Facile Preparation of Monodisperse Cu@Ag Core–Shell Nanoparticles for Conductive Ink in Printing Electronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Cu@Ag Nanoparticles and Conductive Ink
2.3. Characterization
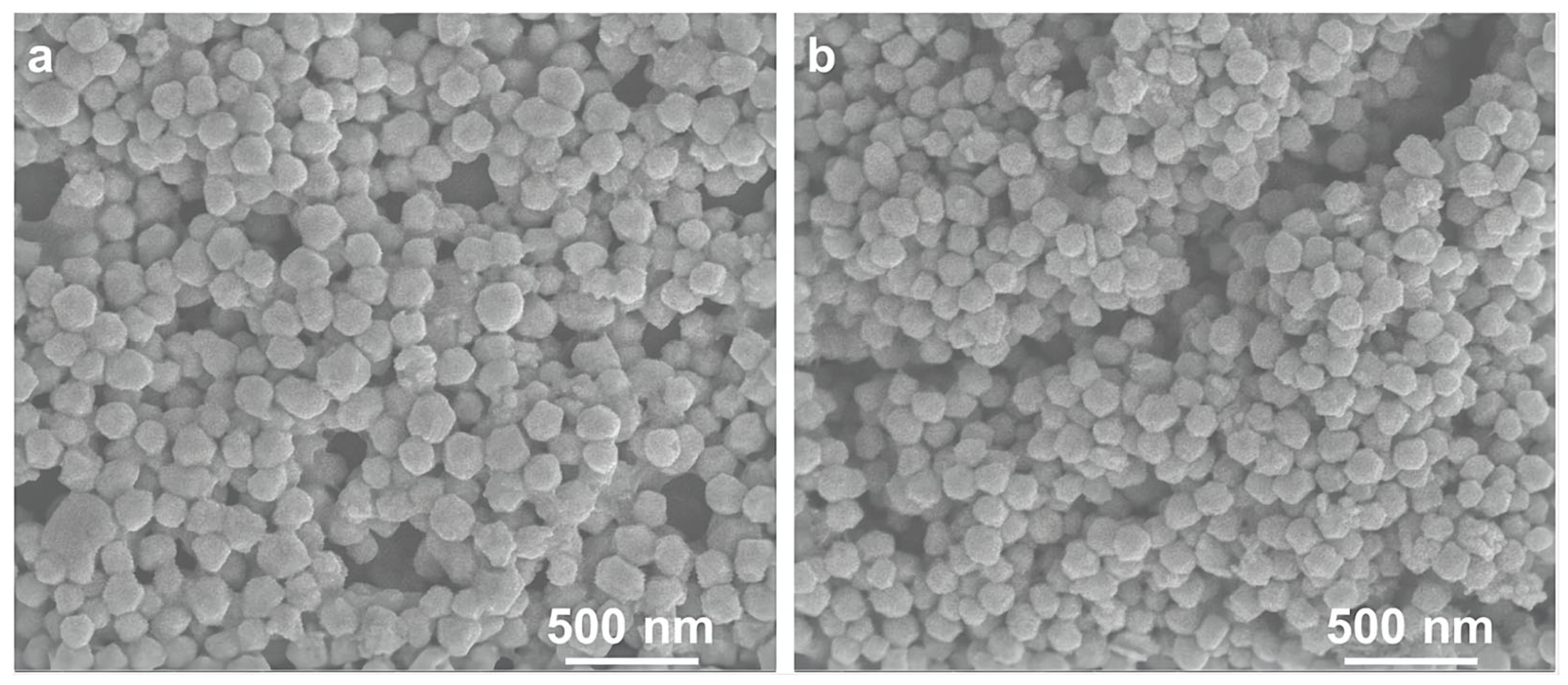
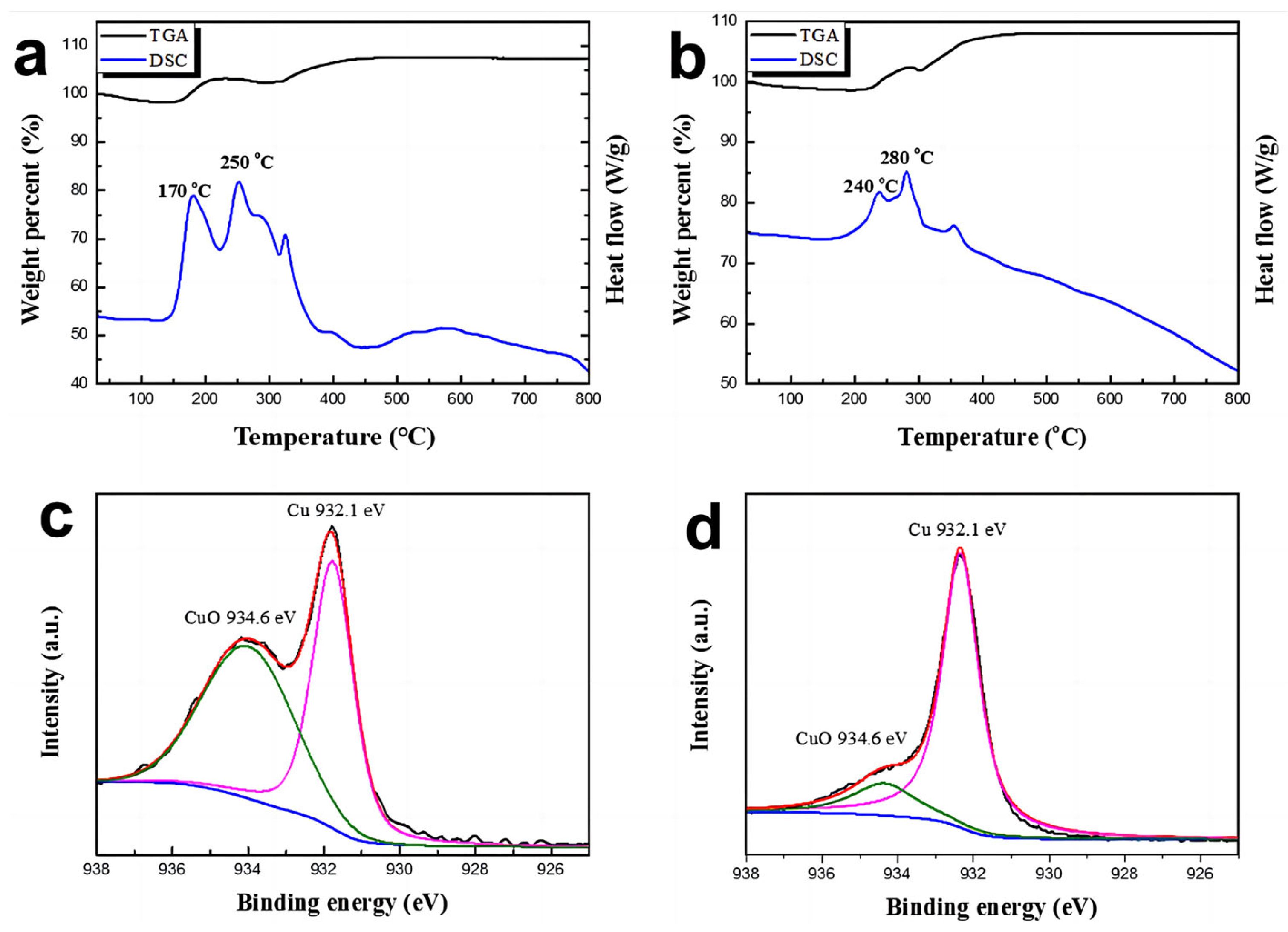
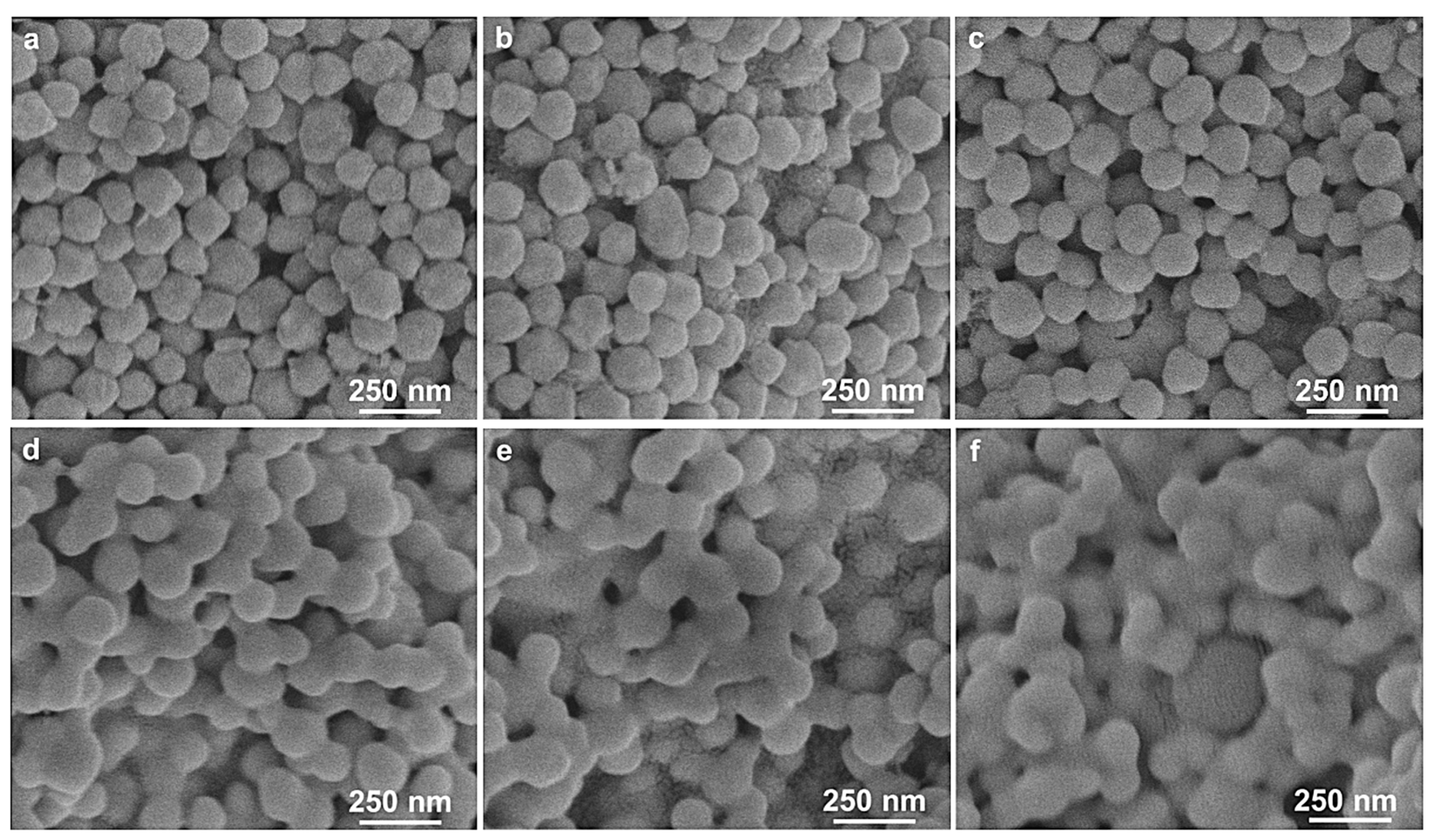
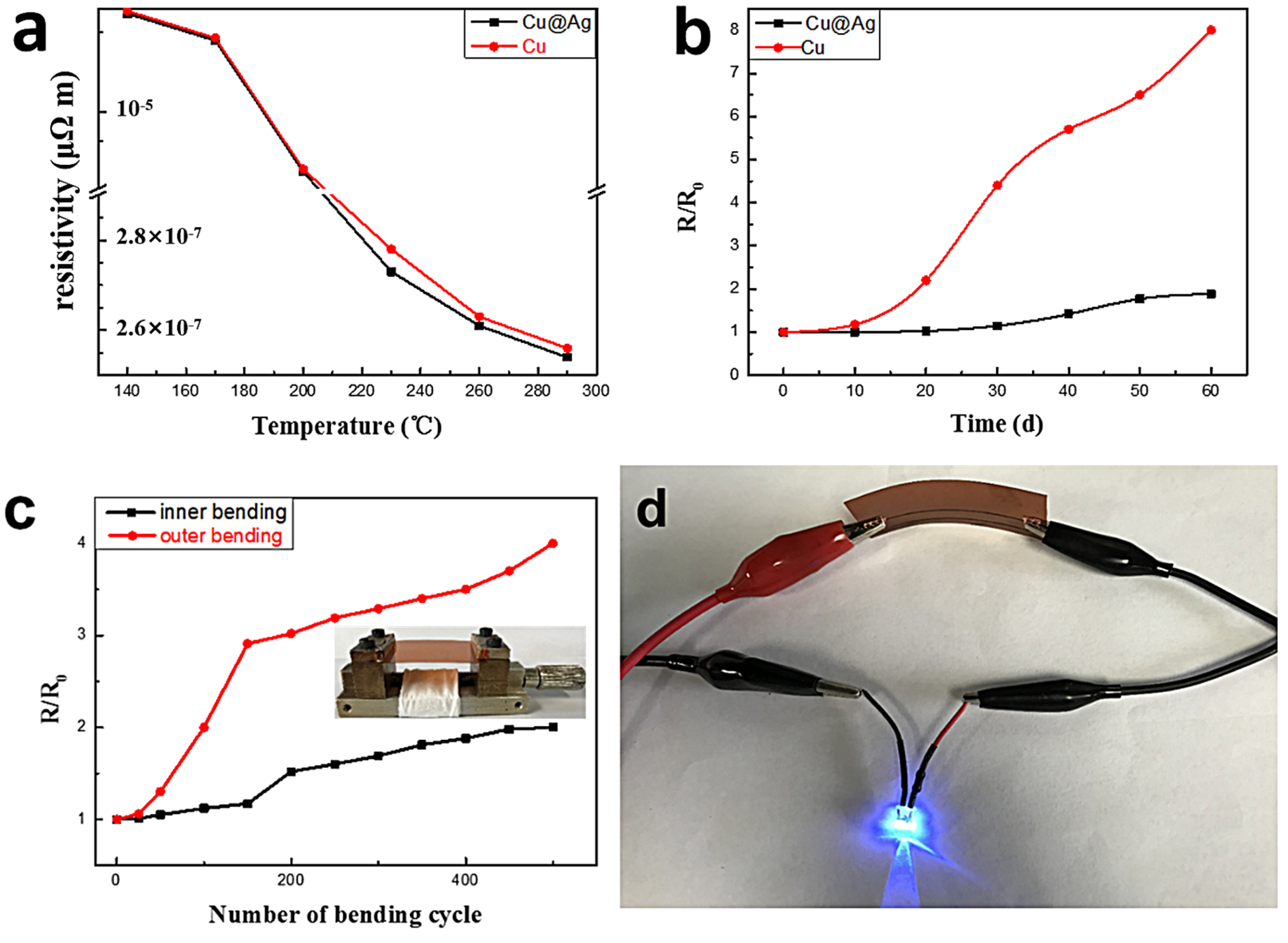
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tomotoshi, D.; Kawasaki, H. Surface and Interface Designs in Copper-Based Conductive Inks for Printed/Flexible Electronics. Nanomaterials 2020, 10, 1689. [Google Scholar] [CrossRef]
- Mo, L.X.; Guo, Z.X.; Yang, L.; Zhang, Q.Q.; Fang, Y.; Xin, Z.Q.; Chen, Z.; Hu, K.; Han, L.; Li, L.H. Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics. Int. J. Mol. Sci. 2019, 20, 2124. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Xu, W.; Zhang, T.; Shi, H.; Wang, T. Room temperature sintering of Cu-Ag core-shell nanoparticles conductive inks for printed electronics. Chem. Eng. J. 2019, 364, 310–319. [Google Scholar] [CrossRef]
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent development in silver-based ink for flexible electronics. J. Sci. Adv. Mater. Devices 2022, 7, 100395. [Google Scholar] [CrossRef]
- Abhinav, K.V.; Rao, R.V.K.; Karthik, P.S.; Singh, S.P. Copper conductive inks: Synthesis and utilization in flexible electronics. RSC Adv. 2015, 5, 63985–64030. [Google Scholar] [CrossRef]
- Craton, M.T.; Konstantinou, X.; Albrecht, J.D.; Chahal, P.; Papapolymerou, J. A Chip-First Microwave Package Using Multimaterial Aerosol Jet Printing. IEEE Trans. Microw. Theory Tech. 2020, 68, 3418–3427. [Google Scholar] [CrossRef]
- Fathi, P.; Shrestha, S.; Yerramilli, R.; Karmakar, N.; Bhattacharya, S. Screen printed chipless RFID tags on packaging substrates. Flex. Print. Electron. 2021, 6, 025009. [Google Scholar] [CrossRef]
- Douglas, S.P.; Mrig, S.; Knapp, C.E. MODs vs. NPs: Vying for the Future of Printed Electronics. Chem.-A Eur. J. 2021, 27, 8062–8081. [Google Scholar] [CrossRef]
- Goncalves, B.F.; Botelho, G.; Lanceros-Mendez, S.; Kolen, Y.V. Eco-friendly and cost-efficient inks for screen-printed fabrication of copper indium gallium diselenide photoabsorber thin films. J. Colloid Interface Sci. 2021, 598, 388–397. [Google Scholar] [CrossRef]
- Nocheseda, C.J.C.; Liza, F.P.; Collera, A.K.M.; Caldona, E.B.; Advincula, R.C. 3D printing of metals using biodegradable cellulose hydrogel inks. Addit. Manuf. 2021, 48, 102380. [Google Scholar] [CrossRef]
- Deng, D.; Cheng, Y.; Jin, Y.; Qi, T.; Xiao, F. Antioxidative effect of lactic acid-stabilized copper nanoparticles prepared in aqueous solution. J. Mater. Chem. 2012, 22, 23989–23995. [Google Scholar] [CrossRef]
- Jeong, S.; Woo, K.; Kim, D.; Lim, S.; Kim, J.S.; Shin, H.; Xia, Y.; Moon, J. Controlling the Thickness of the Surface Oxide Layer on Cu Nanoparticles for the Fabrication of Conductive Structures by Ink-Jet Printing. Adv. Funct. Mater. 2008, 18, 679–686. [Google Scholar] [CrossRef]
- Yang, W.-D.; Liu, C.-Y.; Zhang, Z.-Y.; Liu, Y.; Nie, S.-D. Copper inks formed using short carbon chain organic Cu-precursors. RSC Adv. 2014, 4, 60144–60147. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Kamyshny, A. Copper Nanoparticles for Printed Electronics: Routes towards Achieving Oxidation Stability. Materials 2010, 3, 4626–4638. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-D.; Wang, C.-H.; Arrighi, V.; Liu, C.-Y.; Watson, D. Microstructure and electrical property of copper films on a flexible substrate formed by an organic ink with 9.6% of Cu content. J. Mater. Sci. Mater. Electron. 2015, 26, 8973–8982. [Google Scholar] [CrossRef]
- Paquet, C.; Lacelle, T.; Deore, B.; Kell, A.J.; Liu, X.; Korobkov, I.; Malenfant, P.R. Pyridine-copper(ii) formates for the generation of high conductivity copper films at low temperatures. Chem. Commun. 2016, 52, 2605–2608. [Google Scholar] [CrossRef]
- Farraj, Y.; Smooha, A.; Kamyshny, A.; Magdassi, S. Plasma-Induced Decomposition of Copper Complex Ink for the Formation of Highly Conductive Copper Tracks on Heat-Sensitive Substrates. ACS Appl. Mater. Interfaces 2017, 9, 8766–8773. [Google Scholar] [CrossRef]
- Bromberg, V.; Ma, S.; Egitto, F.D.; Singler, T.J. Highly conductive lines by plasma-induced conversion of inkjet-printed silver nitrate traces. J. Mater. Chem. C 2013, 1, 6842–6849. [Google Scholar] [CrossRef]
- Paquet, C.; James, R.; Kell, A.J.; Mozenson, O.; Ferrigno, J.; Lafrenière, S.; Malenfant, P.R.L. Photosintering and electrical performance of CuO nanoparticle inks. Org. Electron. 2014, 15, 1836–1842. [Google Scholar] [CrossRef]
- Kang, B.; Han, S.; Kim, J.; Ko, S.; Yang, M. One-Step Fabrication of Copper Electrode by Laser-Induced Direct Local Reduction and Agglomeration of Copper Oxide Nanoparticle. J. Phys. Chem. C 2011, 115, 23664–23670. [Google Scholar] [CrossRef]
- Kang, H.; Sowade, E.; Baumann, R.R. Direct intense pulsed light sintering of inkjet-printed copper oxide layers within six milliseconds. ACS Appl. Mater. Interfaces 2014, 6, 1682–1687. [Google Scholar] [CrossRef]
- Wang, B.Y.; Yoo, T.H.; Song, Y.W.; Lim, D.S.; Oh, Y.J. Cu ion ink for a flexible substrate and highly conductive patterning by intensive pulsed light sintering. ACS Appl. Mater. Interfaces 2013, 5, 4113–4119. [Google Scholar] [CrossRef]
- Rager, M.S.; Aytug, T.; Veith, G.M.; Joshi, P. Low-Thermal-Budget Photonic Processing of Highly Conductive Cu Interconnects Based on CuO Nanoinks: Potential for Flexible Printed Electronics. ACS Appl. Mater. Interfaces 2016, 8, 2441–2448. [Google Scholar] [CrossRef]
- Yung, W.K.; Sun, B.; Huang, J.; Jin, Y.; Meng, Z.; Choy, H.S.; Cai, Z.; Li, G.; Ho, C.L.; Yang, J.; et al. Photochemical Copper Coating on 3D Printed Thermoplastics. Sci. Rep. 2016, 6, 31188. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wang, H.; Wu, C.; Lin, N.; Soomro, A.M.; Guo, H.; Liu, C.; Yang, X.; Wu, Y.; Cai, D.; et al. Direct synthesis of graphene 3D-coated Cu nanosilks network for antioxidant transparent conducting electrode. Nanoscale 2015, 7, 10613–10621. [Google Scholar] [CrossRef]
- Cao, W.; Li, W.; Yin, R.; Zhou, W. Controlled fabrication of Cu–Sn core–shell nanoparticles via displacement reaction. Colloid Surf. A 2014, 453, 37–43. [Google Scholar] [CrossRef]
- Grouchko, M.; Kamyshny, A.; Magdassi, S. Formation of air-stable copper–silver core–shell nanoparticles for inkjet printing. J. Mater. Chem. 2009, 19, 3057. [Google Scholar] [CrossRef]
- Chee, S.-S.; Lee, J.-H. Preparation and oxidation behavior of Ag-coated Cu nanoparticles less than 20 nm in size. J. Mater. Chem. C 2014, 2, 5372–5381. [Google Scholar] [CrossRef]
- Hajek, M.; Vesely, J.; Cieslar, M. Precision of electrical resistivity measurements. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2007, 462, 339–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.; Li, G.; Zhao, T.; Fu, X.; Sun, R.; Zhou, F.; Wong, C.P. Facile preparation of monodisperse, impurity-free, and antioxidation copper nanoparticles on a large scale for application in conductive ink. ACS Appl. Mater. Interfaces 2013, 6, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Schlucker, S. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lei, M.; Yang, S.; Zhou, L.; Liu, L.; Xiao, X.; Jiang, C.; Roy, V.A.L. A one-pot route to the synthesis of alloyed Cu/Ag bimetallic nanoparticles with different mass ratios for catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2014, 3, 3450–3455. [Google Scholar] [CrossRef]
- Deng, D.; Jin, Y.; Cheng, Y.; Qi, T.; Xiao, F. Copper nanoparticles: Aqueous phase synthesis and conductive films fabrication at low sintering temperature. ACS Appl. Mater. Interfaces 2013, 5, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, D.; Zhang, X. Preparation and characterization of copper/silver bimetallic nanowires with core-shell structure. Surf. Interface Anal. 2015, 47, 529–534. [Google Scholar] [CrossRef]
- Wünscher, S.; Abbel, R.; Perelaer, J.; Schubert, U.S. Progress of alternative sintering approaches of inkjet-printed metal inks and their application for manufacturing of flexible electronic devices. J. Mater. Chem. C 2014, 2, 10232–10261. [Google Scholar] [CrossRef]
- Jiu, J.; Wang, J.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; Suganuma, K.; Hara, M.; Nakazawa, E.; Uchida, H. The effect of light and humidity on the stability of silver nanowire transparent electrodes. RSC Adv. 2015, 5, 27657–27664. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Gao, Y.; Jiu, J.; Li, C.-F.; Chen, C.; Hu, D.; Goya, Y.; Wang, Y.; Koga, H.; et al. Highly reliable and highly conductive submicron Cu particle patterns fabricated by low temperature heat-welding and subsequent flash light sinter-reinforcement. J. Mater. Chem. C 2017, 5, 1155–1164. [Google Scholar] [CrossRef]
- Jiu, J.; Sugahara, T.; Nogi, M.; Araki, T.; Suganuma, K.; Uchida, H.; Shinozaki, K. High-intensity pulse light sintering of silver nanowire transparent films on polymer substrates: The effect of the thermal properties of substrates on the performance of silver films. Nanoscale 2013, 5, 11820–11828. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yu, X.; Zhang, R.; Ouyang, Q.; Sun, R.; Cao, L.; Zhu, P. Facile Preparation of Monodisperse Cu@Ag Core–Shell Nanoparticles for Conductive Ink in Printing Electronics. Micromachines 2023, 14, 1318. https://doi.org/10.3390/mi14071318
Li G, Yu X, Zhang R, Ouyang Q, Sun R, Cao L, Zhu P. Facile Preparation of Monodisperse Cu@Ag Core–Shell Nanoparticles for Conductive Ink in Printing Electronics. Micromachines. 2023; 14(7):1318. https://doi.org/10.3390/mi14071318
Chicago/Turabian StyleLi, Gang, Xuecheng Yu, Ruoyu Zhang, Qionglin Ouyang, Rong Sun, Liqiang Cao, and Pengli Zhu. 2023. "Facile Preparation of Monodisperse Cu@Ag Core–Shell Nanoparticles for Conductive Ink in Printing Electronics" Micromachines 14, no. 7: 1318. https://doi.org/10.3390/mi14071318
APA StyleLi, G., Yu, X., Zhang, R., Ouyang, Q., Sun, R., Cao, L., & Zhu, P. (2023). Facile Preparation of Monodisperse Cu@Ag Core–Shell Nanoparticles for Conductive Ink in Printing Electronics. Micromachines, 14(7), 1318. https://doi.org/10.3390/mi14071318






