Optofluidic Tweezers: Efficient and Versatile Micro/Nano-Manipulation Tools
Abstract
1. Introduction
2. Static Optical Tweezers
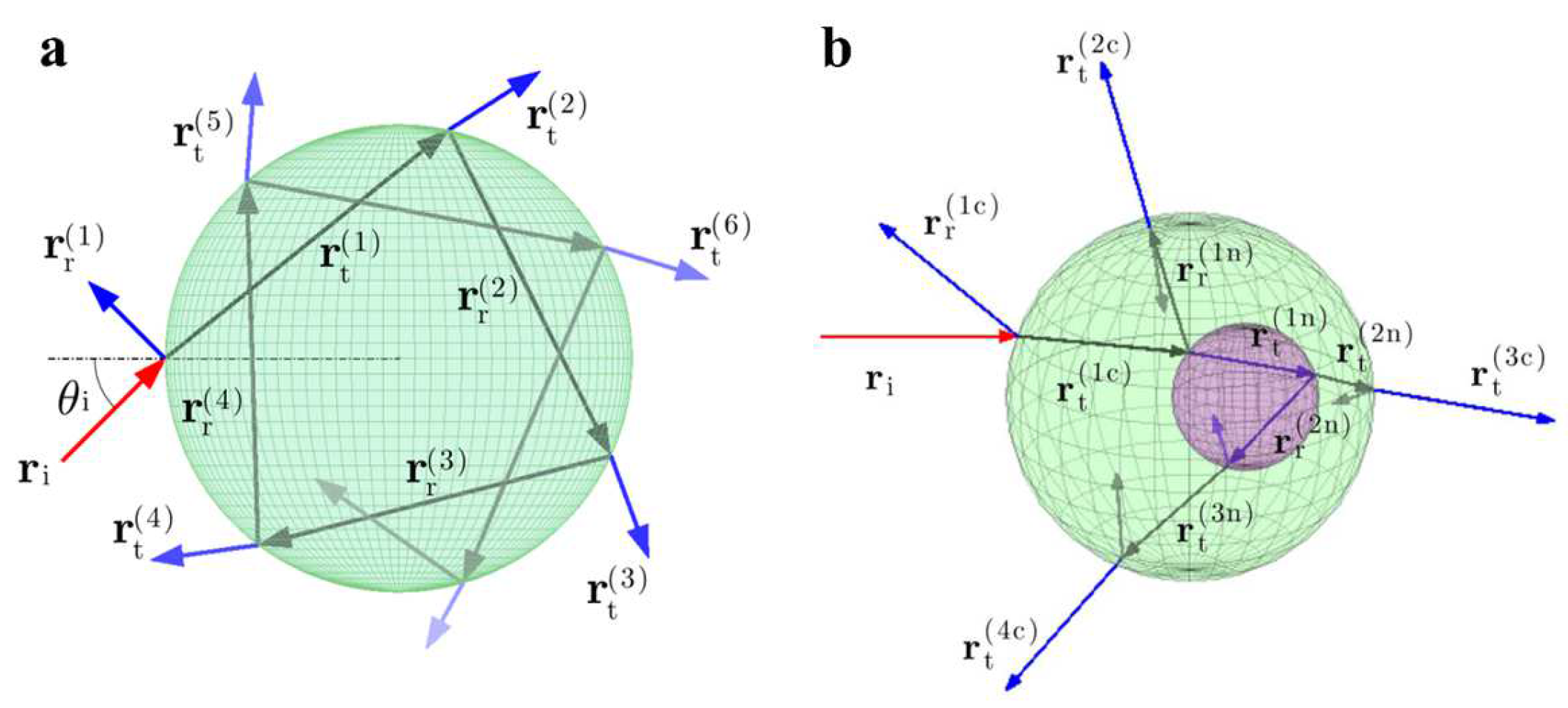
2.1. Geometric Model
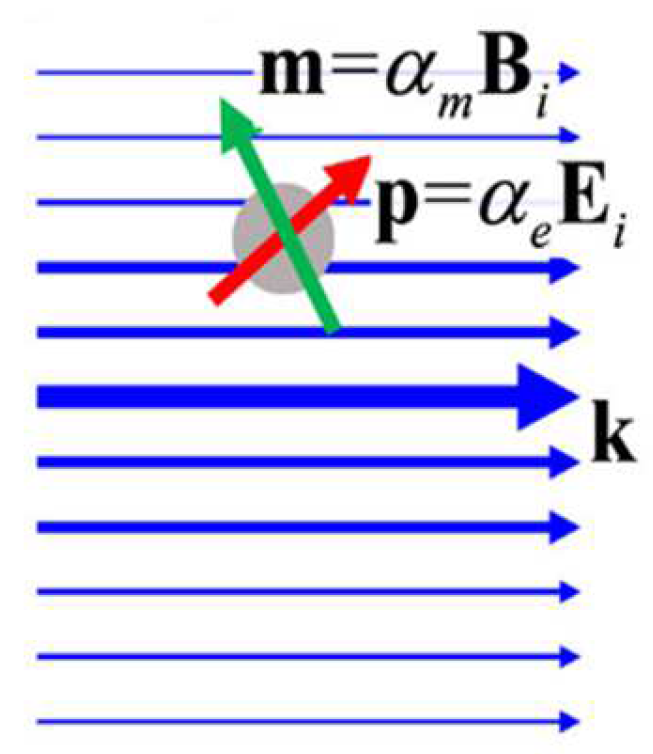
2.2. Dipole-Approximation Model
2.3. Electromagnetic Model
2.4. Forms of Static Optical Tweezers
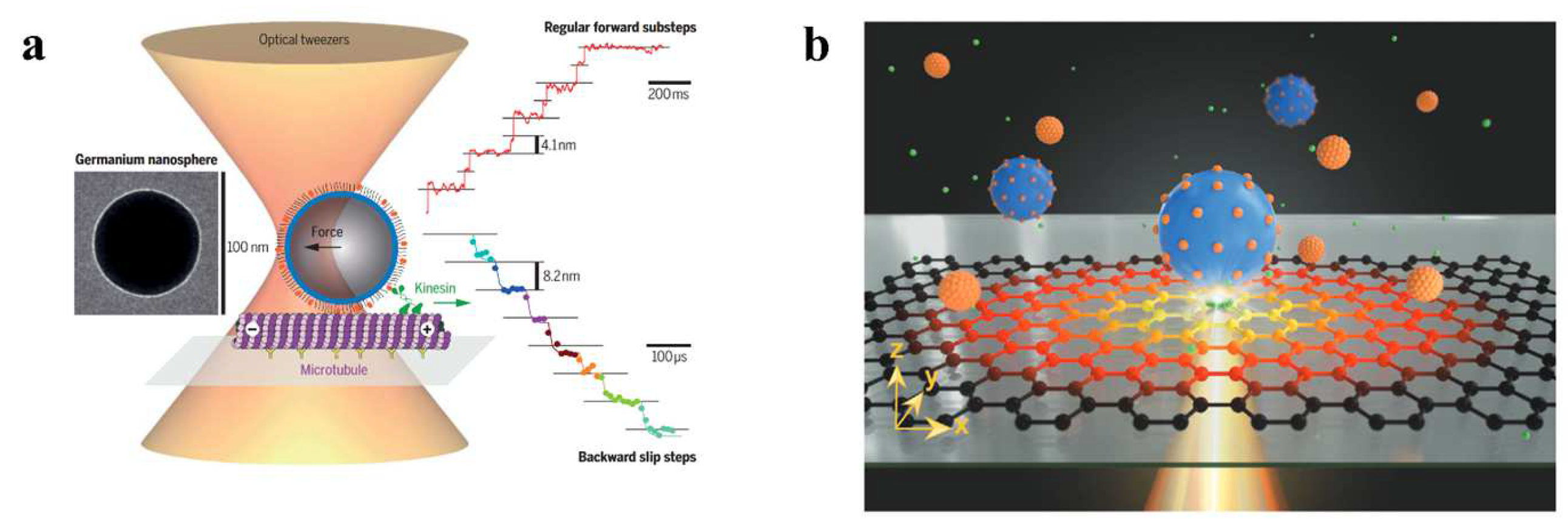
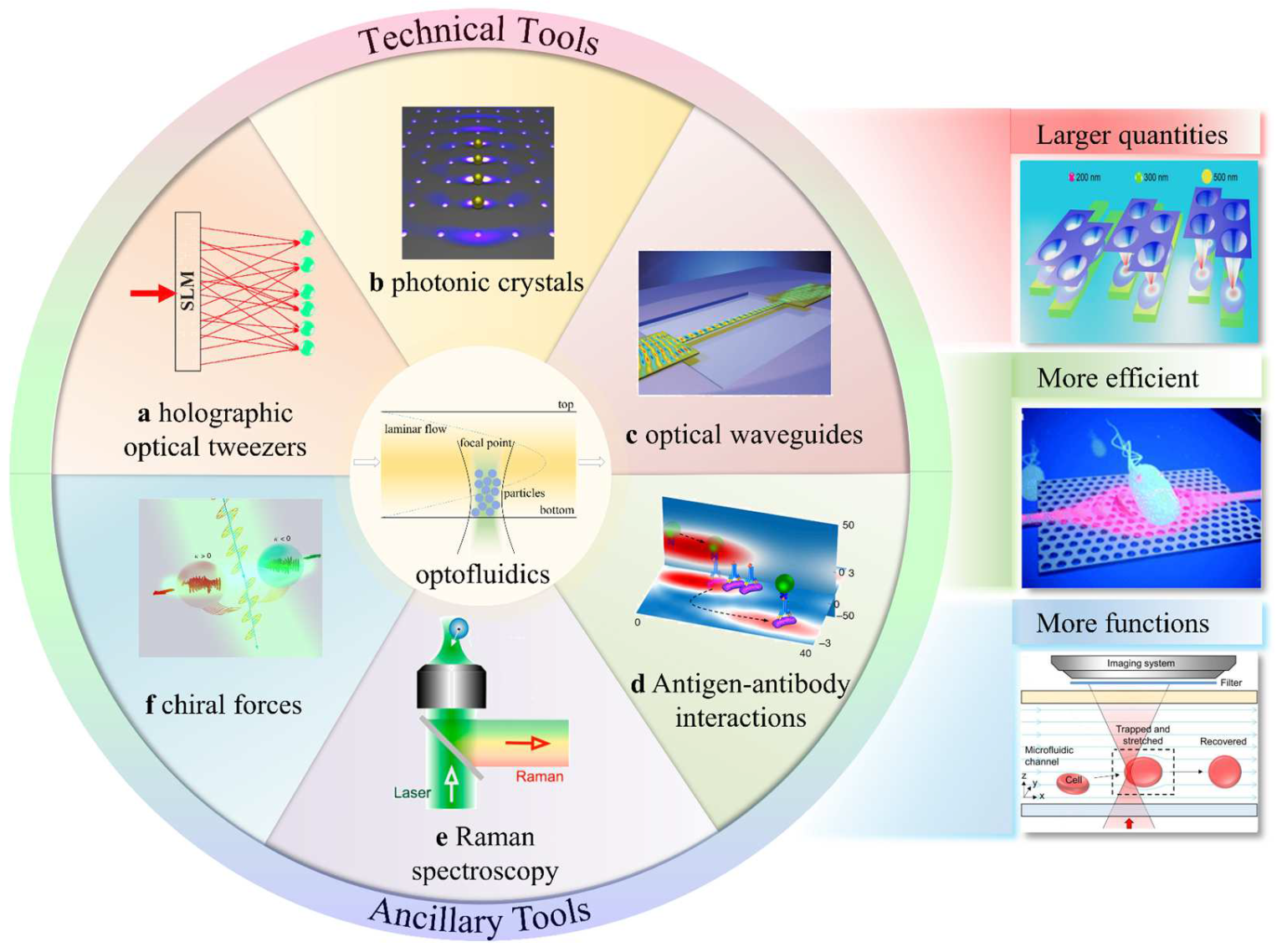
3. Optofluidic Tweezers
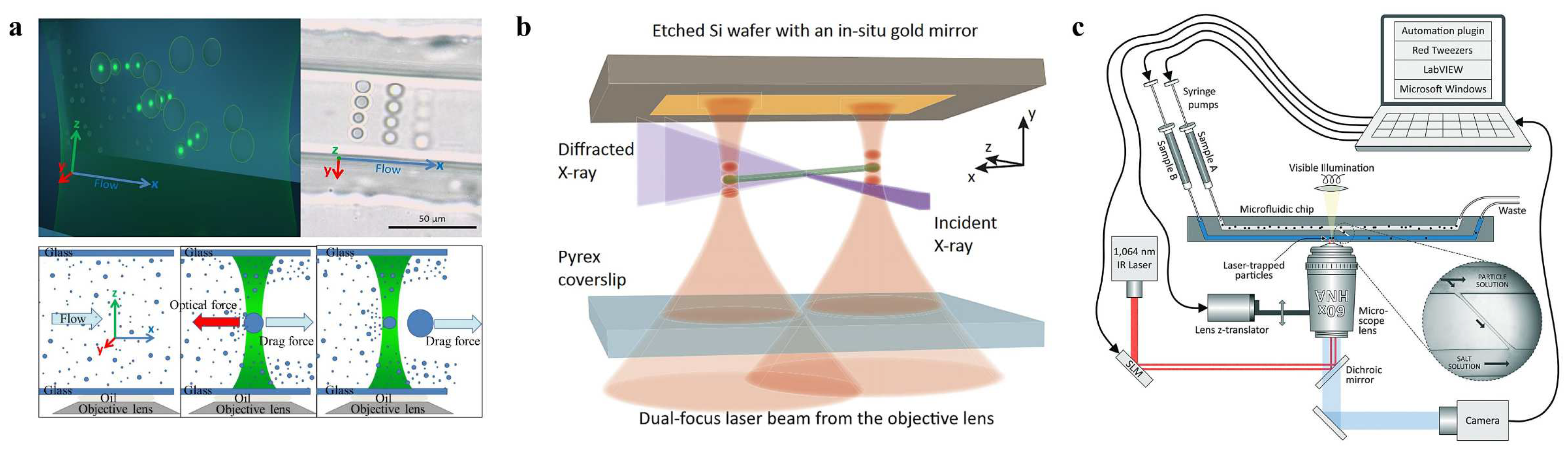
3.1. Holographic Optical Tweezers
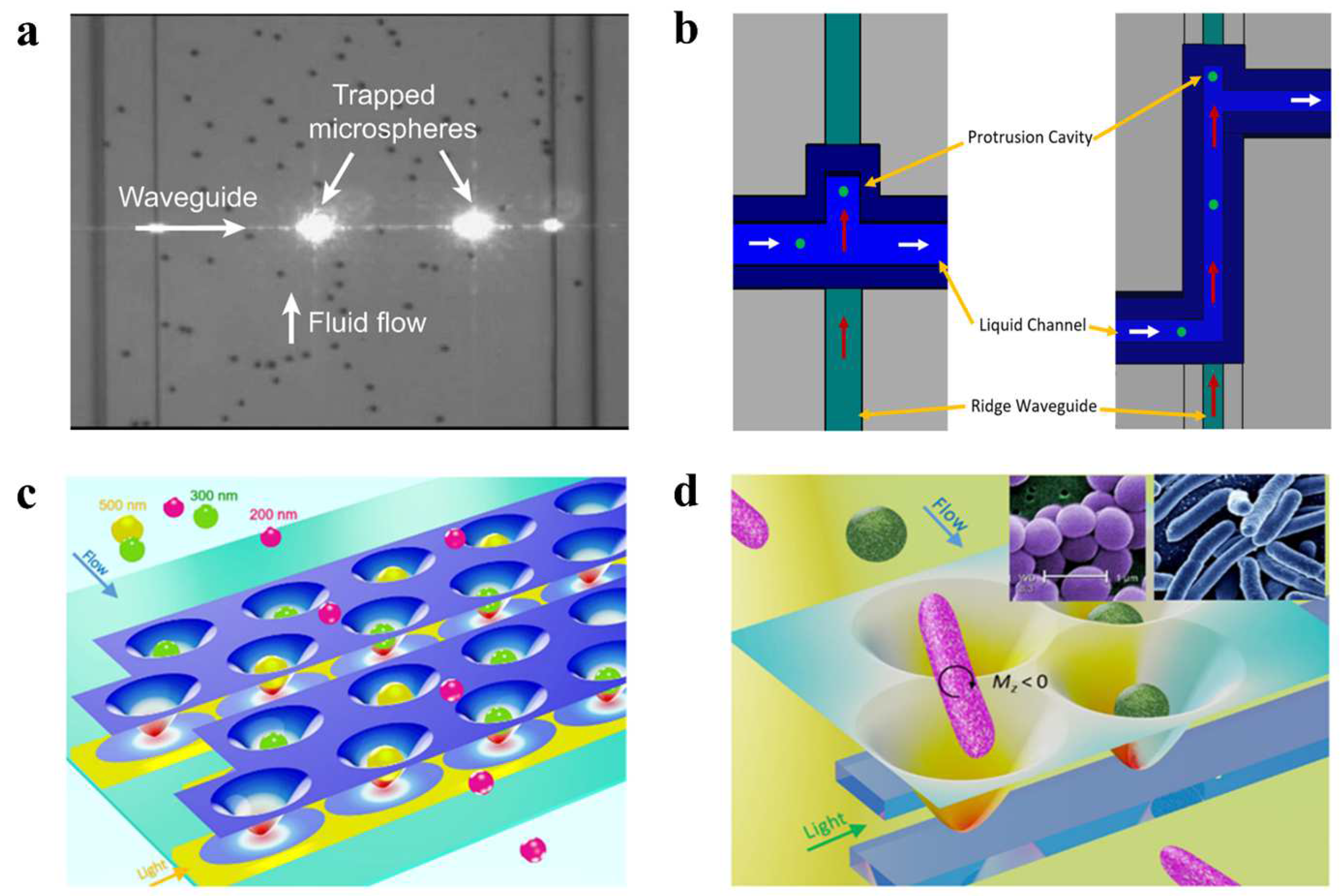
3.2. Photonic-Crystal Optical Tweezers
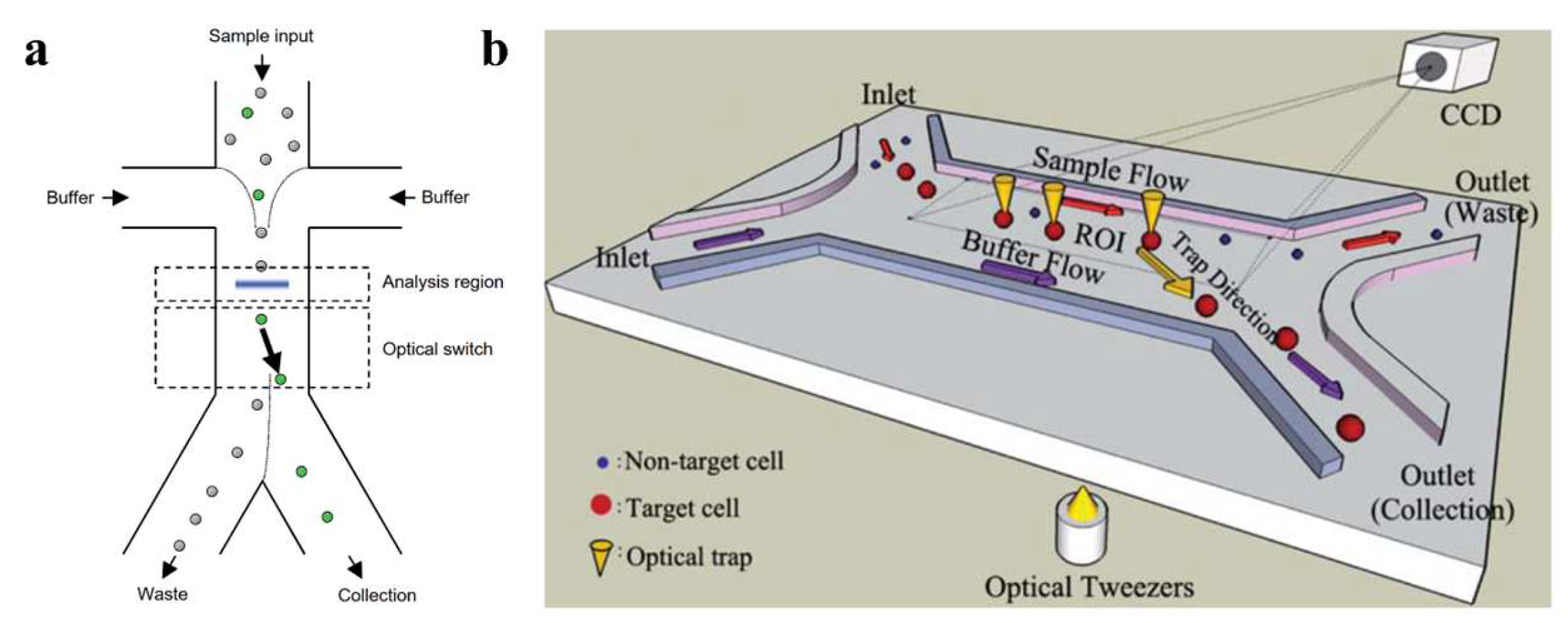
3.3. Waveguide Optical Tweezers
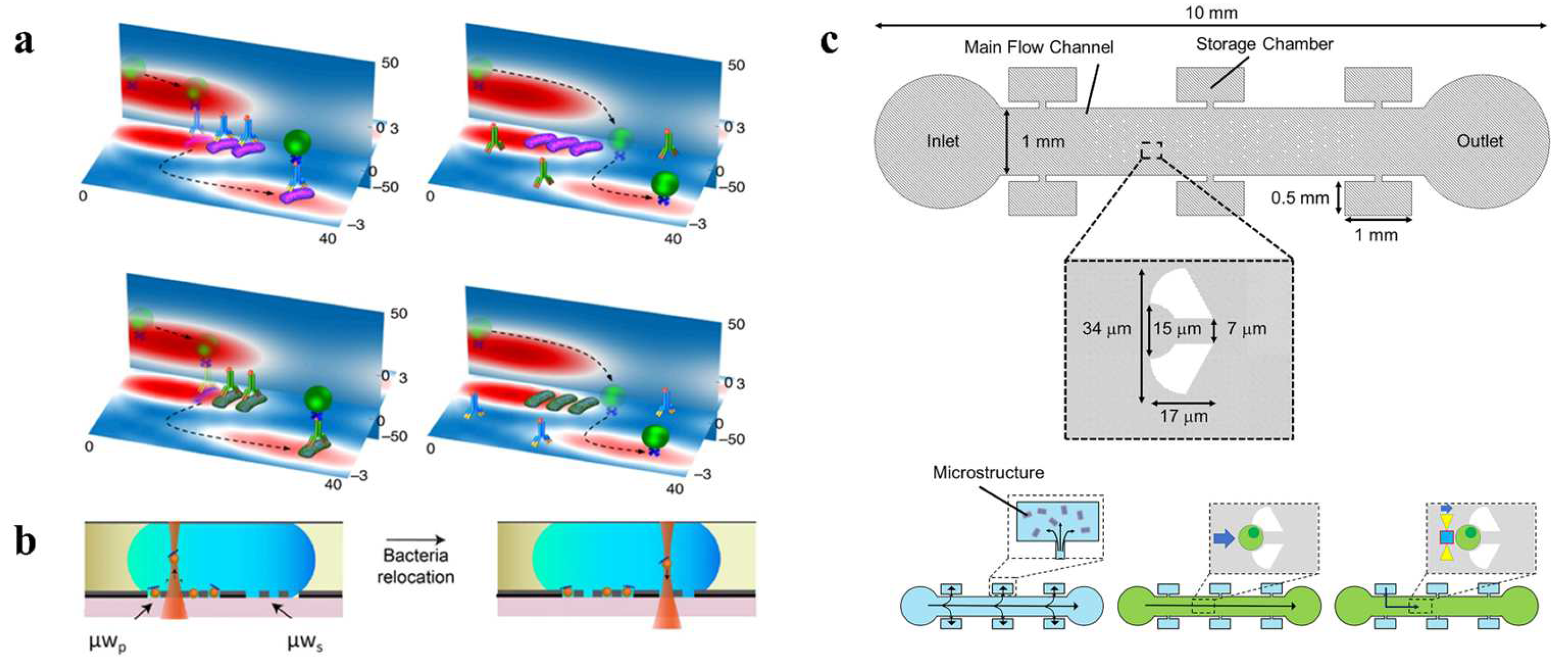
3.4. Antigen/Antibody Interactions
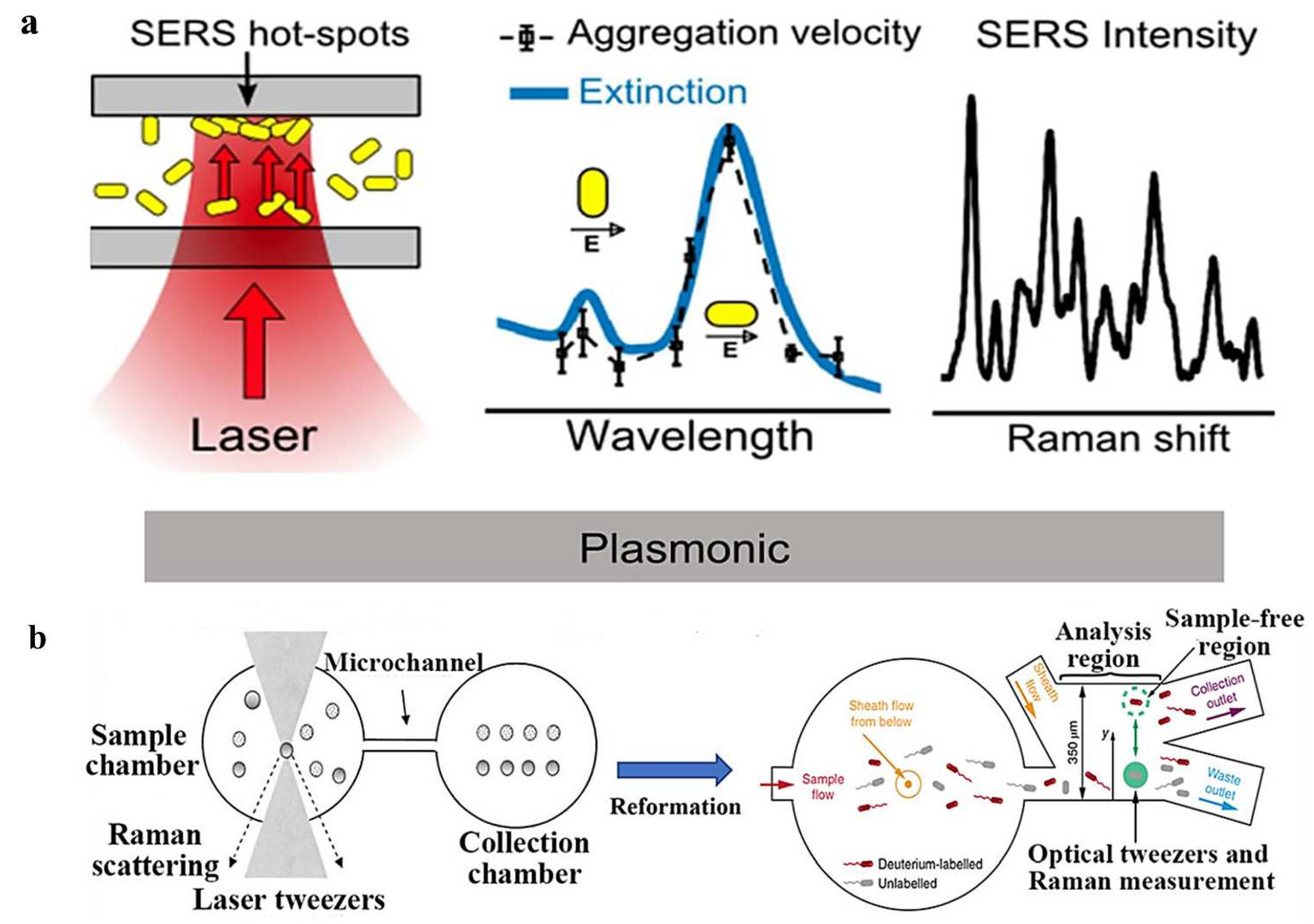
3.5. Raman-Assisted Optical Tweezers
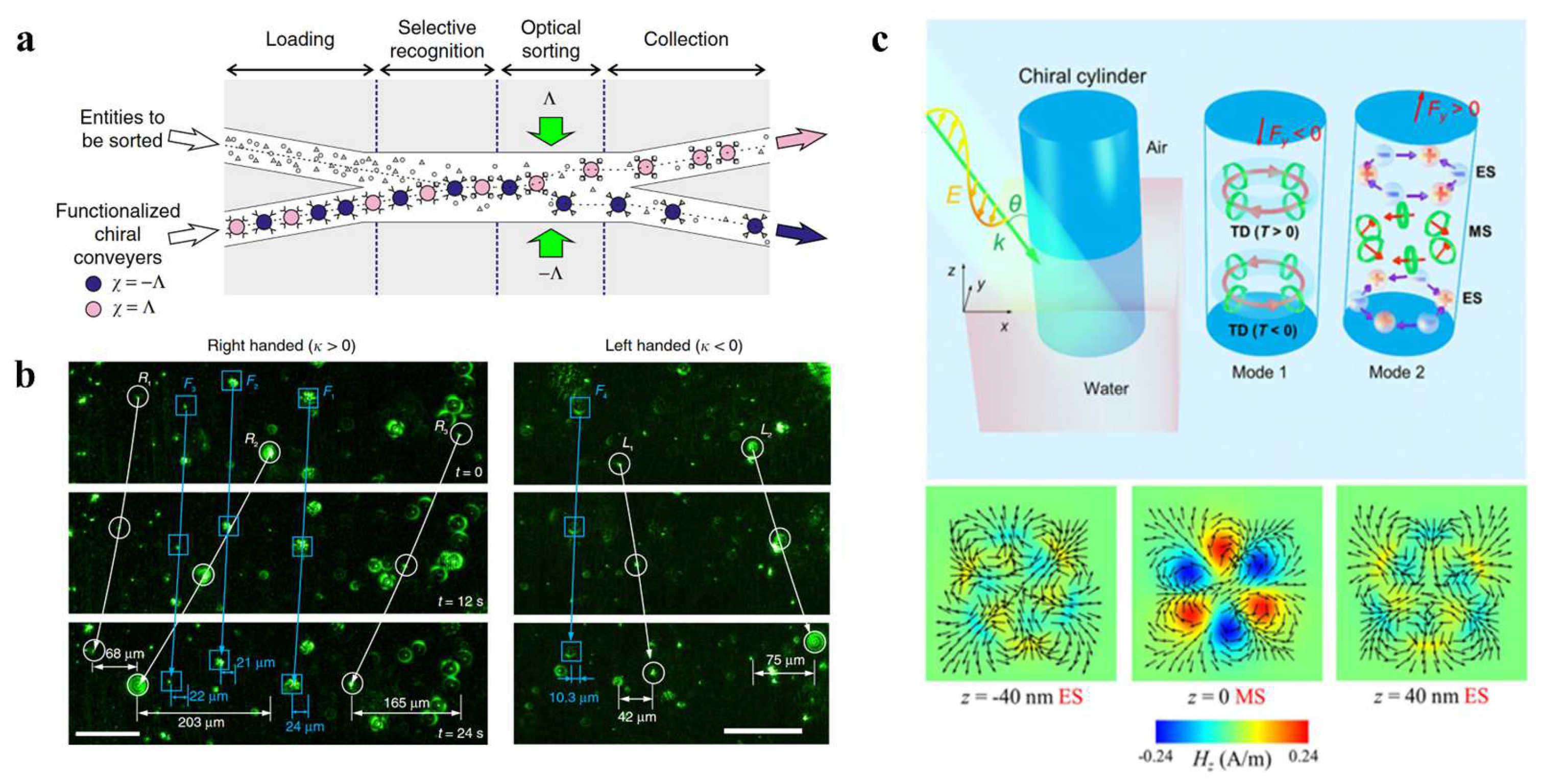
3.6. Chiral Optical Forces
3.7. Other Optofluidic Tweezers
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ye, J.; Zhao, Y.; Zhu, C. Advanced Optical Tweezers on Cell Manipulation and Analysis. Eur. Phys. J. Plus 2022, 137, 1024. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Chemla, Y.R.; Smith, S.B.; Bustamante, C. Recent Advances in Optical Tweezers. Annu. Rev. Biochem. 2008, 77, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Grier, D.G. Optical Tweezers in Colloid and Interface Science. Curr. Opin. Colloid Interface Sci. 1997, 2, 264–270. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Y.-X.; Chen, M.; Arita, Y.; Rosales-Guzmán, C. Optical Trapping with Structured Light: A Review. Adv. Photonics 2021, 3, 034001. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M. Optical Trapping and Manipulation of Viruses and Bacteria. Science 1987, 235, 1517–1520. [Google Scholar] [CrossRef]
- Fazal, F.M.; Block, S.M. Optical Tweezers Study Life under Tension. Nat. Photonics 2011, 5, 318–321. [Google Scholar] [CrossRef]
- LaFratta, C.N. Optical Tweezers for Medical Diagnostics. Anal. Bioanal. Chem. 2013, 405, 5671–5677. [Google Scholar] [CrossRef]
- Chu, S.; Hollberg, L.; Bjorkholm, J.E.; Cable, A.; Ashkin, A. Three-Dimensional Viscous Confinement and Cooling of Atoms by Resonance Radiation Pressure. Phys. Rev. Lett. 1985, 55, 48–51. [Google Scholar] [CrossRef]
- Chu, S.; Bjorkholm, J.E.; Ashkin, A.; Cable, A. Experimental Observation of Optically Trapped Atoms. Phys. Rev. Lett. 1986, 57, 314–317. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Liu, Y.; Zhang, Y.; Xiao, Y.; Li, B. Optical Forces: From Fundamental to Biological Applications. Adv. Mater. 2020, 32, 2001994. [Google Scholar] [CrossRef] [PubMed]
- Asplund, M.C.; Johnson, J.A.; Patterson, J.E. The 2018 Nobel Prize in Physics: Optical Tweezers and Chirped Pulse Amplification. Anal. Bioanal. Chem. 2019, 411, 5001–5005. [Google Scholar] [CrossRef] [PubMed]
- Grigorenko, A.N.; Roberts, N.W.; Dickinson, M.R.; Zhang, Y. Nanometric Optical Tweezers Based on Nanostructured Substrates. Nat. Photonics 2008, 2, 365–370. [Google Scholar] [CrossRef]
- Dholakia, K.; Čižmár, T. Shaping the Future of Manipulation. Nat. Photonics 2011, 5, 335–342. [Google Scholar] [CrossRef]
- Gao, D.; Ding, W.; Nieto-Vesperinas, M.; Ding, X.; Rahman, M.; Zhang, T.; Lim, C.; Qiu, C.-W. Optical Manipulation from the Microscale to the Nanoscale: Fundamentals, Advances and Prospects. Light Sci. Appl. 2017, 6, e17039. [Google Scholar] [CrossRef]
- Zheng, Y.; Ryan, J.; Hansen, P.; Cheng, Y.-T.; Lu, T.-J.; Hesselink, L. Nano-Optical Conveyor Belt, Part II: Demonstration of Handoff Between Near-Field Optical Traps. Nano Lett. 2014, 14, 2971–2976. [Google Scholar] [CrossRef]
- Yang, A.H.J.; Moore, S.D.; Schmidt, B.S.; Klug, M.; Lipson, M.; Erickson, D. Optical Manipulation of Nanoparticles and Biomolecules in Sub-Wavelength Slot Waveguides. Nature 2009, 457, 71–75. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, Y.; Chin, L.K.; Li, Z.; Liu, J.; Chen, M.K.; Wang, S.; Zhang, Y.; Liu, P.Y.; Zhou, X.; et al. Multifunctional Virus Manipulation with Large-Scale Arrays of All-Dielectric Resonant Nanocavities. Laser Photonics Rev. 2022, 16, 2100197. [Google Scholar] [CrossRef]
- Wang, M.M.; Tu, E.; Raymond, D.E.; Yang, J.M.; Zhang, H.; Hagen, N.; Dees, B.; Mercer, E.M.; Forster, A.H.; Kariv, I.; et al. Microfluidic Sorting of Mammalian Cells by Optical Force Switching. Nat. Biotechnol. 2005, 23, 83–87. [Google Scholar] [CrossRef]
- Čižmár, T.; Šiler, M.; Šerý, M.; Zemánek, P.; Garcés-Chávez, V.; Dholakia, K. Optical Sorting and Detection of Submicrometer Objects in a Motional Standing Wave. Phys. Rev. B 2006, 74, 035105. [Google Scholar] [CrossRef]
- Pang, Y.; Song, H.; Kim, J.H.; Hou, X.; Cheng, W. Optical Trapping of Individual Human Immunodeficiency Viruses in Culture Fluid Reveals Heterogeneity with Single-Molecule Resolution. Nat. Nanotechnol. 2014, 9, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, R.; Keuntje, M.; Theophile, K.; Müller, J.; Mielcarek, E.; Ngezahayo, A.; Anders-von Ahlften, A. In Situ Mapping of Nitrifiers and Anammox Bacteria in Microbial Aggregates by Means of Confocal Resonance Raman Microscopy. J. Microbiol. Methods 2008, 72, 241–248. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, A.; Qiu, C.; Wang, Z.; Cheng, X. Research progress on optofluidic optical tweezers. Opt. Precis. Eng. 2022, 30, 2765–2782. [Google Scholar] [CrossRef]
- Shao, L.; Liu, Z.; Hu, J.; Gunawardena, D.; Tam, H.-Y. Optofluidics in Microstructured Optical Fibers. Micromachines 2018, 9, 145. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, X.; Zuo, Y.; Hu, X.; Shi, Y.; Liang, L.; Yang, Y. Optofluidics: The Interaction between Light and Flowing Liquids in Integrated Devices. Opto-Electron. Adv. 2019, 2, 19000701–19000710. [Google Scholar] [CrossRef]
- Chen, S.; Hao, R.; Zhang, Y.; Yang, H. Optofluidics in Bio-Imaging Applications. Photonics Res. 2019, 7, 532. [Google Scholar] [CrossRef]
- Chen, J.; Loo, J.F.; Wang, D.; Zhang, Y.; Kong, S.; Ho, H. Thermal Optofluidics: Principles and Applications. Adv. Opt. Mater. 2020, 8, 1900829. [Google Scholar] [CrossRef]
- Hess, D.; Yang, T.; Stavrakis, S. Droplet-Based Optofluidic Systems for Measuring Enzyme Kinetics. Anal. Bioanal. Chem. 2020, 412, 3265–3283. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M. Optofluidic Microsystems for Chemical and Biological Analysis. Nat. Photonics 2011, 5, 591–597. [Google Scholar] [CrossRef]
- Psaltis, D.; Quake, S.R.; Yang, C. Developing Optofluidic Technology through the Fusion of Microfluidics and Optics. Nature 2006, 442, 381–386. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, X.; Zuo, Y.; Liang, L.; Zhang, S.; Zhang, X.; Yang, Y. Precise Sorting of Gold Nanoparticles in a Flowing System. ACS Photonics 2016, 3, 2497–2504. [Google Scholar] [CrossRef]
- Lasnoy, E.; Wagner, O.; Edri, E.; Shpaisman, H. Drag Controlled Formation of Polymeric Colloids with Optical Traps. Lab. Chip 2019, 19, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Kwan, C.C.; Poon, A.W. An Optofluidic “Tweeze-and-Drag” Cell Stretcher in a Microfluidic Channel. Lab. Chip 2020, 20, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Geonzon, L.C.; Kobayashi, M.; Sugimoto, T.; Adachi, Y. Adsorption Kinetics of Polyacrylamide-Based Polyelectrolyte onto a Single Silica Particle Studied Using Microfluidics and Optical Tweezers. J. Colloid Interface Sci. 2023, 630, 846–854. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Xiong, S.; Zhang, Y.; Chin, L.K.; Chen, Y.-Y.; Zhang, J.B.; Zhang, T.H.; Ser, W.; Larrson, A.; Lim, S.H.; et al. Sculpting Nanoparticle Dynamics for Single-Bacteria-Level Screening and Direct Binding-Efficiency Measurement. Nat. Commun. 2018, 9, 815. [Google Scholar] [CrossRef]
- Pilát, Z.; Bernatová, S.; Ježek, J.; Kirchhoff, J.; Tannert, A.; Neugebauer, U.; Samek, O.; Zemánek, P. Microfluidic Cultivation and Laser Tweezers Raman Spectroscopy of E. Coli under Antibiotic Stress. Sensors 2018, 18, 1623. [Google Scholar] [CrossRef]
- Bernatová, S.; Donato, M.G.; Ježek, J.; Pilát, Z.; Samek, O.; Magazzù, A.; Maragò, O.M.; Zemánek, P.; Gucciardi, P.G. Wavelength-Dependent Optical Force Aggregation of Gold Nanorods for SERS in a Microfluidic Chip. J. Phys. Chem. C 2019, 123, 5608–5615. [Google Scholar] [CrossRef]
- Dai, X.; Fu, W.; Chi, H.; Mesias, V.S.D.; Zhu, H.; Leung, C.W.; Liu, W.; Huang, J. Optical Tweezers-Controlled Hotspot for Sensitive and Reproducible Surface-Enhanced Raman Spectroscopy Characterization of Native Protein Structures. Nat. Commun. 2021, 12, 1292. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y.; Zhou, L.-M.; Xu, X.; Zhu, T.; Shi, Y.; Qiu, C.-W.; Ding, W. Optical Pulling Forces and Their Applications. Adv. Opt. Photonics 2020, 12, 288. [Google Scholar] [CrossRef]
- Huang, N.; Martínez, L.J.; Jaquay, E.; Nakano, A.; Povinelli, M.L. Optical Epitaxial Growth of Gold Nanoparticle Arrays. Nano Lett. 2015, 15, 5841–5845. [Google Scholar] [CrossRef]
- Li, M.; Pernice, W.H.P.; Xiong, C.; Baehr-Jones, T.; Hochberg, M.; Tang, H.X. Harnessing Optical Forces in Integrated Photonic Circuits. Nature 2008, 456, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Gillibert, R.; Balakrishnan, G.; Deshoules, Q.; Tardivel, M.; Magazzù, A.; Donato, M.G.; Maragò, O.M.; Lamy De La Chapelle, M.; Colas, F.; Lagarde, F.; et al. Raman Tweezers for Small Microplastics and Nanoplastics Identification in Seawater. Environ. Sci. Technol. 2019, 53, 9003–9013. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhu, T.; Zhang, T.; Mazzulla, A.; Tsai, D.P.; Ding, W.; Liu, A.Q.; Cipparrone, G.; Sáenz, J.J.; Qiu, C.-W. Chirality-Assisted Lateral Momentum Transfer for Bidirectional Enantioselective Separation. Light Sci. Appl. 2020, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, H.; Chin, L.K.; Zhang, Y.; Yap, P.H.; Ser, W.; Qiu, C.-W.; Liu, A.Q. Optical Potential-Well Array for High-Selectivity, Massive Trapping and Sorting at Nanoscale. Nano Lett. 2020, 20, 5193–5200. [Google Scholar] [CrossRef]
- van Leest, T.; Caro, J. Cavity-Enhanced Optical Trapping of Bacteria Using a Silicon Photonic Crystal. Lab. Chip 2013, 13, 4358. [Google Scholar] [CrossRef] [PubMed]
- Lenton, I.C.D.; Armstrong, D.J.; Stilgoe, A.B.; Nieminen, T.A.; Rubinsztein-Dunlop, H. Orientation of Swimming Cells with Annular Beam Optical Tweezers. Opt. Commun. 2020, 459, 124864. [Google Scholar] [CrossRef]
- Ashkin, A. Forces of a Single-Beam Gradient Laser Trap on a Dielectric Sphere in the Ray Optics Regime. Biophys. J. 1992, 61, 569–582. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M. Radiation Pressure on a Free Liquid Surface. Phys. Rev. Lett. 1973, 30, 139–142. [Google Scholar] [CrossRef]
- Chang, Y.-R.; Hsu, L.; Chi, S. Optical Trapping of a Spherically Symmetric Sphere in the Ray-Optics Regime: A Model for Optical Tweezers upon Cells. Appl. Opt. 2006, 45, 3885. [Google Scholar] [CrossRef]
- Jones, P.H.; Maragò, O.M.; Volpe, G. Optical Tweezers: Principles and Applications, 1st ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 42–75. [Google Scholar]
- Callegari, A.; Mijalkov, M.; Gököz, A.B.; Volpe, G. Computational Toolbox for Optical Tweezers in Geometrical Optics. J. Opt. Soc. Am. B 2015, 32, B11. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Crosse, J.A.; Danesh, M.; Qiu, C.-W.; Danner, A.J.; Soukoulis, C.M. Interplay of Optical Force and Ray-Optic Behavior between Luneburg Lenses. ACS Photonics 2015, 2, 1384–1390. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Danesh, M.; Qiu, C.-W.; Danner, A.J. Tracing Optical Force Fields within Graded-Index Media. New J. Phys. 2014, 16, 053035. [Google Scholar] [CrossRef]
- Chaumet, P.C.; Nieto-Vesperinas, M. Coupled Dipole Method Determination of the Electromagnetic Force on a Particle over a Flat Dielectric Substrate. Phys. Rev. B 2000, 61, 14119–14127. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Frontmatter. Absorption and Scattering of Light by Small Particles, 1st ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1998; pp. 130–157. [Google Scholar]
- Nieto-Vesperinas, M.; Saenz, J.J.; Gomez-Medina, R. Optical Forces on Small Magnetodielectric Particles. Opt. Express 2010, 18, 11428–11443. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Vesperinas, M.; Gomez-Medina, R.; Saenz, J.J. Angle-Suppressed Scattering and Optical Forces on Submicrometer Dielectric Particles. J. Opt. Soc. Am. A 2011, 28, 54. [Google Scholar] [CrossRef] [PubMed]
- Chaumet, P.C.; Rahmani, A. Electromagnetic Force and Torque on Magnetic and Negative-Index Scatterers. Opt. Express 2009, 17, 2224. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, J.; Ao, R.; Ma, Y.; Fu, B. Numerical Simulation of Deformed Red Blood Cell by Utilizing Neural Network Approach and Finite Element Analysis. Comput. Methods Biomech. Biomed. Engin. 2020, 23, 1190–1200. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Li, R.; Liu, H.; Ge, Y.; Xu, Z. Plasmonic Optical Trapping of Nanoparticles Using T-Shaped Copper Nanoantennas. Opt. Express 2021, 29, 9826. [Google Scholar] [CrossRef]
- Gauthier, R.C. Computation of the Optical Trapping Force Using an FDTD Based Technique. Opt. Express 2005, 13, 3707. [Google Scholar] [CrossRef]
- Nalimov, A.G.; Kotlyar, V.V. Rotation of an Ellipsoidal Dielectric Particle in the Focus of a Circularly Polarized Gaussian Beam. Optik 2020, 222, 165479. [Google Scholar] [CrossRef]
- Khonina, S.N.; Krasnov, S.V.; Ustinov, A.V.; Degtyarev, S.A.; Porfirev, A.P.; Kuchmizhak, A.; Kudryashov, S.I. Refractive Twisted Microaxicons. Opt. Lett. 2020, 45, 1334. [Google Scholar] [CrossRef] [PubMed]
- Yurkin, M.A.; Hoekstra, A.G. The Discrete Dipole Approximation: An Overview and Recent Developments. J. Quant. Spectrosc. Radiat. Transf. 2007, 106, 558–589. [Google Scholar] [CrossRef]
- Nieminen, T.A.; Rubinsztein-Dunlop, H.; Heckenberg, N.R. Calculation of the T-Matrix: General Considerations and Application of the Point-Matching Method. J. Quant. Spectrosc. Radiat. Transf. 2003, 79, 1019–1029. [Google Scholar] [CrossRef]
- Waterman, P.C. Symmetry, Unitarity, and Geometry in Electromagnetic Scattering. Phys. Rev. D 1971, 3, 825–839. [Google Scholar] [CrossRef]
- Polimeno, P.; Iatì, M.A.; Degli Esposti Boschi, C.; Simpson, S.H.; Svak, V.; Brzobohatý, O.; Zemánek, P.; Maragò, O.M.; Saija, R. T-Matrix Calculations of Spin-Dependent Optical Forces in Optically Trapped Nanowires. Eur. Phys. J. Plus 2021, 136, 86. [Google Scholar] [CrossRef]
- Polimeno, P.; Magazzù, A.; Iatì, M.A.; Saija, R.; Folco, L.; Bronte Ciriza, D.; Donato, M.G.; Foti, A.; Gucciardi, P.G.; Saidi, A.; et al. Optical Tweezers in a Dusty Universe: Modeling Optical Forces for Space Tweezers Applications. Eur. Phys. J. Plus 2021, 136, 339. [Google Scholar] [CrossRef]
- Masliyah, J.H.; Bhattacharjee, S. Electrokinetic and Colloid Transport Phenomena; Wiley-Interscience: Hoboken, NJ, USA, 2006; ISBN 978-0-471-78882-9.70. [Google Scholar]
- Hansen, P.M.; Tolić-Nørrelykke, I.M.; Flyvbjerg, H.; Berg-Sørensen, K. Tweezercalib 2.0: Faster Version of MatLab Package for Precise Calibration of Optical Tweezers. Comput. Phys. Commun. 2006, 174, 518–520. [Google Scholar] [CrossRef]
- Dienerowitz, M.; Gibson, G.; Dienerowitz, F.; Padgett, M. Expanding the Toolbox for Nanoparticle Trapping and Spectroscopy with Holographic Optical Tweezers. J. Opt. 2012, 14, 045003. [Google Scholar] [CrossRef]
- Taylor, M.A.; Bowen, W.P. A Computational Tool to Characterize Particle Tracking Measurements in Optical Tweezers. J. Opt. 2013, 15, 085701. [Google Scholar] [CrossRef]
- Lenton, I.C.D.; Stilgoe, A.B.; Nieminen, T.A.; Rubinsztein-Dunlop, H. OTSLM Toolbox for Structured Light Methods. Comput. Phys. Commun. 2020, 253, 107199. [Google Scholar] [CrossRef]
- Zhong, M.-C.; Wei, X.-B.; Zhou, J.-H.; Wang, Z.-Q.; Li, Y.-M. Trapping Red Blood Cells in Living Animals Using Optical Tweezers. Nat. Commun. 2013, 4, 1768. [Google Scholar] [CrossRef]
- Sudhakar, S.; Abdosamadi, M.K.; Jachowski, T.J.; Bugiel, M.; Jannasch, A.; Schäffer, E. Germanium Nanospheres for Ultraresolution Picotensiometry of Kinesin Motors. Science 2021, 371, eabd9944. [Google Scholar] [CrossRef]
- Favre-Bulle, I.A.; Stilgoe, A.B.; Rubinsztein-Dunlop, H.; Scott, E.K. Optical Trapping of Otoliths Drives Vestibular Behaviours in Larval Zebrafish. Nat. Commun. 2017, 8, 630. [Google Scholar] [CrossRef]
- Wang, M.D.; Yin, H.; Landick, R.; Gelles, J.; Block, S.M. Stretching DNA with Optical Tweezers. Biophys. J. 1997, 72, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Heller, I.; Sitters, G.; Broekmans, O.D.; Farge, G.; Menges, C.; Wende, W.; Hell, S.W.; Peterman, E.J.G.; Wuite, G.J.L. STED Nanoscopy Combined with Optical Tweezers Reveals Protein Dynamics on Densely Covered DNA. Nat. Methods 2013, 10, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.; Bryant, Z.; Nöllmann, M.; Le, M.U.; Cozzarelli, N.R.; Bustamante, C. DNA Overwinds When Stretched. Nature 2006, 442, 836–839. [Google Scholar] [CrossRef]
- Curtis, J.E.; Koss, B.A.; Grier, D.G. Dynamic Holographic Optical Tweezers. Opt. Commun. 2002, 207, 169–175. [Google Scholar] [CrossRef]
- Leach, J.; Sinclair, G.; Jordan, P.; Courtial, J.; Padgett, M.J.; Cooper, J.; Laczik, Z.J. 3D Manipulation of Particles into Crystal Structures Using Holographic Optical Tweezers. Opt. Express 2004, 12, 220. [Google Scholar] [CrossRef]
- Dufresne, E.R.; Spalding, G.C.; Dearing, M.T.; Sheets, S.A.; Grier, D.G. Computer-Generated Holographic Optical Tweezer Arrays. Rev. Sci. Instrum. 2001, 72, 1810. [Google Scholar] [CrossRef]
- Grier, D.G.; Roichman, Y. Holographic Optical Trapping. Appl. Opt. 2006, 45, 880. [Google Scholar] [CrossRef] [PubMed]
- Siviloglou, G.A.; Broky, J.; Dogariu, A.; Christodoulides, D.N. Observation of Accelerating Airy Beams. Phys. Rev. Lett. 2007, 99, 213901. [Google Scholar] [CrossRef]
- Baumgartl, J.; Mazilu, M.; Dholakia, K. Optically Mediated Particle Clearing Using Airy Wavepackets. Nat. Photonics 2008, 2, 675–678. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, K.; Lu, X. Radiation Force of Abruptly Autofocusing Airy Beams on a Rayleigh Particle. Opt. Express 2013, 21, 24413. [Google Scholar] [CrossRef]
- Garcés-Chávez, V.; McGloin, D.; Melville, H.; Sibbett, W.; Dholakia, K. Simultaneous Micromanipulation in Multiple Planes Using a Self-Reconstructing Light Beam. Nature 2002, 419, 145–147. [Google Scholar] [CrossRef]
- Milne, G.; Dholakia, K.; McGloin, D.; Volke-Sepulveda, K.; Zemánek, P. Transverse Particle Dynamics in a Bessel Beam. Opt. Express 2007, 15, 13972. [Google Scholar] [CrossRef]
- Curtis, J.E.; Grier, D.G. Structure of Optical Vortices. Phys. Rev. Lett. 2003, 90, 133901. [Google Scholar] [CrossRef]
- Ng, J.; Lin, Z.; Chan, C.T. Theory of Optical Trapping by an Optical Vortex Beam. Phys. Rev. Lett. 2010, 104, 103601. [Google Scholar] [CrossRef] [PubMed]
- Padgett, M.; Bowman, R. Tweezers with a Twist. Nat. Photonics 2011, 5, 343–348. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, X.; Xie, Z.; Min, C.; Fu, X.; Liu, Q.; Gong, M.; Yuan, X. Optical Vortices 30 Years on: OAM Manipulation from Topological Charge to Multiple Singularities. Light Sci. Appl. 2019, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Jaquay, E.; Martínez, L.J.; Mejia, C.A.; Povinelli, M.L. Light-Assisted, Templated Self-Assembly Using a Photonic-Crystal Slab. Nano Lett. 2013, 13, 2290–2294. [Google Scholar] [CrossRef]
- Jaquay, E.; Martínez, L.J.; Huang, N.; Mejia, C.A.; Sarkar, D.; Povinelli, M.L. Light-Assisted, Templated Self-Assembly of Gold Nanoparticle Chains. Nano Lett. 2014, 14, 5184–5188. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Serey, X.; Erickson, D. Nanomanipulation Using Silicon Photonic Crystal Resonators. Nano Lett. 2010, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Min, C.; Dou, X.; Wang, X.; Urbach, H.P.; Somekh, M.G.; Yuan, X. Plasmonic Tweezers: For Nanoscale Optical Trapping and Beyond. Light Sci. Appl. 2021, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.L.; Righini, M.; Quidant, R. Plasmon Nano-Optical Tweezers. Nat. Photonics 2011, 5, 349–356. [Google Scholar] [CrossRef]
- Righini, M.; Ghenuche, P.; Cherukulappurath, S.; Myroshnychenko, V.; García De Abajo, F.J.; Quidant, R. Nano-Optical Trapping of Rayleigh Particles and Escherichia Coli Bacteria with Resonant Optical Antennas. Nano Lett. 2009, 9, 3387–3391. [Google Scholar] [CrossRef]
- Crozier, K.B. Quo Vadis, Plasmonic Optical Tweezers? Light Sci. Appl. 2019, 8, 35. [Google Scholar] [CrossRef]
- Li, Y.; Xin, H.; Zhang, Y.; Lei, H.; Zhang, T.; Ye, H.; Saenz, J.J.; Qiu, C.-W.; Li, B. Living Nanospear for Near-Field Optical Probing. ACS Nano 2018, 12, 10703–10711. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, N.; Shi, Y.; Xin, H.; Li, B. Optical Fiber Tweezers: A Versatile Tool for Optical Trapping and Manipulation. Micromachines 2020, 11, 114. [Google Scholar] [CrossRef]
- Xin, H.; Zhao, N.; Wang, Y.; Zhao, X.; Pan, T.; Shi, Y.; Li, B. Optically Controlled Living Micromotors for the Manipulation and Disruption of Biological Targets. Nano Lett. 2020, 20, 7177–7185. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, B. Single-Cell Biomagnifier for Optical Nanoscopes and Nanotweezers. Light Sci. Appl. 2019, 8, 61. [Google Scholar] [CrossRef]
- Lin, L.; Wang, M.; Peng, X.; Lissek, E.N.; Mao, Z.; Scarabelli, L.; Adkins, E.; Coskun, S.; Unalan, H.E.; Korgel, B.A.; et al. Opto-Thermoelectric Nanotweezers. Nat. Photonics 2018, 12, 195–201. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, Y.; Xie, X.; Zhang, Y.; Min, C.; Yuan, X. Graphene-Based Opto-Thermoelectric Tweezers. Adv. Mater. 2022, 34, 2107691. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Peng, X.; Wu, Z.; Coughlan, A.C.H.; Mao, Z.; Bevan, M.A.; Zheng, Y. Opto-Thermophoretic Assembly of Colloidal Matter. Sci. Adv. 2017, 3, e1700458. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Peng, X.; Zheng, Y. Reconfigurable Opto-Thermoelectric Printing of Colloidal Particles. Chem. Commun. 2017, 53, 7357–7360. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.H.; Li, J.; Lin, L.; Liu, Y.; Zheng, Y. Opto-Thermophoretic Attraction, Trapping, and Dynamic Manipulation of Lipid Vesicles. Langmuir 2018, 34, 13252–13262. [Google Scholar] [CrossRef]
- Lin, L.; Peng, X.; Wei, X.; Mao, Z.; Xie, C.; Zheng, Y. Thermophoretic Tweezers for Low-Power and Versatile Manipulation of Biological Cells. ACS Nano 2017, 11, 3147–3154. [Google Scholar] [CrossRef]
- Horvath, H. Photophoresis–A Forgotten Force?? KONA Powder Part. J. 2014, 31, 181–199. [Google Scholar] [CrossRef]
- Bera, S.K.; Kumar, A.; Sil, S.; Saha, T.K.; Saha, T.; Banerjee, A. Simultaneous Measurement of Mass and Rotation of Trapped Absorbing Particles in Air. Opt. Lett. 2016, 41, 4356. [Google Scholar] [CrossRef]
- Lin, J.; Deng, J.; Wei, R.; Li, Y.; Wang, Y. Measurement of Mass by Optical Forced Oscillation of Absorbing Particles Trapped in Air. J. Opt. Soc. Am. B 2017, 34, 1242. [Google Scholar] [CrossRef]
- Carlse, G.; Borsos, K.B.; Beica, H.C.; Vacheresse, T.; Pouliot, A.; Perez-Garcia, J.; Vorozcovs, A.; Barron, B.; Jackson, S.; Marmet, L.; et al. Technique for Rapid Mass Determination of Airborne Microparticles Based on Release and Recapture from an Optical Dipole Force Trap. Phys. Rev. Appl. 2020, 14, 024017. [Google Scholar] [CrossRef]
- Lutz, C.; Kollmann, M.; Leiderer, P.; Bechinger, C. Diffusion of Colloids in One-Dimensional Light Channels. J. Phys. Condens. Matter 2004, 16, S4075–S4083. [Google Scholar] [CrossRef]
- Gubbiotti, A.; Chinappi, M.; Casciola, C.M. Confinement Effects on the Dynamics of a Rigid Particle in a Nanochannel. Phys. Rev. E 2019, 100, 053307. [Google Scholar] [CrossRef]
- Delong, S.; Balboa Usabiaga, F.; Donev, A. Brownian Dynamics of Confined Rigid Bodies. J. Chem. Phys. 2015, 143, 144107. [Google Scholar] [CrossRef]
- Grier, D.G. A Revolution in Optical Manipulation. Nature 2003, 424, 810–816. [Google Scholar] [CrossRef]
- Dufresne, E.R.; Grier, D.G. Optical Tweezer Arrays and Optical Substrates Created with Diffractive Optics. Rev. Sci. Instrum. 1998, 69, 1974–1977. [Google Scholar] [CrossRef]
- Cordero, M.L.; Burnham, D.R.; Baroud, C.N.; McGloin, D. Thermocapillary Manipulation of Droplets Using Holographic Beam Shaping: Microfluidic Pin Ball. Appl. Phys. Lett. 2008, 93, 034107. [Google Scholar] [CrossRef]
- Haraszti, T.; Schulz, S.; Uhrig, K.; Kurre, R.; Roos, W.; Schmitz, C.H.J.; Curtis, J.E.; Maier, T.; Clemen, A.E.-M.; Spatz, J.P. Biomimetic Model of the Actin Cortex. Biophys. Rev. Lett. 2009, 4, 17–32. [Google Scholar] [CrossRef]
- Bolognesi, G.; Hargreaves, A.; Ward, A.D.; Kirby, A.K.; Bain, C.D.; Ces, O. Microfluidic Generation of Monodisperse Ultra-Low Interfacial Tension Oil Droplets in Water. RSC Adv. 2015, 5, 8114–8121. [Google Scholar] [CrossRef]
- Gao, Y.; Harder, R.; Southworth, S.H.; Guest, J.R.; Huang, X.; Yan, Z.; Ocola, L.E.; Yifat, Y.; Sule, N.; Ho, P.J.; et al. Three-Dimensional Optical Trapping and Orientation of Microparticles for Coherent X-Ray Diffraction Imaging. Proc. Natl. Acad. Sci. USA 2019, 116, 4018–4024. [Google Scholar] [CrossRef]
- Chupeau, M.; Gladrow, J.; Chepelianskii, A.; Keyser, U.F.; Trizac, E. Optimizing Brownian Escape Rates by Potential Shaping. Proc. Natl. Acad. Sci. USA 2020, 117, 1383–1388. [Google Scholar] [CrossRef]
- Ward, R.; Ravindran, S.; Otazo, M.R.; Cradock, B.; Avci, E.; Gillies, G.; Coker, C.; Williams, M.A.K. Inside the Ensemble: Unlocking the Potential of One-at-a-Time Experiments with Lab-on-a-Chip Automation. Lab. Chip 2021, 21, 4401–4413. [Google Scholar] [CrossRef] [PubMed]
- Joannopoulos, J.D.; Villeneuve, P.R.; Fan, S. Photonic Crystals: Putting a New Twist on Light. Nature 1997, 386, 143–149. [Google Scholar] [CrossRef]
- Biswas, U.; Nayak, C.; Rakshit, J.K. Fabrication Techniques and Applications of Two-Dimensional Photonic Crystal: History and the Present Status. Opt. Eng. 2022, 62, 010901. [Google Scholar] [CrossRef]
- Kang, P.; Schein, P.; Serey, X.; O’Dell, D.; Erickson, D. Nanophotonic Detection of Freely Interacting Molecules on a Single Influenza Virus. Sci. Rep. 2015, 5, 12087. [Google Scholar] [CrossRef]
- Gholizadeh, E.; Jafari, B.; Golmohammadi, S. Graphene-Based Optofluidic Tweezers for Refractive-Index and Size-Based Nanoparticle Sorting, Manipulation, and Detection. Sci. Rep. 2023, 13, 1975. [Google Scholar] [CrossRef]
- Descharmes, N.; Dharanipathy, U.P.; Diao, Z.; Tonin, M.; Houdré, R. Single Particle Detection, Manipulation and Analysis with Resonant Optical Trapping in Photonic Crystals. Lab. Chip 2013, 13, 3268. [Google Scholar] [CrossRef]
- Therisod, R.; Tardif, M.; Marcoux, P.R.; Picard, E.; Jager, J.-B.; Hadji, E.; Peyrade, D.; Houdré, R. Gram-Type Differentiation of Bacteria with 2D Hollow Photonic Crystal Cavities. Appl. Phys. Lett. 2018, 113, 111101. [Google Scholar] [CrossRef]
- Nitkowski, A.; Gondarenko, A.; Lipson, M. On-Chip Supercontinuum Optical Trapping and Resonance Excitation of Microspheres. Opt. Lett. 2010, 35, 1626. [Google Scholar] [CrossRef]
- Walker, Z.J.; Wells, T.; Belliston, E.; Walker, S.B.; Zeller, C.; Sampad, M.J.N.; Saiduzzaman, S.M.; Schmidt, H.; Hawkins, A.R. Optofluidic Particle Manipulation: Optical Trapping in a Thin-Membrane Microchannel. Biosensors 2022, 12, 690. [Google Scholar] [CrossRef]
- Kühn, S.; Measor, P.; Lunt, E.J.; Phillips, B.S.; Deamer, D.W.; Hawkins, A.R.; Schmidt, H. Loss-Based Optical Trap for on-Chip Particle Analysis. Lab. Chip 2009, 9, 2212. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, H.; Nguyen, K.T.; Zhang, Y.; Chin, L.K.; Zhu, T.; Yu, Y.; Cai, H.; Yap, P.H.; Liu, P.Y.; et al. Nanophotonic Array-Induced Dynamic Behavior for Label-Free Shape-Selective Bacteria Sieving. ACS Nano 2019, 13, 12070–12080. [Google Scholar] [CrossRef] [PubMed]
- Oostindie, S.C.; Lazar, G.A.; Schuurman, J.; Parren, P.W.H.I. Avidity in Antibody Effector Functions and Biotherapeutic Drug Design. Nat. Rev. Drug Discov. 2022, 21, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.N. Affinity Enhancement of Antibodies: How Low-Affinity Antibodies Produced Early in Immune Responses Are Followed by High-Affinity Antibodies Later and in Memory B-Cell Responses. Cancer Immunol. Res. 2014, 2, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xu, W.; Wang, G.; Zhang, X.; Wang, F.; Li, B.; Yu, C.; Zhang, X.; Zhang, X. Nanoparticles Manipulation on Plasmonic Metasurface for Determination of Antibody in Lab-on-a-Chip Devices. In Proceedings of the Optoelectronic Devices and Integration VII, Proc; SPIE: Beijing, China, 2018; Volume 10814. [Google Scholar] [CrossRef]
- Tewari Kumar, P.; Decrop, D.; Safdar, S.; Passaris, I.; Kokalj, T.; Puers, R.; Aertsen, A.; Spasic, D.; Lammertyn, J. Digital Microfluidics for Single Bacteria Capture and Selective Retrieval Using Optical Tweezers. Micromachines 2020, 11, 308. [Google Scholar] [CrossRef]
- Mori, S.; Ito, T.; Takao, H.; Shimokawa, F.; Terao, K. Optically Driven Microtools with an Antibody-immobilised Surface for On-site Cell Assembly. IET Nanobiotechnol. 2023, 17, 197–203. [Google Scholar] [CrossRef]
- Yan, S.; Qiu, J.; Guo, L.; Li, D.; Xu, D.; Liu, Q. Development Overview of Raman-Activated Cell Sorting Devoted to Bacterial Detection at Single-Cell Level. Appl. Microbiol. Biotechnol. 2021, 105, 1315–1331. [Google Scholar] [CrossRef]
- Casabella, S.; Scully, P.; Goddard, N.; Gardner, P. Automated Analysis of Single Cells Using Laser Tweezers Raman Spectroscopy. Analyst 2016, 141, 689–696. [Google Scholar] [CrossRef]
- Lee, K.S.; Palatinszky, M.; Pereira, F.C.; Nguyen, J.; Fernandez, V.I.; Mueller, A.J.; Menolascina, F.; Daims, H.; Berry, D.; Wagner, M.; et al. An Automated Raman-Based Platform for the Sorting of Live Cells by Functional Properties. Nat. Microbiol. 2019, 4, 1035–1048. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, T.; Liu, J.; Tsai, D.P.; Zhang, H.; Wang, S.; Chan, C.T.; Wu, P.C.; Zayats, A.V.; Nori, F.; et al. Stable Optical Lateral Forces from Inhomogeneities of the Spin Angular Momentum. Sci. Adv. 2022, 8, eabn2291. [Google Scholar] [CrossRef]
- Tkachenko, G.; Brasselet, E. Optofluidic Sorting of Material Chirality by Chiral Light. Nat. Commun. 2014, 5, 3577. [Google Scholar] [CrossRef]
- Zhu, T.; Shi, Y.; Ding, W.; Tsai, D.P.; Cao, T.; Liu, A.Q.; Nieto-Vesperinas, M.; Sáenz, J.J.; Wu, P.C.; Qiu, C.-W. Extraordinary Multipole Modes and Ultra-Enhanced Optical Lateral Force by Chirality. Phys. Rev. Lett. 2020, 125, 043901. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chen, A.; Lin, C.-H. Microfluidic Cell Counter/Sorter Utilizing Multiple Particle Tracing Technique and Optically Switching Approach. Biomed. Microdevices 2008, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Kong, M.; Wang, Z.; Costa, K.D.; Li, R.A.; Sun, D. Enhanced Cell Sorting and Manipulation with Combined Optical Tweezer and Microfluidic Chip Technologies. Lab. Chip 2011, 11, 3656. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, Y.; Han, X.; Kan, L.; Ren, J.; Sun, L.; Diao, Z.; Ji, Y.; Zhu, P.; Xu, J.; et al. Versatile, Facile and Low-Cost Single-Cell Isolation, Culture and Sequencing by Optical Tweezer-Assisted Pool-Screening. Lab. Chip 2023, 23, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.; Yan, Z. Creating Multifunctional Optofluidic Potential Wells for Nanoparticle Manipulation. Nano Lett. 2018, 18, 7400–7406. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, S.; Chin, L.K.; Zhang, J.; Ser, W.; Wu, J.; Chen, T.; Yang, Z.; Hao, Y.; Liedberg, B.; et al. Nanometer-Precision Linear Sorting with Synchronized Optofluidic Dual Barriers. Sci. Adv. 2018, 4, eaao0773. [Google Scholar] [CrossRef]
- Zheng, B.; Kang, Y.-F.; Zhang, T.; Li, C.-Y.; Huang, S.; Zhang, Z.-L.; Wu, Q.-S.; Qi, C.-B.; Pang, D.-W.; Tang, H.-W. Improving Flow Bead Assay: Combination of Near-Infrared Optical Tweezers Stabilizing and Upconversion Luminescence Encoding. Anal. Chem. 2020, 92, 5258–5266. [Google Scholar] [CrossRef]
- Zhou, L.-M.; Shi, Y.; Zhu, X.; Hu, G.; Cao, G.; Hu, J.; Qiu, C.-W. Recent Progress on Optical Micro/Nanomanipulations: Structured Forces, Structured Particles, and Synergetic Applications. ACS Nano 2022, 16, 13264–13278. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; You, M.; Shi, Y.; Huang, H.; Wei, Z.; He, T.; Xiong, S.; Wang, Z.; Cheng, X. Optofluidic Tweezers: Efficient and Versatile Micro/Nano-Manipulation Tools. Micromachines 2023, 14, 1326. https://doi.org/10.3390/mi14071326
Zhu Y, You M, Shi Y, Huang H, Wei Z, He T, Xiong S, Wang Z, Cheng X. Optofluidic Tweezers: Efficient and Versatile Micro/Nano-Manipulation Tools. Micromachines. 2023; 14(7):1326. https://doi.org/10.3390/mi14071326
Chicago/Turabian StyleZhu, Yuchen, Minmin You, Yuzhi Shi, Haiyang Huang, Zeyong Wei, Tao He, Sha Xiong, Zhanshan Wang, and Xinbin Cheng. 2023. "Optofluidic Tweezers: Efficient and Versatile Micro/Nano-Manipulation Tools" Micromachines 14, no. 7: 1326. https://doi.org/10.3390/mi14071326
APA StyleZhu, Y., You, M., Shi, Y., Huang, H., Wei, Z., He, T., Xiong, S., Wang, Z., & Cheng, X. (2023). Optofluidic Tweezers: Efficient and Versatile Micro/Nano-Manipulation Tools. Micromachines, 14(7), 1326. https://doi.org/10.3390/mi14071326







