Acoustic Atomization-Induced Pumping Based on a Vibrating Sharp-Tip Capillary
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
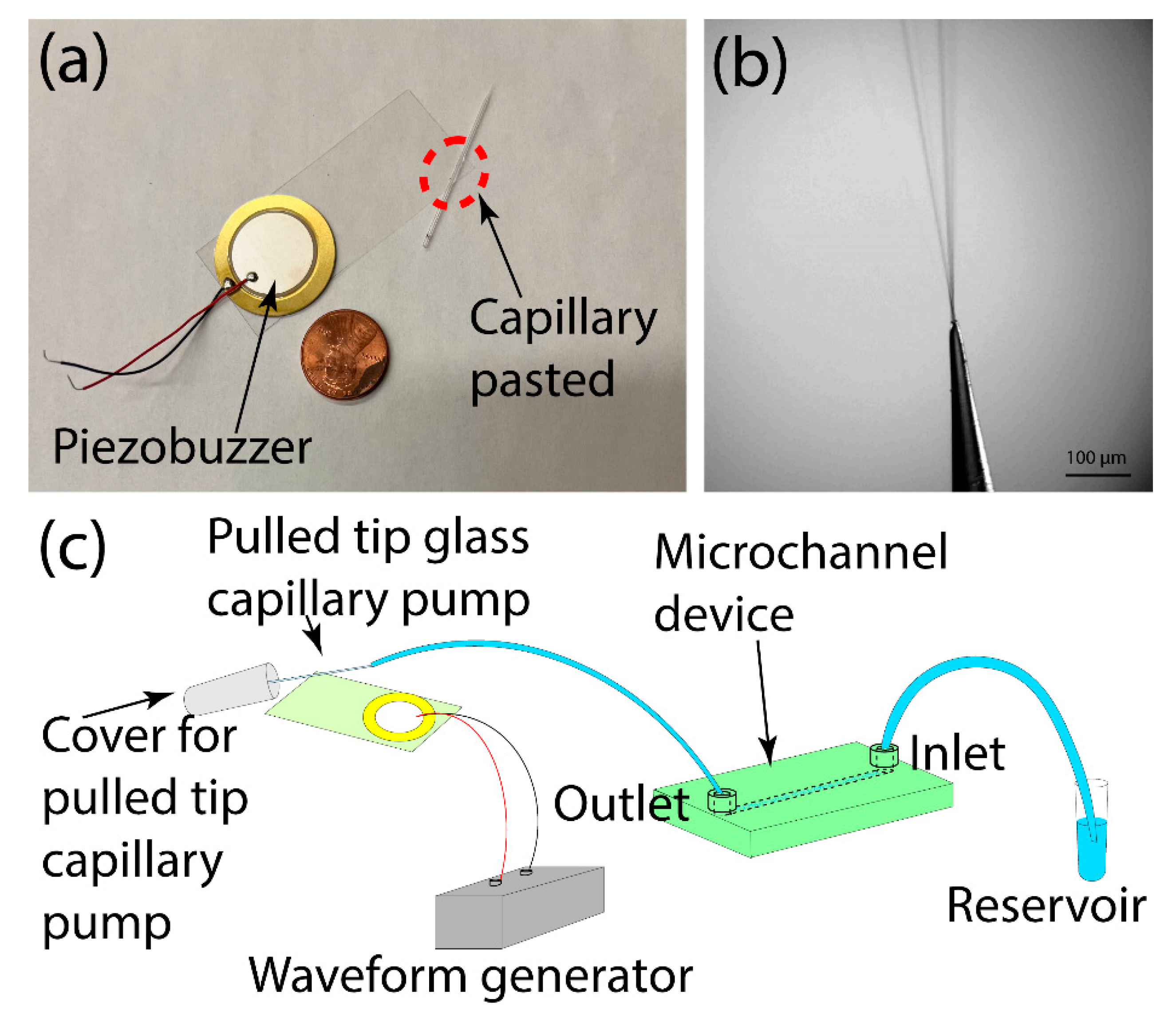
2.2. Device Design and Fabrication
2.3. Pump Operation
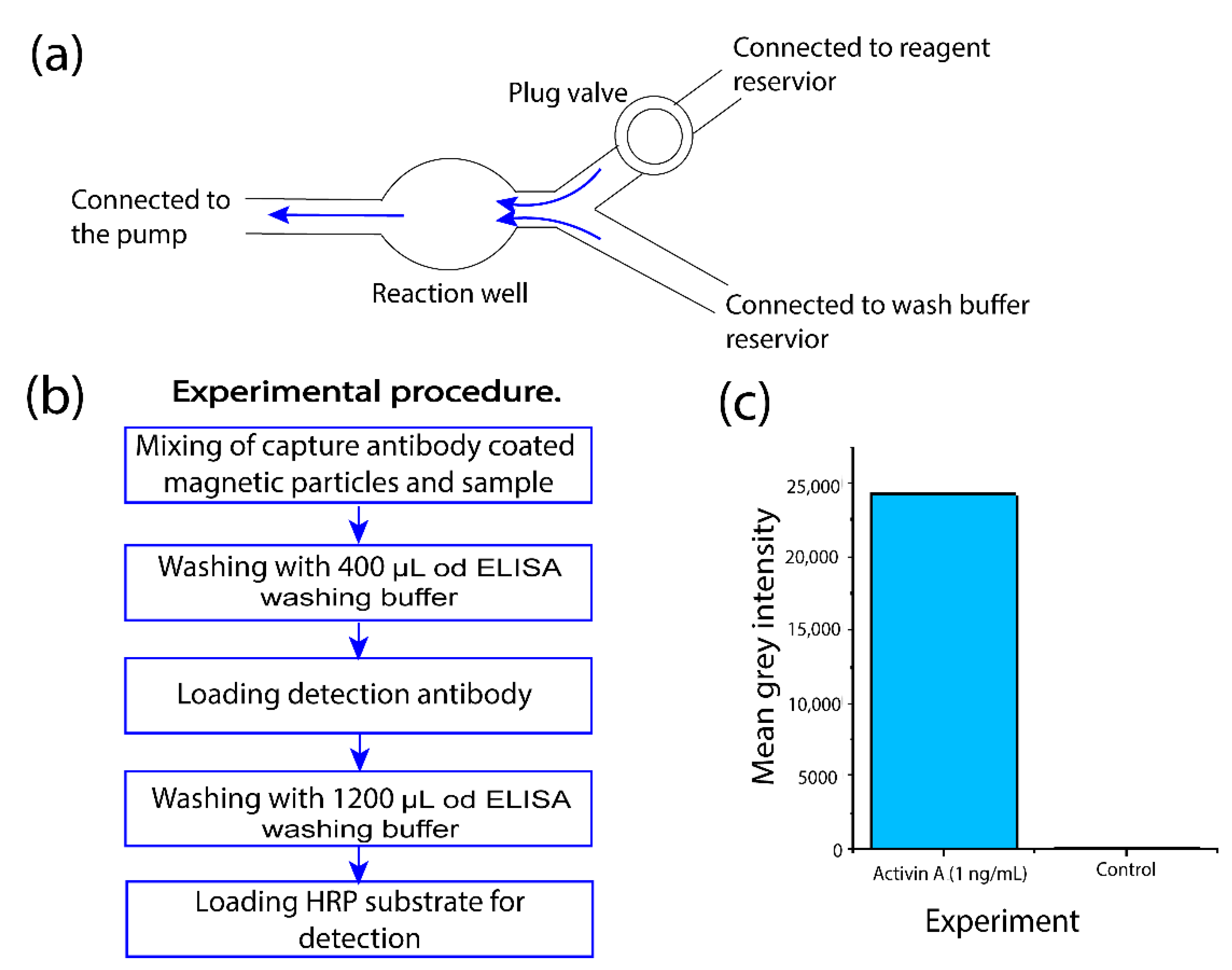
2.4. On-Chip Magnetic Bead-Based ELISA
3. Results and Discussion
3.1. Operation Principle
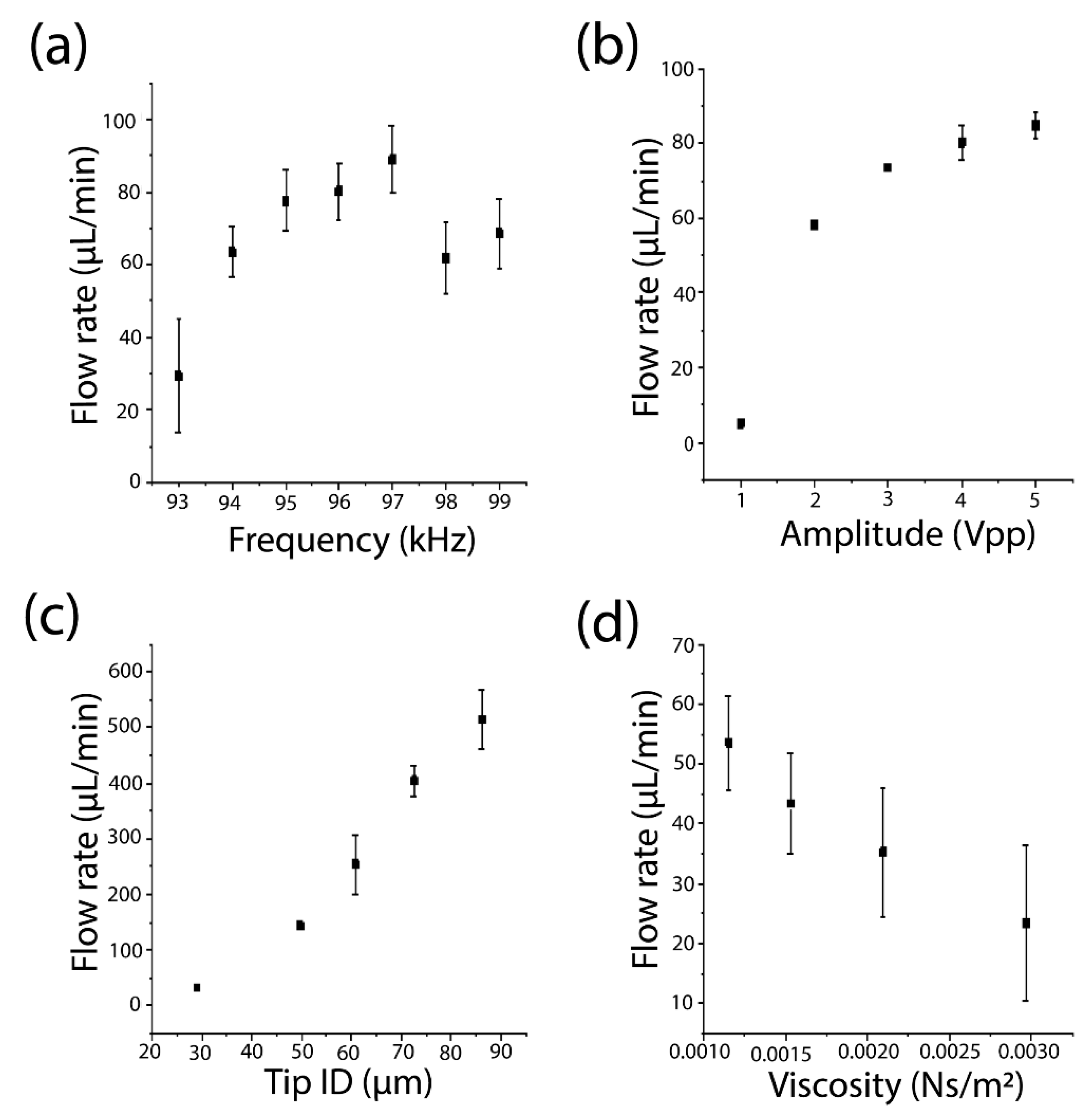
3.2. Characterization of Important Operational and Design Parameters on Pumping Performance
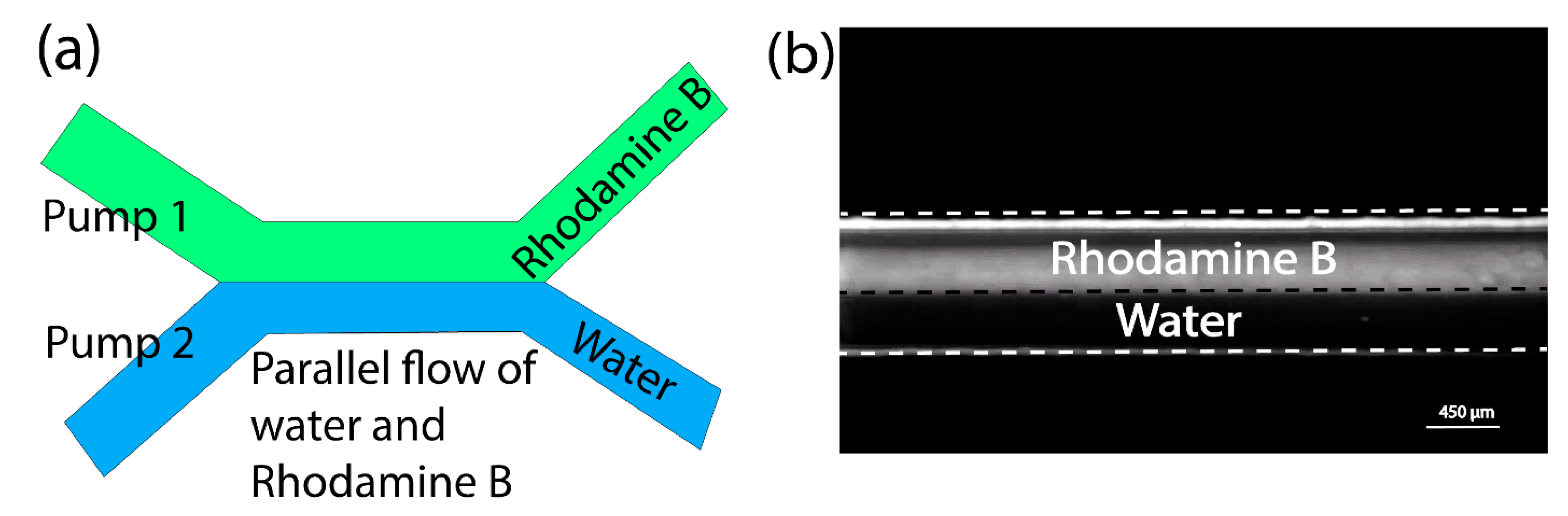
3.3. Multi-Pump Operations
3.4. Performing Sequential Fluid Operations to Complete ELISA Protocol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, N.; Liu, Y.; Wang, S.; Tang, X.; Ge, P.; Nan, J.; Zhang, J.; Yang, B. Pressure-Controlled Microfluidic Sub-Picoliter Ultramicro-Volume Syringes Based on Integrated Micro-Nanostructure Arrays. Lab Chip 2019, 19, 3368–3374. [Google Scholar] [CrossRef]
- Li, Z.; Mak, S.Y.; Sauret, A.; Shum, H.C. Syringe-Pump-Induced Fluctuation in All-Aqueous Microfluidic System Implications for Flow Rate Accuracy. Lab Chip 2014, 14, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A Microfluidic ExoSearch Chip for Multiplexed Exosome Detection towards Blood-Based Ovarian Cancer Diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-W.; Lee, S.; Lee, D.-H.; Kim, J.; deMello, A.J.; Chang, S.-I. Integrated Pneumatic Micro-Pumps for High-Throughput Droplet-Based Microfluidics. RSC Adv. 2014, 4, 20341–20345. [Google Scholar] [CrossRef] [Green Version]
- Clime, L.; Brassard, D.; Geissler, M.; Veres, T. Active Pneumatic Control of Centrifugal Microfluidic Flows for Lab-on-a-Chip Applications. Lab Chip 2015, 15, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Beebe, D.J. A Passive Pumping Method for Microfluidic Devices. Lab Chip 2002, 2, 131. [Google Scholar] [CrossRef]
- Marimuthu, M.; Kim, S. Pumpless Steady-Flow Microfluidic Chip for Cell Culture. Anal. Biochem. 2013, 437, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Jia, Z.; Zhu, Z.; He, C.; Wang, W.; Morgan, A.; Lu, J.J.; Liu, S. Miniaturized Electroosmotic Pump Capable of Generating Pressures of More than 1200 Bar. Anal. Chem. 2012, 84, 9609–9614. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-K.; Lee, J.-G.; Park, J.-M.; Lee, B.-S.; Lee, Y.; Ko, C. One-Step Pathogen Specific DNA Extraction from Whole Blood on a Centrifugal Microfluidic Device. Lab Chip 2007, 7, 565. [Google Scholar] [CrossRef]
- Atencia, J.; Beebe, D.J. Magnetically-Driven Biomimetic Micro Pumping Using Vortices. Lab Chip 2004, 4, 598. [Google Scholar] [CrossRef]
- Maruo, S.; Inoue, H. Optically Driven Viscous Micropump Using a Rotating Microdisk. Appl. Phys. Lett. 2007, 91, 084101. [Google Scholar] [CrossRef]
- Leach, J.; Mushfique, H.; di Leonardo, R.; Padgett, M.; Cooper, J. An Optically Driven Pump for Microfluidics. Lab Chip 2006, 6, 735. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Effenhauser, C.S.; Burggraf, N.; Harrison, D.J.; Seiler, K.; Fluri, K. Electroosmotic Pumping and Electrophoretic Separations for Miniaturized Chemical Analysis Systems. J. Micromech. Microeng. 1994, 4, 257–265. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Zhu, X.; Wang, Y.; Xue, J. Low-Voltage Electroosmotic Pumps Fabricated from Track-Etched Polymer Membranes. Lab Chip 2012, 12, 1710. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ni, Q.; Crane, N.; Guldiken, R. Surface Acoustic Wave Based Pumping in a Microchannel. Microsyst. Technol. 2017, 23, 1335–1342. [Google Scholar] [CrossRef]
- Huang, P.-H.; Nama, N.; Mao, Z.; Li, P.; Rufo, J.; Chen, Y.; Xie, Y.; Wei, C.-H.; Wang, L.; Huang, T.J. A Reliable and Programmable Acoustofluidic Pump Powered by Oscillating Sharp-Edge Structures. Lab Chip 2014, 14, 4319–4323. [Google Scholar] [CrossRef] [Green Version]
- Ozcelik, A.; Aslan, Z. A Practical Microfluidic Pump Enabled by Acoustofluidics and 3D Printing. Microfluid. Nanofluid. 2021, 25, 5. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, Y.; Wu, M.; Zhou, R.; Chung, D.; Caraveo, G.; Xu, J. Acoustofluidic Stick-and-Play Micropump Built on Foil for Single-Cell Trapping. Lab Chip 2019, 19, 3045–3053. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Liu, B.; Chang, Y.; Pang, W.; Duan, X. A Self-Contained Acoustofluidic Platform for Biomarker Detection. Lab Chip 2022, 22, 3817–3826. [Google Scholar] [CrossRef]
- Kiebert, F.; Wege, S.; Massing, J.; König, J.; Cierpka, C.; Weser, R.; Schmidt, H. 3D Measurement and Simulation of Surface Acoustic Wave Driven Fluid Motion: A Comparison. Lab Chip 2017, 17, 2104–2114. [Google Scholar] [CrossRef]
- Bachman, H.; Huang, P.-H.; Zhao, S.; Yang, S.; Zhang, P.; Fu, H.; Huang, T.J. Acoustofluidic Devices Controlled by Cell Phones. Lab Chip 2018, 18, 433–441. [Google Scholar] [CrossRef]
- Dentry, M.B.; Friend, J.R.; Yeo, L.Y. Continuous Flow Actuation between External Reservoirs in Small-Scale Devices Driven by Surface Acoustic Waves. Lab Chip 2014, 14, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Rife, J.C.; Bell, M.I.; Horwitz, J.S.; Kabler, M.N.; Auyeung, R.C.Y.; Kim, W.J. Miniature Valveless Ultrasonic Pumps and Mixers. Sens. Actuators Phys. 2000, 86, 135–140. [Google Scholar] [CrossRef]
- Tovar, A.R.; Patel, M.V.; Lee, A.P. Lateral Air Cavities for Microfluidic Pumping with the Use of Acoustic Energy. Microfluid. Nanofluidics 2011, 10, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Bachman, H.; Fu, H.; Huang, P.-H.; Tian, Z.; Embry-Seckler, J.; Rufo, J.; Xie, Z.; Hartman, J.H.; Zhao, S.; Yang, S.; et al. Open Source Acoustofluidics. Lab Chip 2019, 19, 2404–2414. [Google Scholar] [CrossRef]
- Du, X.Y.; Fu, Y.Q.; Luo, J.K.; Flewitt, A.J.; Milne, W.I. Microfluidic Pumps Employing Surface Acoustic Waves Generated in ZnO Thin Films. J. Appl. Phys. 2009, 105, 024508. [Google Scholar] [CrossRef]
- Destgeer, G.; Sung, H.J. Recent Advances in Microfluidic Actuation and Micro-Object Manipulation via Surface Acoustic Waves. Lab Chip 2015, 15, 2722–2738. [Google Scholar] [CrossRef]
- Huang, A.; Connacher, W.; Stambaugh, M.; Zhang, N.; Zhang, S.; Mei, J.; Jain, A.; Alluri, S.; Leung, V.; Rajapaksa, A.E.; et al. Practical Microcircuits for Handheld Acoustofluidics. Lab Chip 2021, 21, 1352–1363. [Google Scholar] [CrossRef]
- Novelli, F.; Hoberg, C.; Adams, E.M.; Klopf, J.M.; Havenith, M. Terahertz Pump–Probe of Liquid Water at 12.3 THz. Phys. Chem. Chem. Phys. 2022, 24, 653–665. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface Acoustic Wave Microfluidics. Lab Chip 2013, 13, 3626. [Google Scholar] [CrossRef]
- Hintermüller, M.A.; Jakoby, B.; Reichel, E.K. Acoustic Streaming via a Flexible PCB for Micropumping Applications. Procedia Eng. 2016, 168, 856–859. [Google Scholar] [CrossRef]
- Connacher, W.; Zhang, N.; Huang, A.; Mei, J.; Zhang, S.; Gopesh, T.; Friend, J. Micro/Nano Acoustofluidics: Materials, Phenomena, Design, Devices, and Applications. Lab Chip 2018, 18, 1952–1996. [Google Scholar] [CrossRef]
- Mohanty, S.; Siciliani de Cumis, U.; Solsona, M.; Misra, S. Bi-Directional Transportation of Micro-Agents Induced by Symmetry-Broken Acoustic Streaming. AIP Adv. 2019, 9, 035352. [Google Scholar] [CrossRef] [Green Version]
- Nama, N.; Huang, P.-H.; Huang, T.J.; Costanzo, F. Investigation of Acoustic Streaming Patterns around Oscillating Sharp Edges. Lab Chip 2014, 14, 2824–2836. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Guo, X.; Brunet, P.; Costalonga, M.; Royon, L. Acoustic Streaming near a Sharp Structure and Its Mixing Performance Characterization. Microfluid. Nanofluid. 2019, 23, 104. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, X.; Royon, L.; Brunet, P. Unveiling of the Mechanisms of Acoustic Streaming Induced by Sharp Edges. Phys. Rev. E 2020, 102, 043110. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, M.; Zhou, J.; Yalamanchili, S. Acoustic Streaming of a Sharp Edge. J. Acoust. Soc. Am. 2014, 136, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Pavlic, A.; Harshbarger, C.L.; Rosenthaler, L.; Snedeker, J.G.; Dual, J. Sharp-Edge-Based Acoustofluidic Chip Capable of Programmable Pumping, Mixing, Cell Focusing, and Trapping. Phys. Fluids 2023, 35, 022006. [Google Scholar] [CrossRef]
- Wu, Z.; Cai, H.; Ao, Z.; Nunez, A.; Liu, H.; Bondesson, M.; Guo, S.; Guo, F. A Digital Acoustofluidic Pump Powered by Localized Fluid-Substrate Interactions. Anal. Chem. 2019, 91, 7097–7103. [Google Scholar] [CrossRef]
- Collins, D.J.; Manor, O.; Winkler, A.; Schmidt, H.; Friend, J.R.; Yeo, L.Y. Atomization off thin water films generated by high-frequency substrate wave vibrations. Phys. Rev. E 2012, 86, 056312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurosawa, M.; Watanabe, T.; Futami, A.; Higuchi, T. Surface acoustic wave atomizer. Sens. Actuators A Phys. 1995, 50, 69–74. [Google Scholar] [CrossRef]
- Winkler, A.; Harazim, S.; Collins, D.J.; Brünig, R.; Schmidt, H.; Menzel, S.B. Compact SAW aerosol generator. Biomed. Microdevices 2017, 19, 9. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, N.; Li, C.; Suder, T.; Karanji, A.K.; Li, X.; He, Z.; Valentine, S.J.; Li, P. Capillary Vibrating Sharp-Edge Spray Ionization (CVSSI) for Voltage-Free Liquid Chromatography-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.; Li, P. A Small Footprint and Robust Interface for Solid Phase Microextraction and Mass Spectrometry Based on Vibrating Sharp-Edge Spray Ionization. J. Am. Soc. Mass Spectrom. 2022, 33, 304–314. [Google Scholar] [CrossRef]
- Pursell, M.E.; Sharif, D.; DeBastiani, A.; Li, C.; Majuta, S.; Li, P.; Valentine, S.J. Development of CVSSI-APCI for the Improvement of Ion Suppression and Matrix Effects in Complex Mixtures. Anal. Chem. 2022, 94, 9226–9233. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Attanayake, K.; Valentine, S.J.; Li, P. Facile Improvement of Negative Ion Mode Electrospray Ionization Using Capillary Vibrating Sharp-Edge Spray Ionization. Anal. Chem. 2020, 92, 2492–2502. [Google Scholar] [CrossRef]
- Li, X.; Attanayake, K.; Valentine, S.J.; Li, P. Vibrating Sharp-edge Spray Ionization (VSSI) for Voltage-free Direct Analysis of Samples Using Mass Spectrometry. Rapid Commun. Mass Spectrom. 2021, 35, e8232. [Google Scholar] [CrossRef]
- Li, X.; Huffman, J.; Ranganathan, N.; He, Z.; Li, P. Acoustofluidic enzyme-linked immunosorbent assay (ELISA) platform enabled by coupled acoustic streaming. Anal. Chim. Acta 2019, 1079, 129–138. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendis, B.L.; He, Z.; Li, X.; Wang, J.; Li, C.; Li, P. Acoustic Atomization-Induced Pumping Based on a Vibrating Sharp-Tip Capillary. Micromachines 2023, 14, 1212. https://doi.org/10.3390/mi14061212
Mendis BL, He Z, Li X, Wang J, Li C, Li P. Acoustic Atomization-Induced Pumping Based on a Vibrating Sharp-Tip Capillary. Micromachines. 2023; 14(6):1212. https://doi.org/10.3390/mi14061212
Chicago/Turabian StyleMendis, Balapuwaduge Lihini, Ziyi He, Xiaojun Li, Jing Wang, Chong Li, and Peng Li. 2023. "Acoustic Atomization-Induced Pumping Based on a Vibrating Sharp-Tip Capillary" Micromachines 14, no. 6: 1212. https://doi.org/10.3390/mi14061212
APA StyleMendis, B. L., He, Z., Li, X., Wang, J., Li, C., & Li, P. (2023). Acoustic Atomization-Induced Pumping Based on a Vibrating Sharp-Tip Capillary. Micromachines, 14(6), 1212. https://doi.org/10.3390/mi14061212





