao, T.; Vecchione, T.; Wong, J.; et al. Direct observation of bi-alkali antimonide photocathodes growth via in operando x-ray diffraction studies. APL Mater. 2014, 2, 121101. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, P.; Ghosh, S. Anistropic growth and high performance organic thin film transistor. AIP Conf. Proc. 2012, 1447, 30–33. [Google Scholar] [CrossRef]
- Sommer, A.H.; Marton, L. Photoemissive Materials: Preparation, Properties, and Uses; Wiley: New York, NY, USA, 1968. [Google Scholar]
- Spicer, W.E. Photoemissive, Photoconductive, and Optical Absorption Studies of Alkali-Antimony Compounds. Phys. Rev. 1958, 112, 114–122. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Montgomery, E. Title of Dissertation: CHARACTERIZATION OF QUANTUM EFFICIENCY AND ROBUSTNESS OF CESIUM-BASED PHOTOCATHODES. Available online: https://psec.uchicago.edu/library/photocathodes/Dissertation1.pdf (accessed on 15 May 2023).
- Montgomery, E.J.; Feldman, D.W.; O’Shea, P.G.; Pan, Z.; Moody, N.A.; Jensen, K.L. Fabrication and measurement of efficient, robust cesiated dispenser photocathodes. In Proceedings of the 2007 Particle Accelerator Conference (PAC), Albuquerque, NM, USA, 25–29 June 2007; pp. 1206–1208. [Google Scholar] [CrossRef]
- di Bona, A.; Sabary, F.; Joly, S.; Michelato, P.; Sertore, D.; Pagani, C.; Valeri, S. Development, operation and analysis of bialkali antimonide photocathodes for high-brightness photo-injectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1997, 385, 385–390. [Google Scholar] [CrossRef]
- Mammei, R.R.; Suleiman, R.; Feingold, J.; Adderley, P.A.; Clark, J.; Covert, S.; Grames, J.; Hansknecht, J.; Machie, D.; Poelker, M.; et al. Charge lifetime measurements at high average current using aK2CsSbphotocathode inside a dc high voltage photogun. Phys. Rev. Spéc. Top. Accel. Beams 2013, 16, 033401. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The Compressibility of Media under Extreme Pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244–247. [Google Scholar] [CrossRef]
- McCarroll, W.H. Chemical and structural characteristics of the potassium-cesium-antimony photocathode. J. Phys. Chem. Solids 1965, 26, 191–195. [Google Scholar] [CrossRef]
- Kalarasse, L.; Bennecer, B. Optical properties of the alkali antimonide semiconductors Cs3Sb, Cs2KSb, CsK2Sb and K3Sb. J. Phys. Chem. Solids 2010, 71, 314–322. [Google Scholar] [CrossRef]
- Murtaza, G.; Ullah, M.; Ullah, N.; Rani, M.; Muzammil, M.; Khenata, R.; Ramay, S.M.; Khan, U. Structural, elastic, electronic and optical properties of bi-alkali antimonides. Bull. Mater. Sci. 2016, 39, 1581–1591. [Google Scholar] [CrossRef]
- Cocchi, C.; Mistry, S.; Schmeißer, M.; Amador, R.; Kühn, J.; Kamps, T. Electronic structure and core electron fingerprints of caesium-based multi-alkali antimonides for ultra-bright electron sources. Sci. Rep. 2019, 9, 18276. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Hamann, D.R. Optimized norm-conserving Vanderbilt pseudopotentials. Phys. Rev. B 2013, 88, 085117. [Google Scholar] [CrossRef]
- Marzari, N.; Mostofi, A.A.; Yates, J.R.; Souza, I.; Vanderbilt, D. Maximally localized Wannier functions: Theory and applications. Rev. Mod. Phys. 2012, 84, 1419–1475. [Google Scholar] [CrossRef]
- Mostofi, A.A.; Yates, J.R.; Lee, Y.-S.; Souza, I.; Vanderbilt, D.; Marzari, N. wannier90: A tool for obtaining maximally-localised Wannier functions. Comput. Phys. Commun. 2008, 178, 685–699, reprinted in Comput. Phys. Commun. 2014, 185, 2309–2310. [Google Scholar] [CrossRef]
- Antoniuk, E.R.; Schindler, P.; Schroeder, W.A.; Dunham, B.; Pianetta, P.; Vecchione, T.; Reed, E.J. Novel Ultrabright and Air-Stable Photocathodes Discovered from Machine Learning and Density Functional Theory Driven Screening. Adv. Mater. 2021, 33, 2104081. [Google Scholar] [CrossRef]
- Brener, N.E. Random-phase-approximation dielectric function for diamond, with local field effects included. Phys. Rev. B 1975, 12, 1487–1492. [Google Scholar] [CrossRef]
- Benassi, A.; Ferretti, A.; Cavazzoni, C. PWSCF.’s Epsilon.x User’s Manual. Available online: www.quantum-espresso.org/Doc/pp_user_guide.pdf (accessed on 1 April 2023).
- Cocchi, C.; Mistry, S.; Schmeißer, M.; Kühn, J.; Kamps, T. First-principles many-body study of the electronic and optical properties of CsK2Sb, a semiconducting material for ultra-bright electron sources. J. Phys. Condens. Matter 2018, 31, 014002. [Google Scholar] [CrossRef] [PubMed]
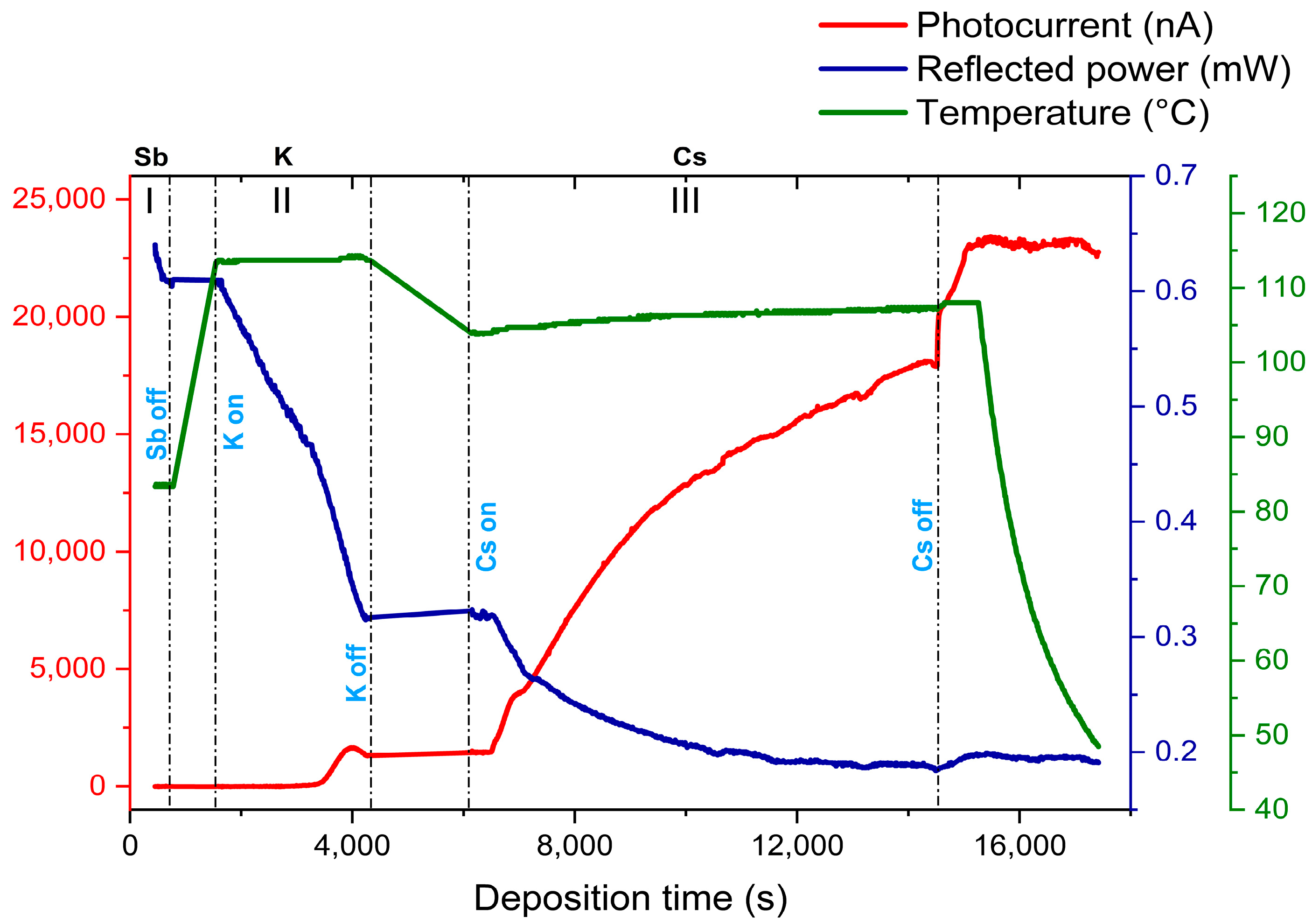
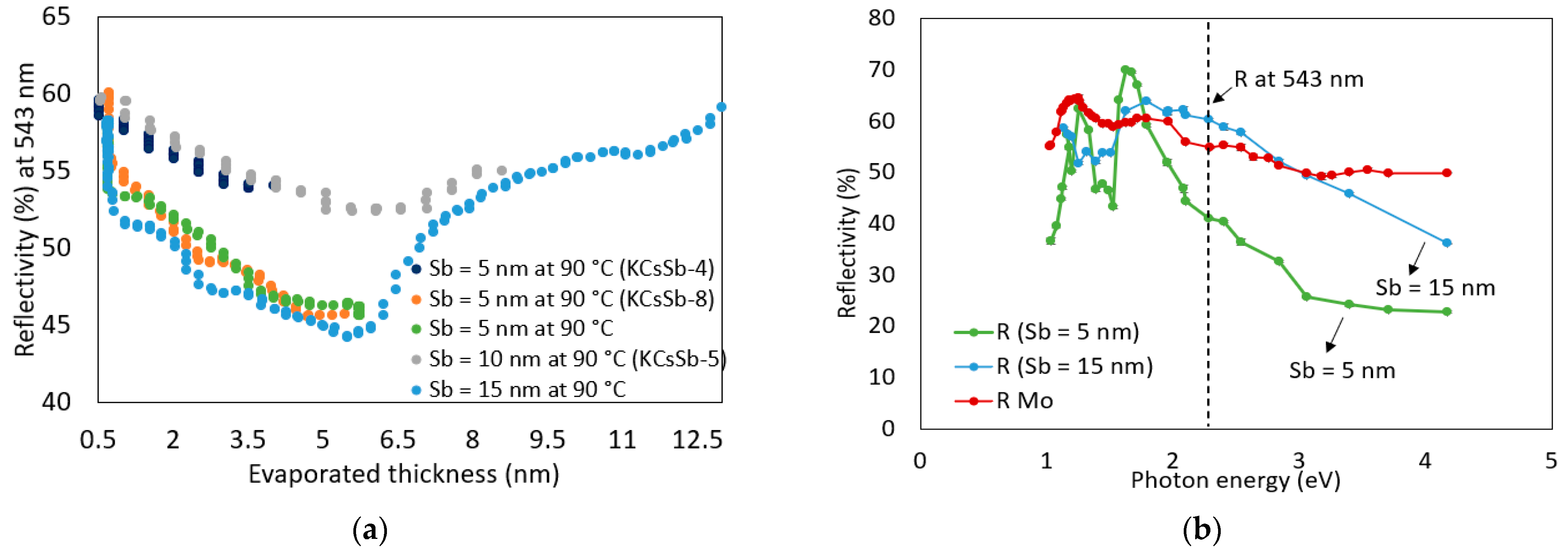
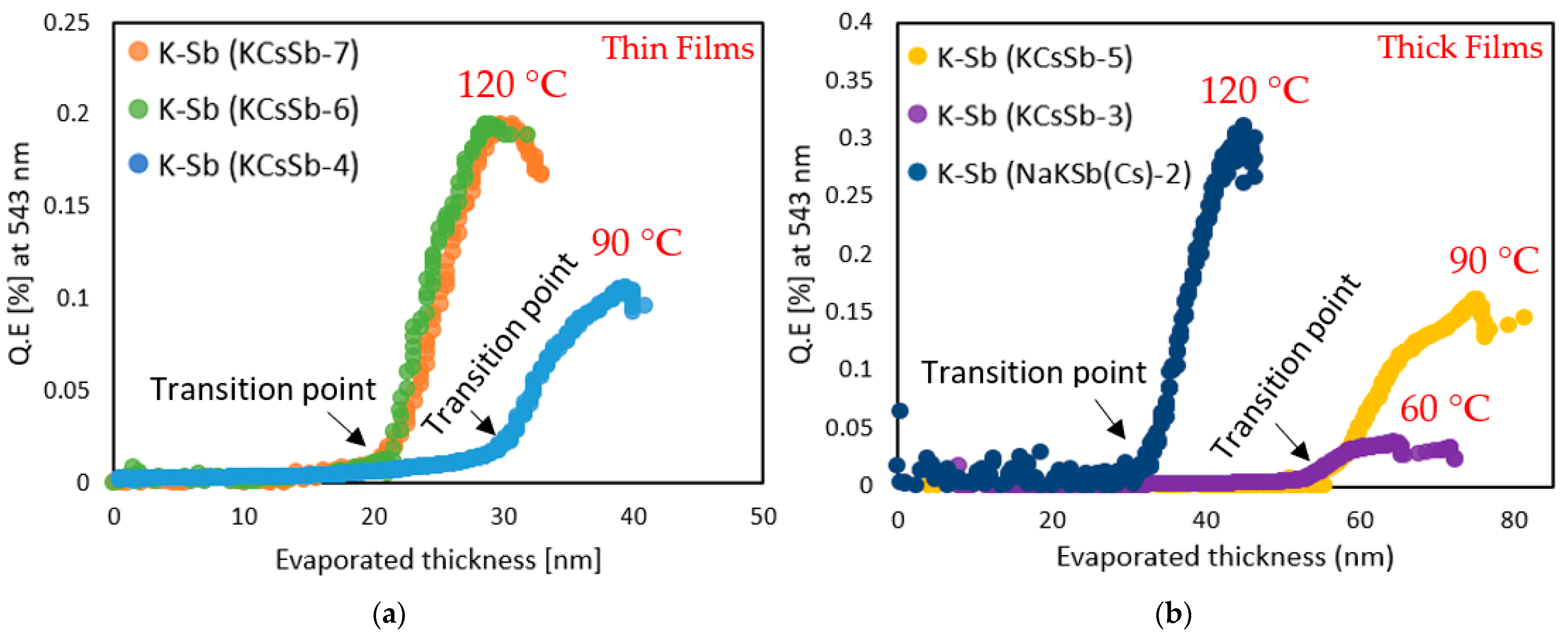
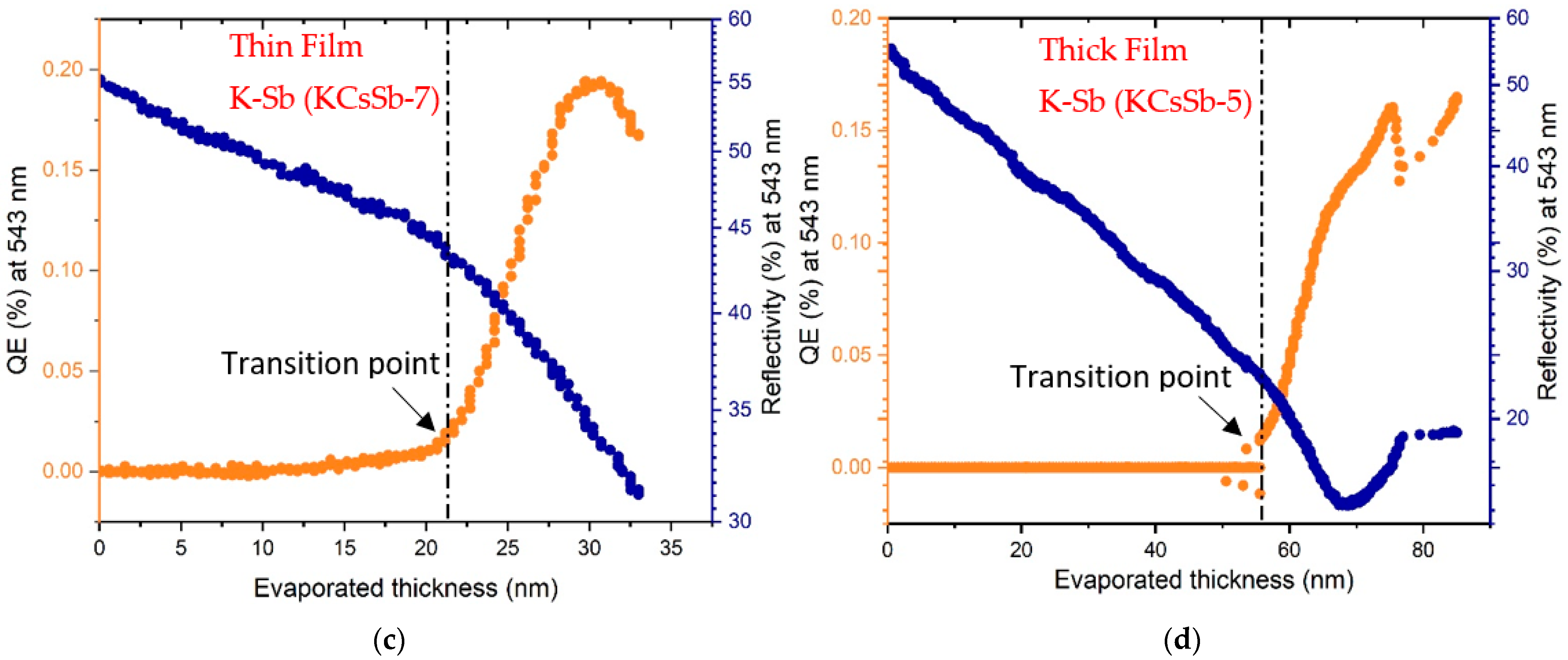
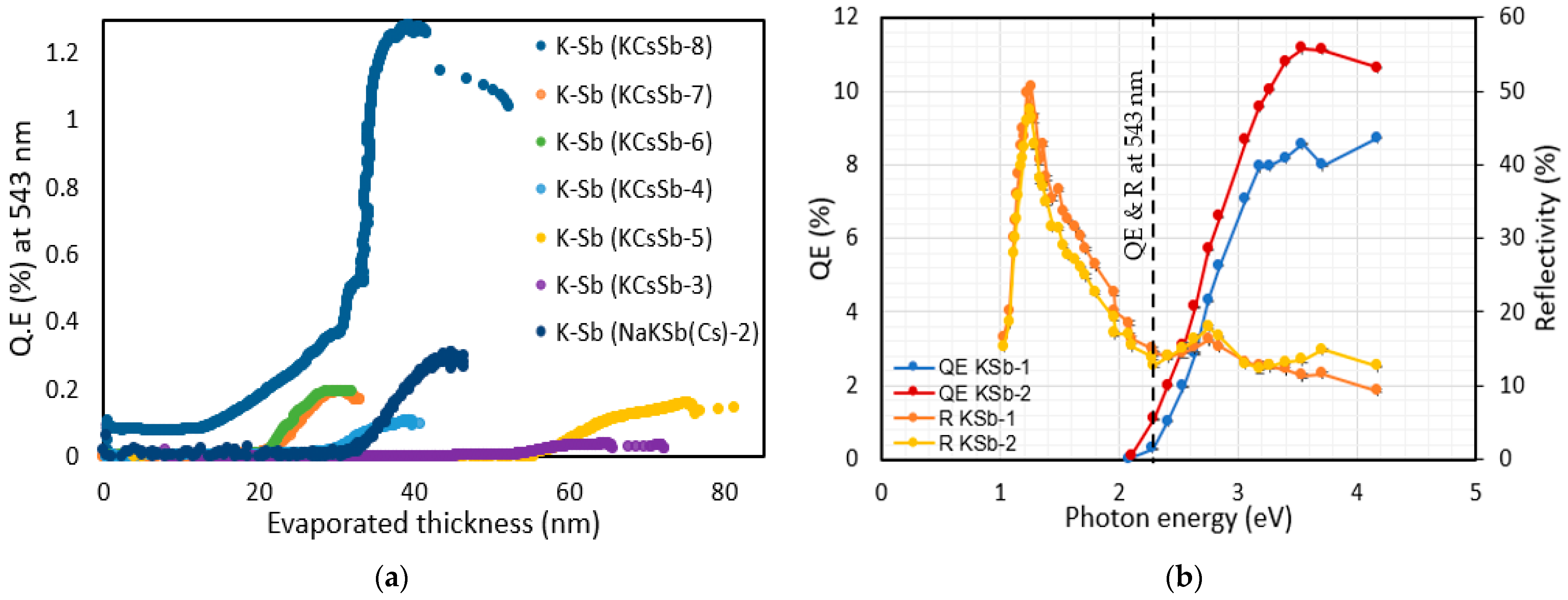
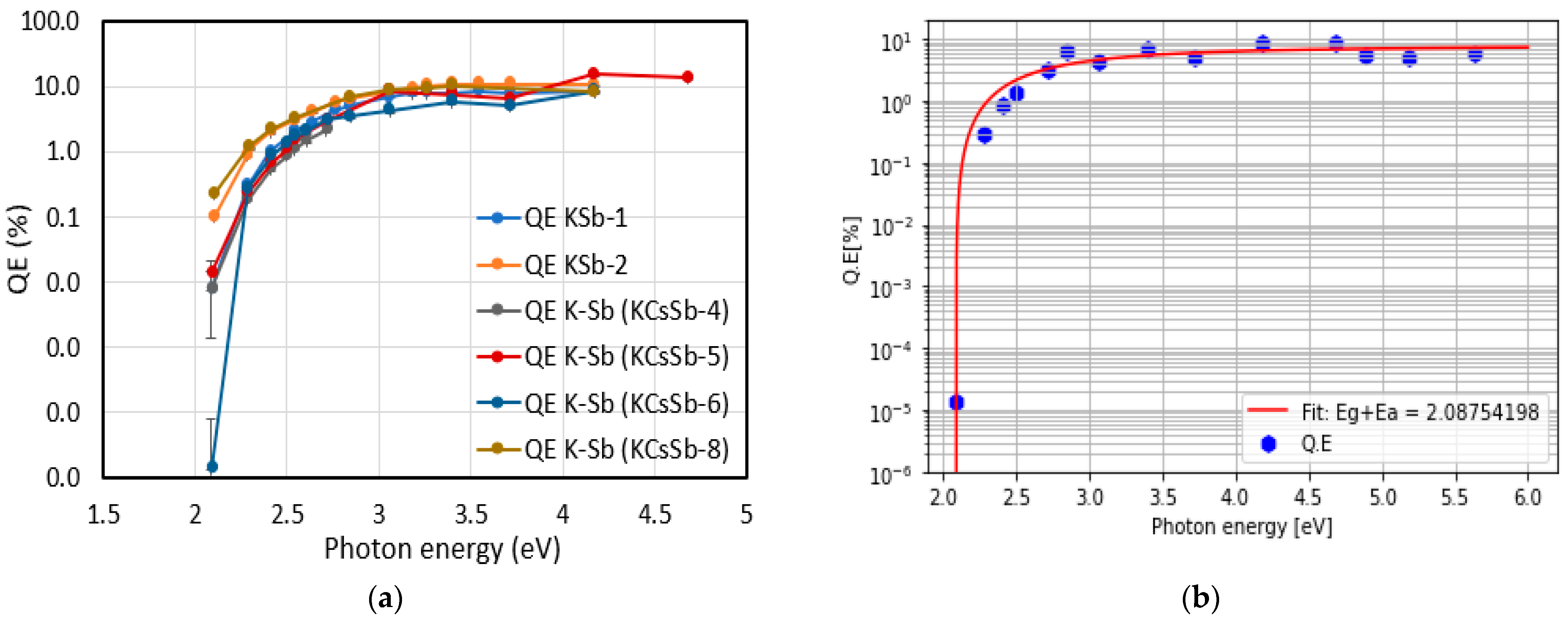
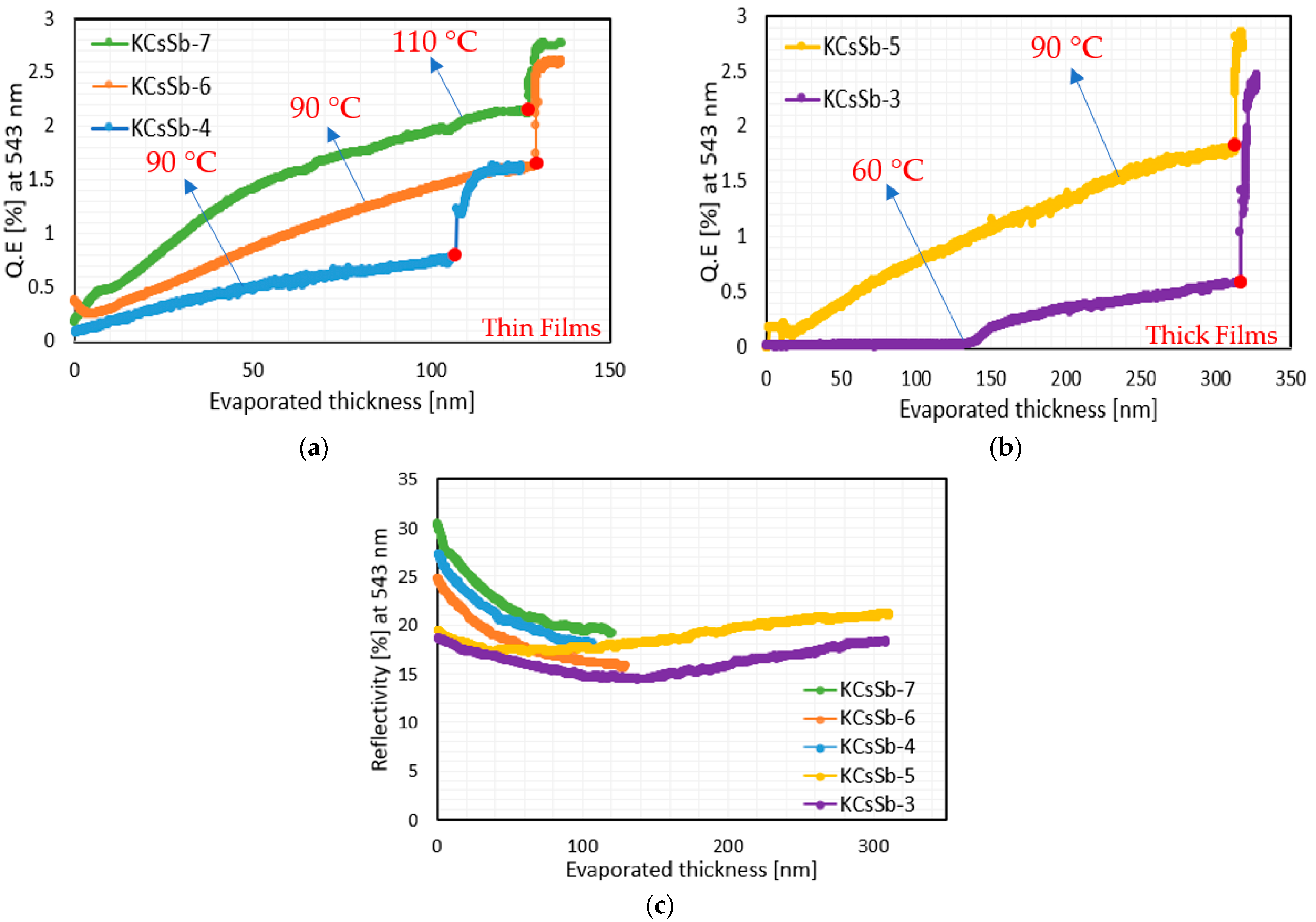
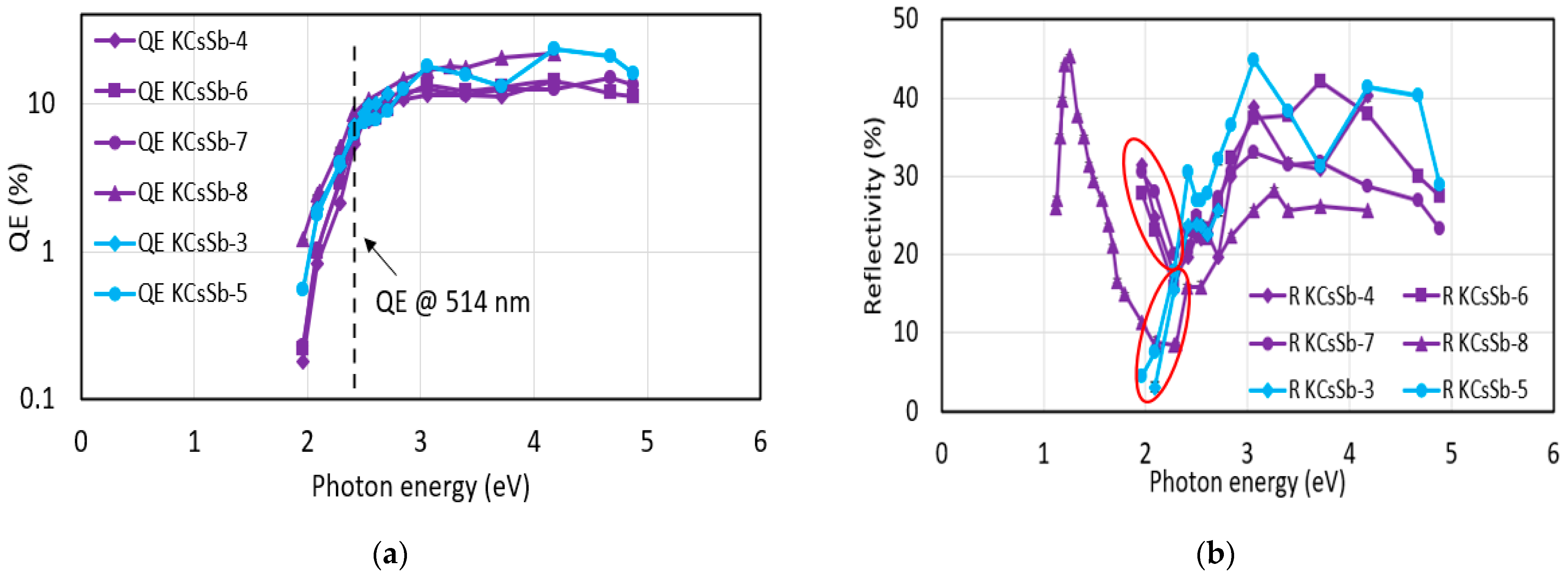

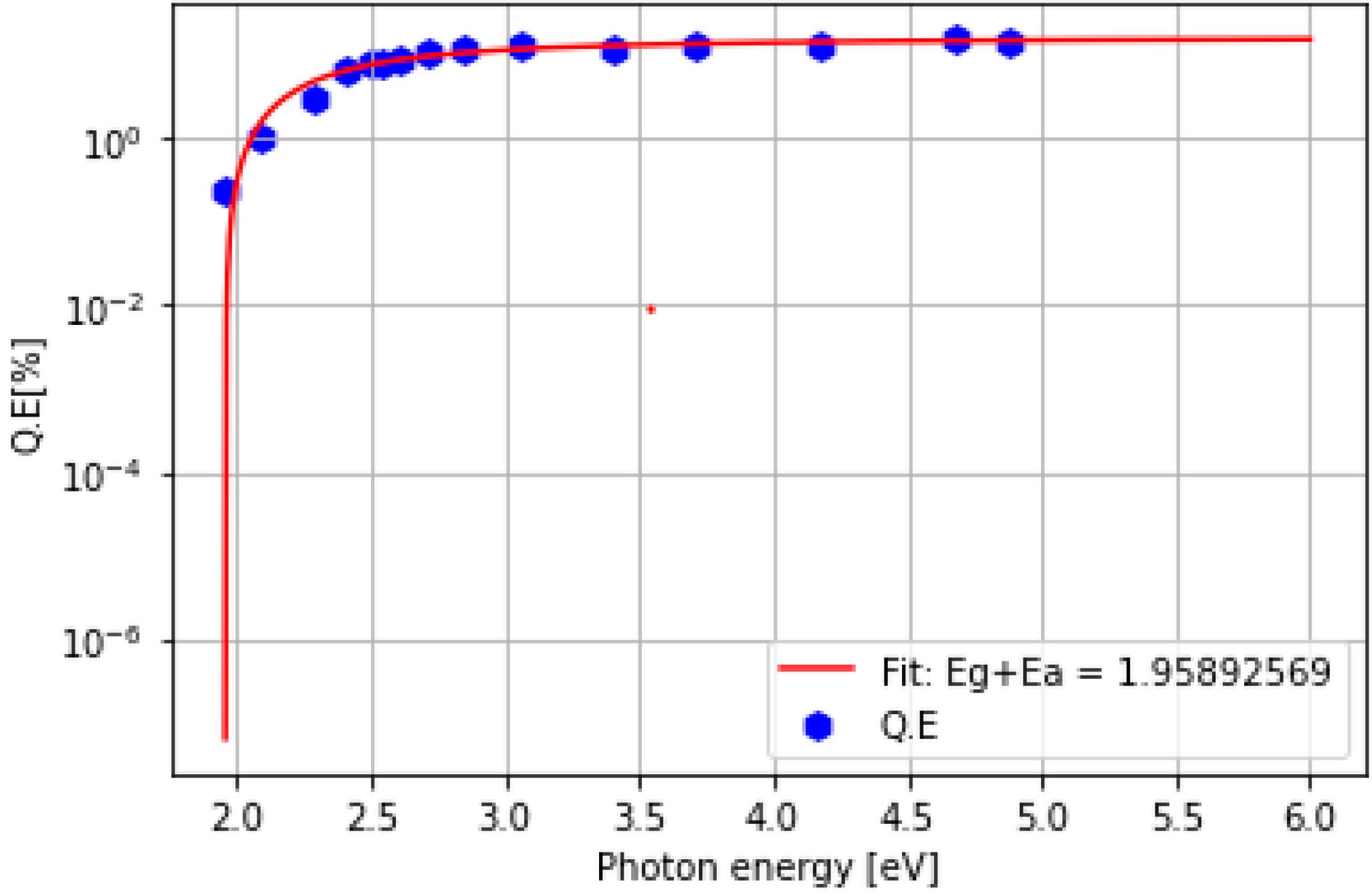
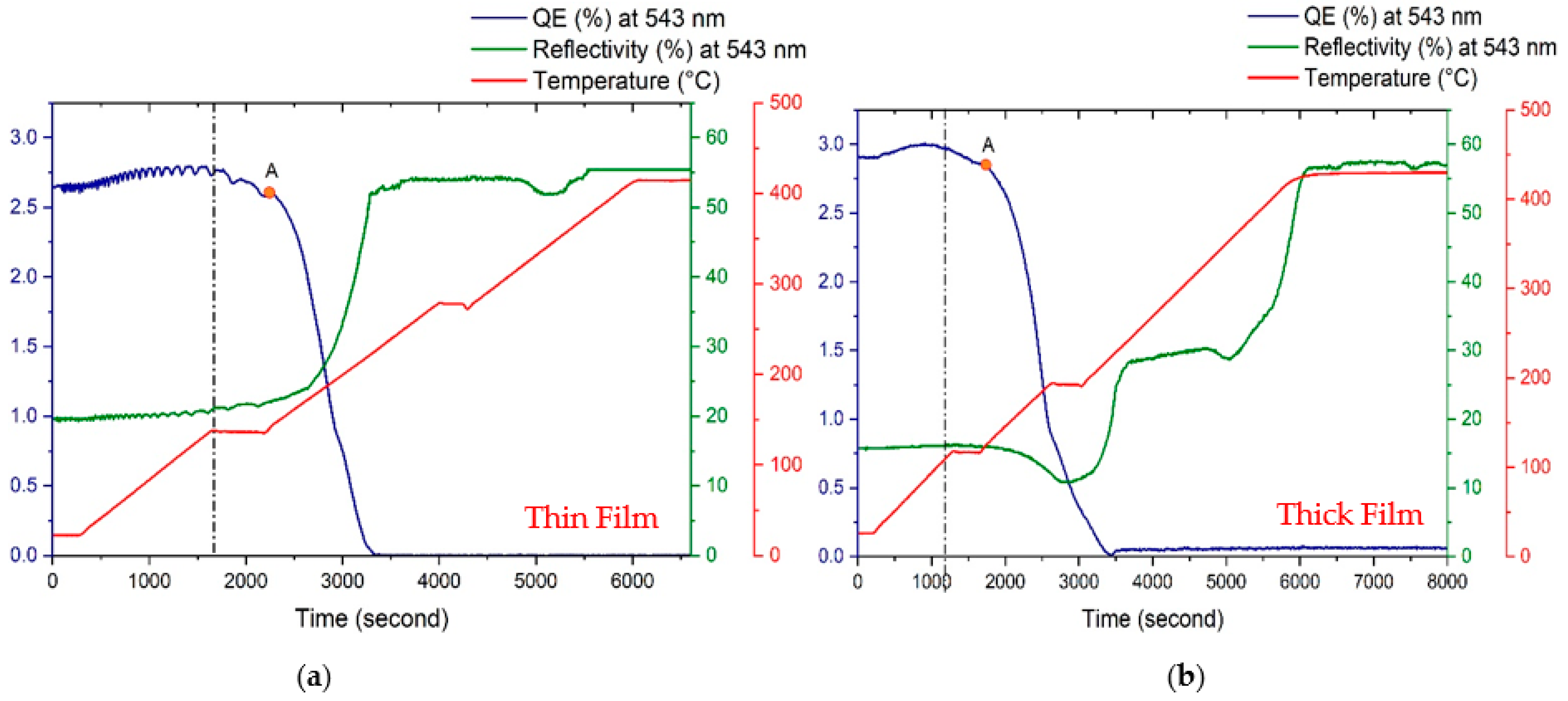
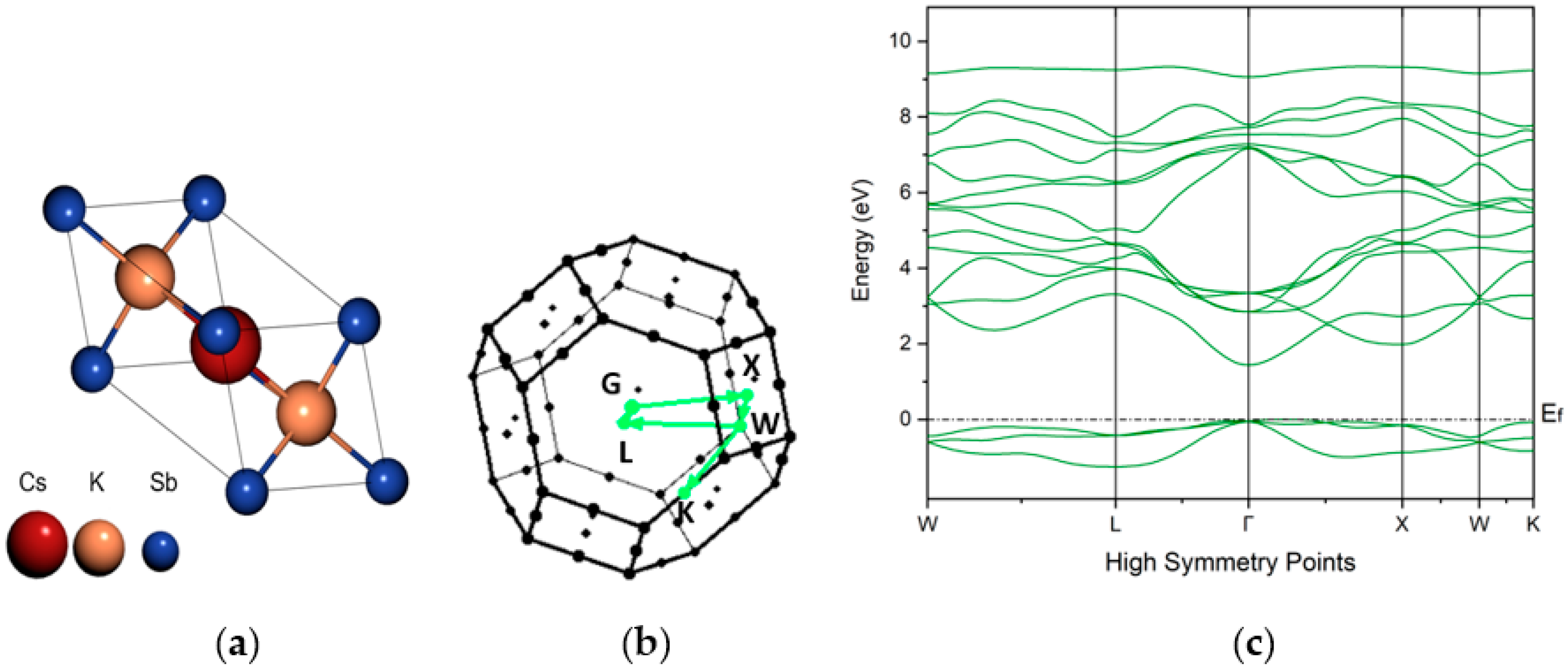

| Cathode | Sb (nm) | K (nm) | Cs (nm) | K/Sb Ratio | Cs/Sb Ratio | TSb-TK-TCs (°C) | QE (%) (514 nm) | ΔR (%) Variation after K (543 nm) | ΔR (%) Variation after Cs (543 nm) |
|---|---|---|---|---|---|---|---|---|---|
| KCsSb-4 | 5 ± 0.9 | 41 ± 0.1 | 106 ± 0.5 | 8.2 | 21.2 | 90-90-90 | 3.9 | 47 | 37 |
| KCsSb-6 | 5 ± 0.9 | 32 ± 0.1 | 117 ± 0.5 | 6.4 | 23.4 | 90-120-90 | 4.6 | 48.5 | 36.6 |
| KCsSb-7 | 5 ± 0.9 | 34 ± 0.5 | 121 ± 0.5 | 6.8 | 24.2 | 90-120-110 | 5.4 | 48.33 | 38.7 |
| KCsSb-8 | 5 ± 0.9 | 43 ± 0.5 | 31 ± 0.5 * | 8.6 | 6.2 * | 90-130-120 | 8.84 | 61.19 | 46.6 * |
| KCsSb-3 | 10 ± 0.9 | 66 ± 0.5 | 313 ± 0.9 | 6.6 | 31.3 | 60-60-60 ** | 5.2 | 65.4 | 0 |
| KCsSb-5 | 10 ± 0.5 | 75 ± 0.1 | 316 ± 0.5 | 7.5 | 31.6 | 90-90-90 | 4.6 | 66.6 | −7 |
| NaKSb-2 | 9 ± 0.5 | 46 ± 0.1 | - | 5.1 | - | 90-120 | 0.3 # | 68.8 | - |
| Cathode | Temperature (°C) during Cs Deposition | Absolute % QE Increase (after Turning off the Cs Source) |
|---|---|---|
| KCsSb-3 | 60 * | 308 |
| KCsSb-4 | 90 | 57 |
| KCsSb-5 | 90 | 55 |
| KCsSb-6 | 90 | 63 |
| KCsSb-7 | 110 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, S.K.; Krasilnikov, M.; Oppelt, A.; Stephan, F.; Sertore, D.; Monaco, L.; Pagani, C.; Hillert, W. Development and Characterization of Multi-Alkali Antimonide Photocathodes for High-Brightness RF Photoinjectors. Micromachines 2023, 14, 1182. https://doi.org/10.3390/mi14061182
Mohanty SK, Krasilnikov M, Oppelt A, Stephan F, Sertore D, Monaco L, Pagani C, Hillert W. Development and Characterization of Multi-Alkali Antimonide Photocathodes for High-Brightness RF Photoinjectors. Micromachines. 2023; 14(6):1182. https://doi.org/10.3390/mi14061182
Chicago/Turabian StyleMohanty, Sandeep Kumar, Mikhail Krasilnikov, Anne Oppelt, Frank Stephan, Daniele Sertore, Laura Monaco, Carlo Pagani, and Wolfgang Hillert. 2023. "Development and Characterization of Multi-Alkali Antimonide Photocathodes for High-Brightness RF Photoinjectors" Micromachines 14, no. 6: 1182. https://doi.org/10.3390/mi14061182






