Fabrication of Spiral Low-Cost Microchannel with Trapezoidal Cross Section for Cell Separation Using a Grayscale Approach
Abstract
:1. Introduction
2. Materials and Methods
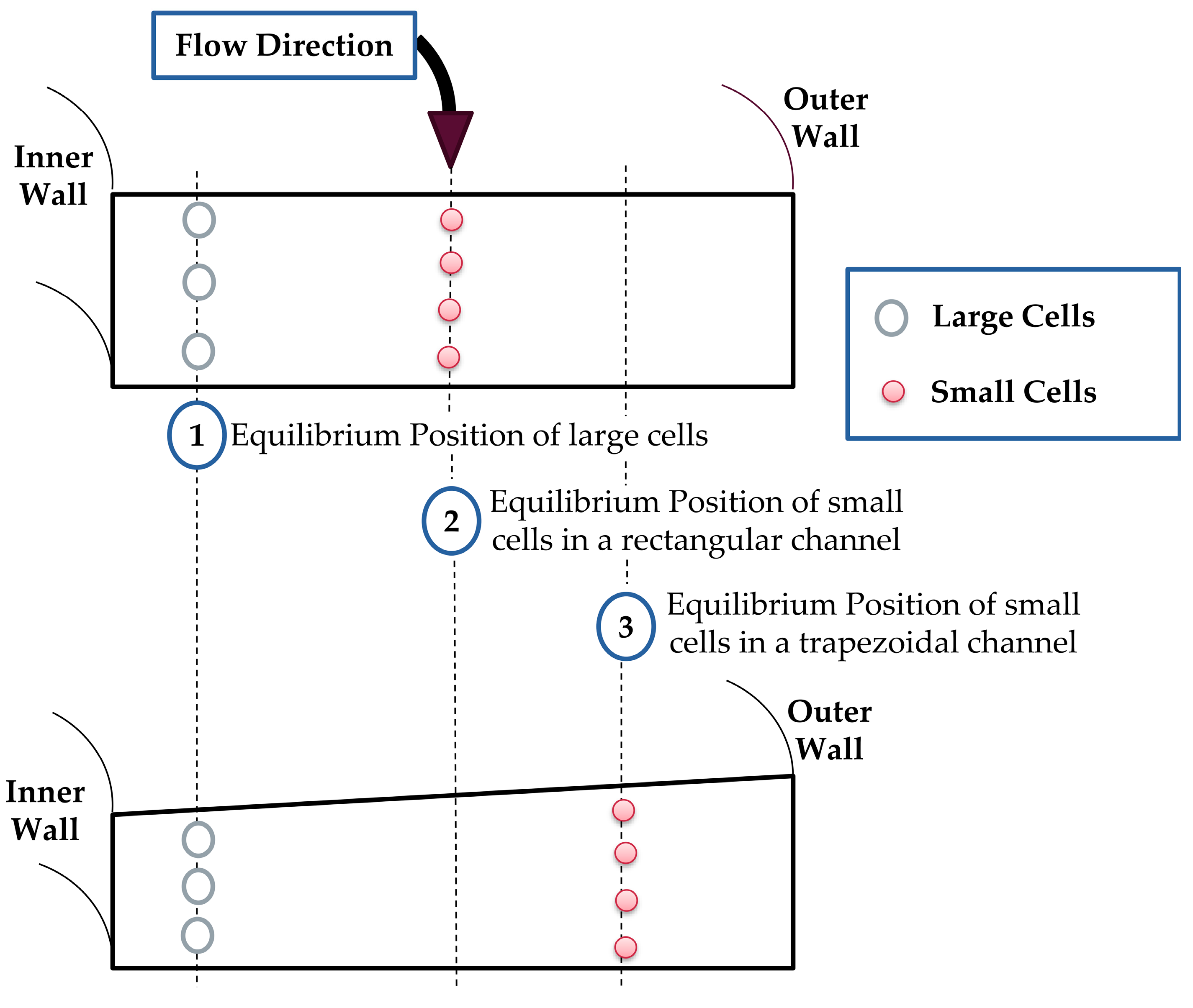
2.1. Spiral Channel Operational Concept
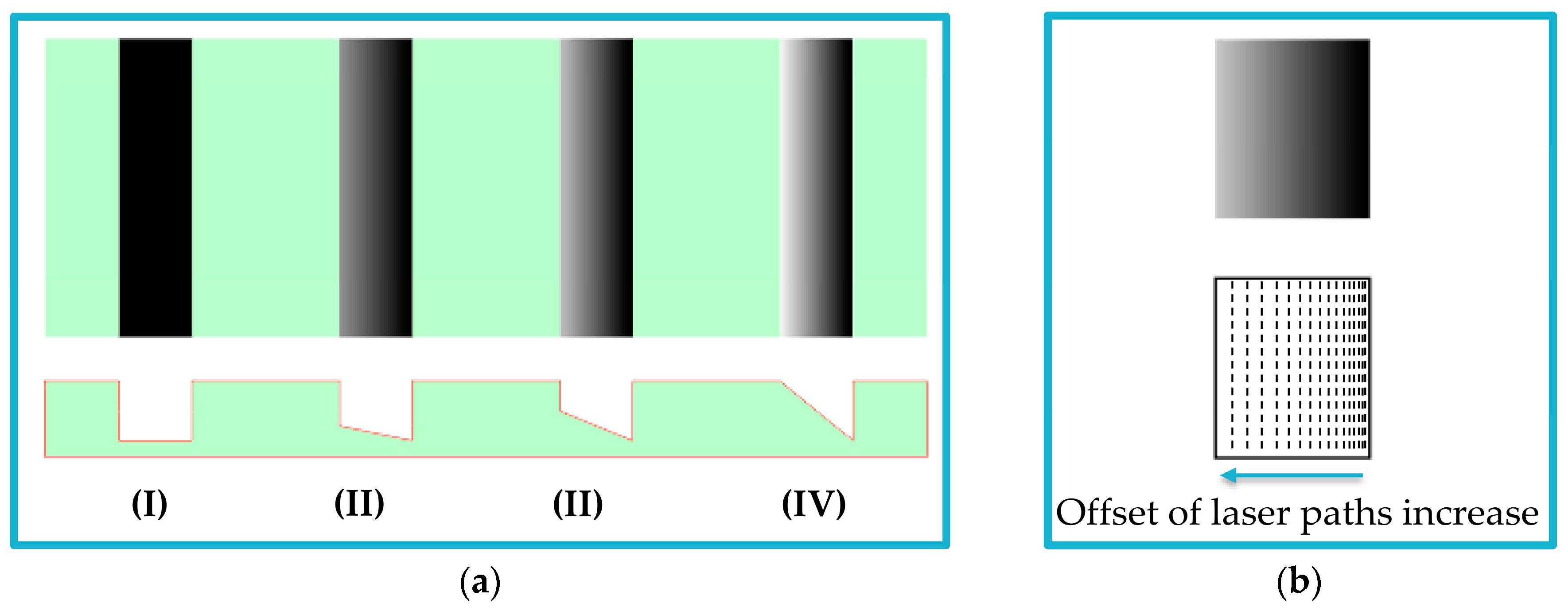
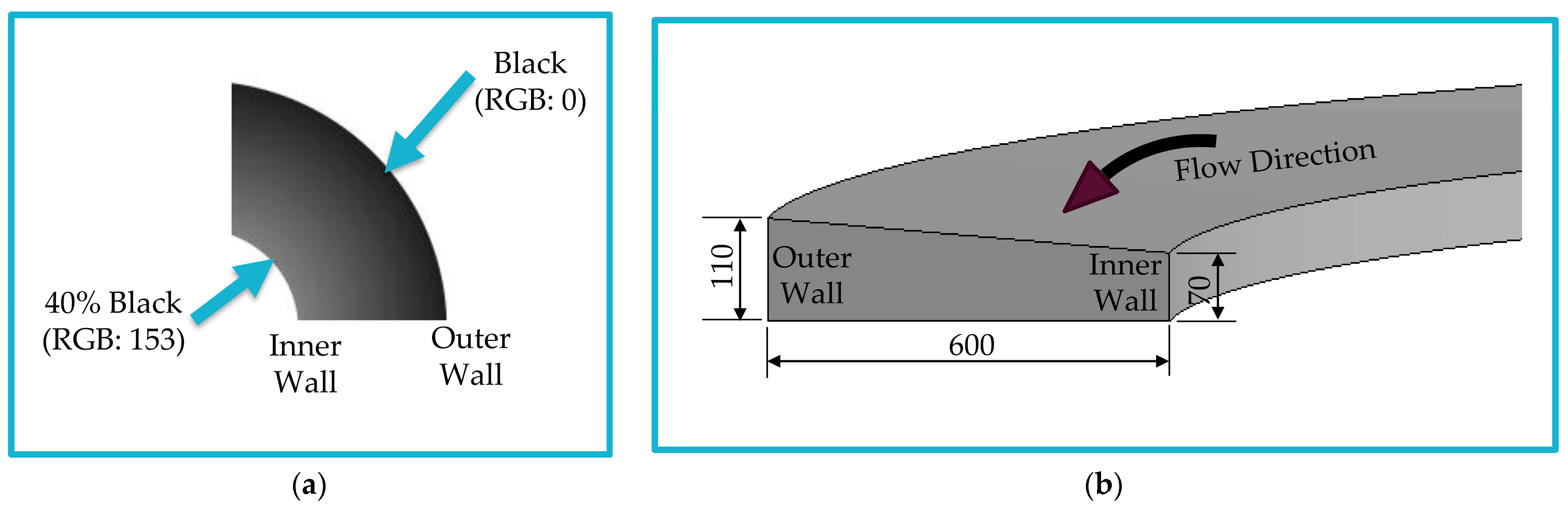
2.2. Concept of Fabrication Using Grayscale Patterning
2.3. Microfluidic Chip Design and Fabrication
2.4. WBCs Separation from Whole Blood
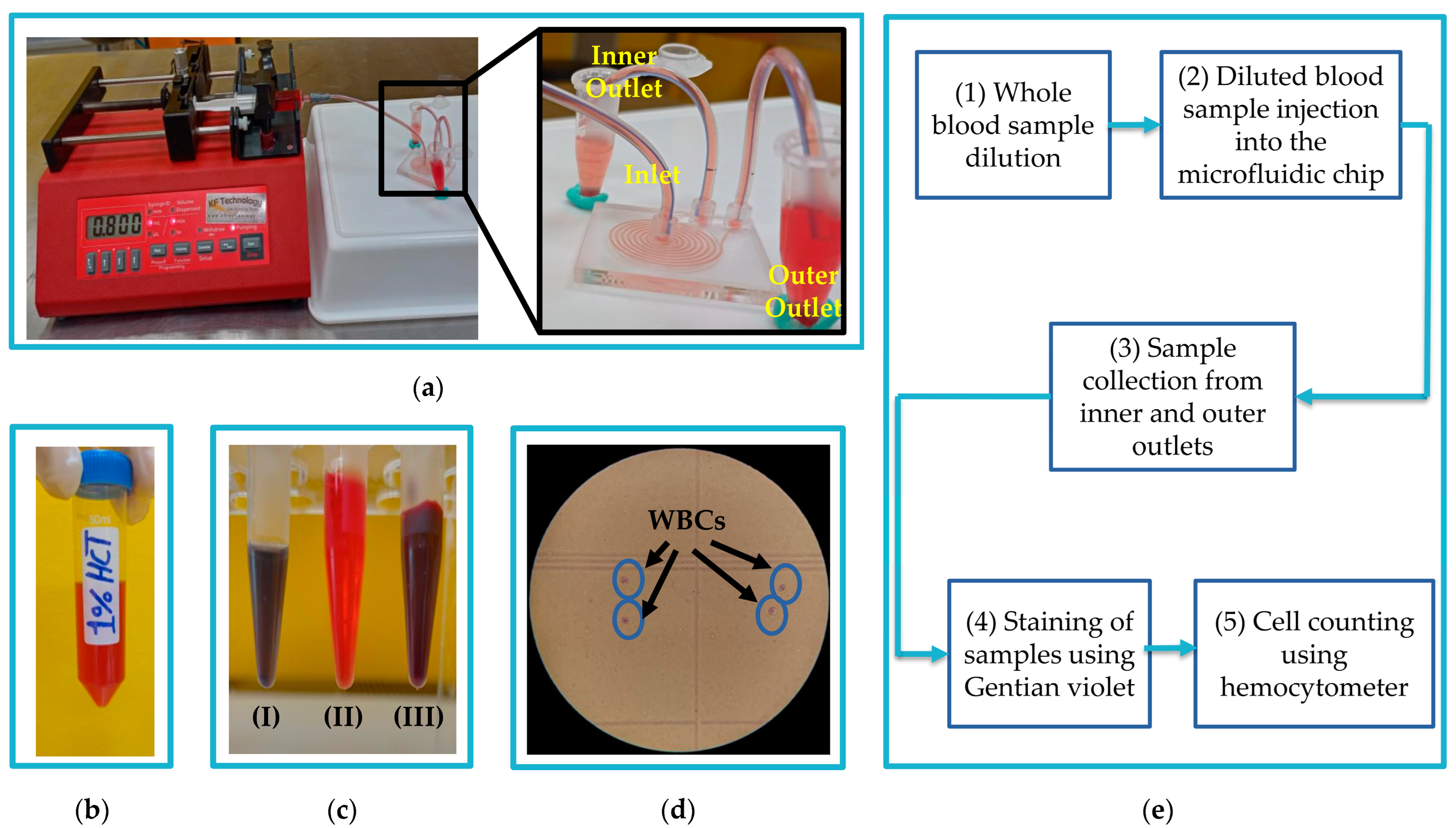
2.4.1. Sample Preparation
2.4.2. Experimental Separation Process
3. Results and Discussion
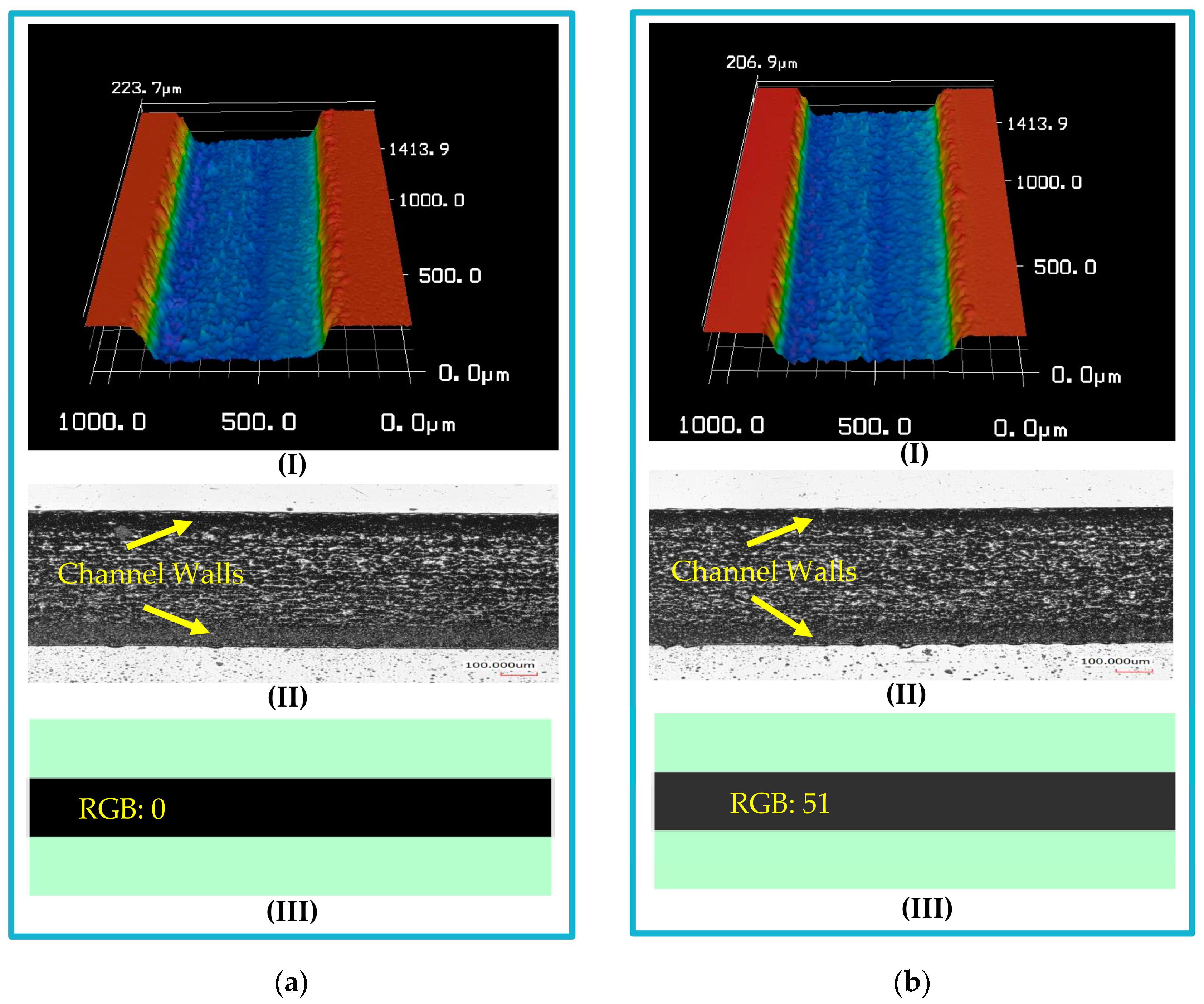
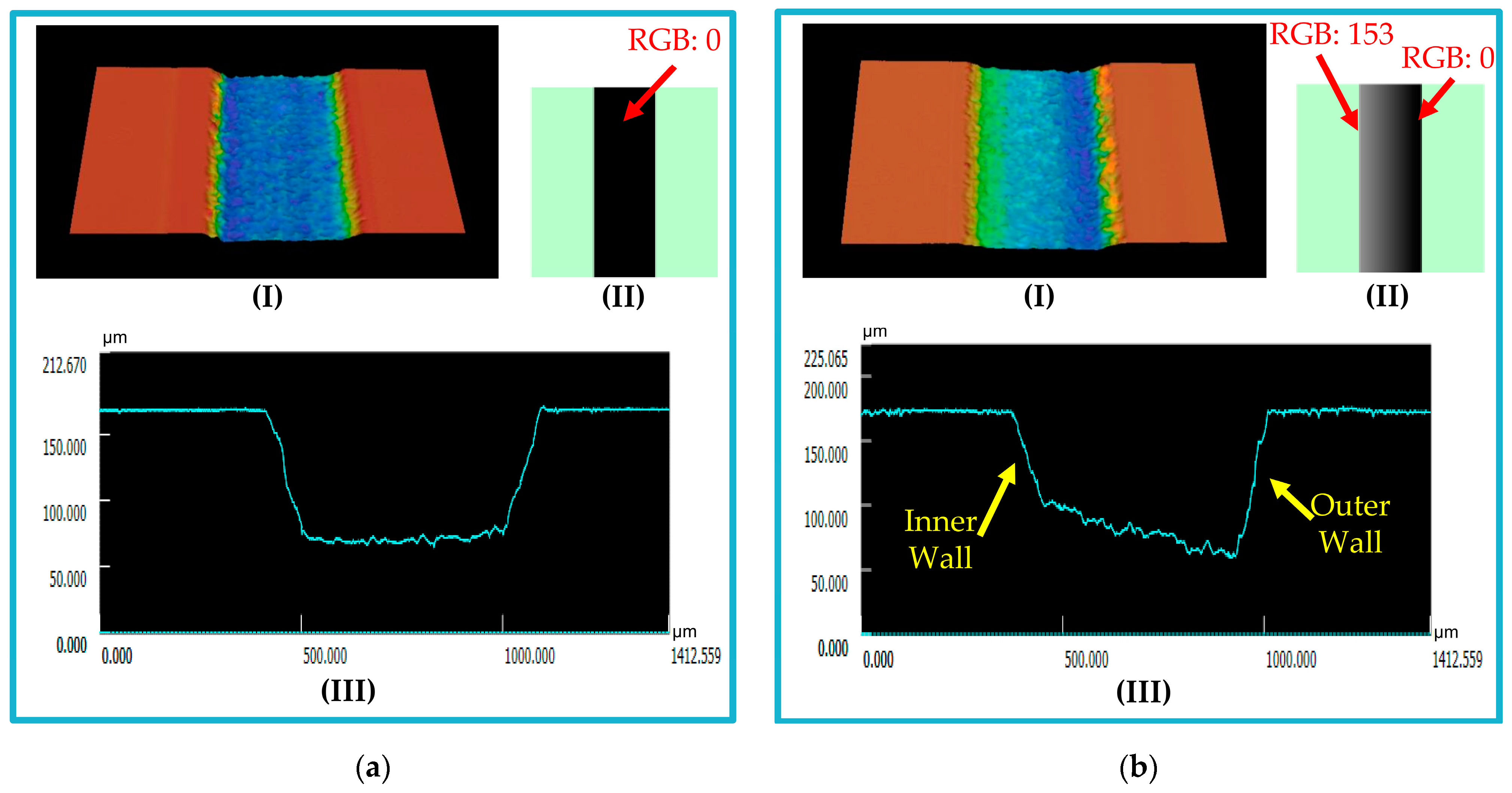
3.1. Microfluidic Platform Fabrication
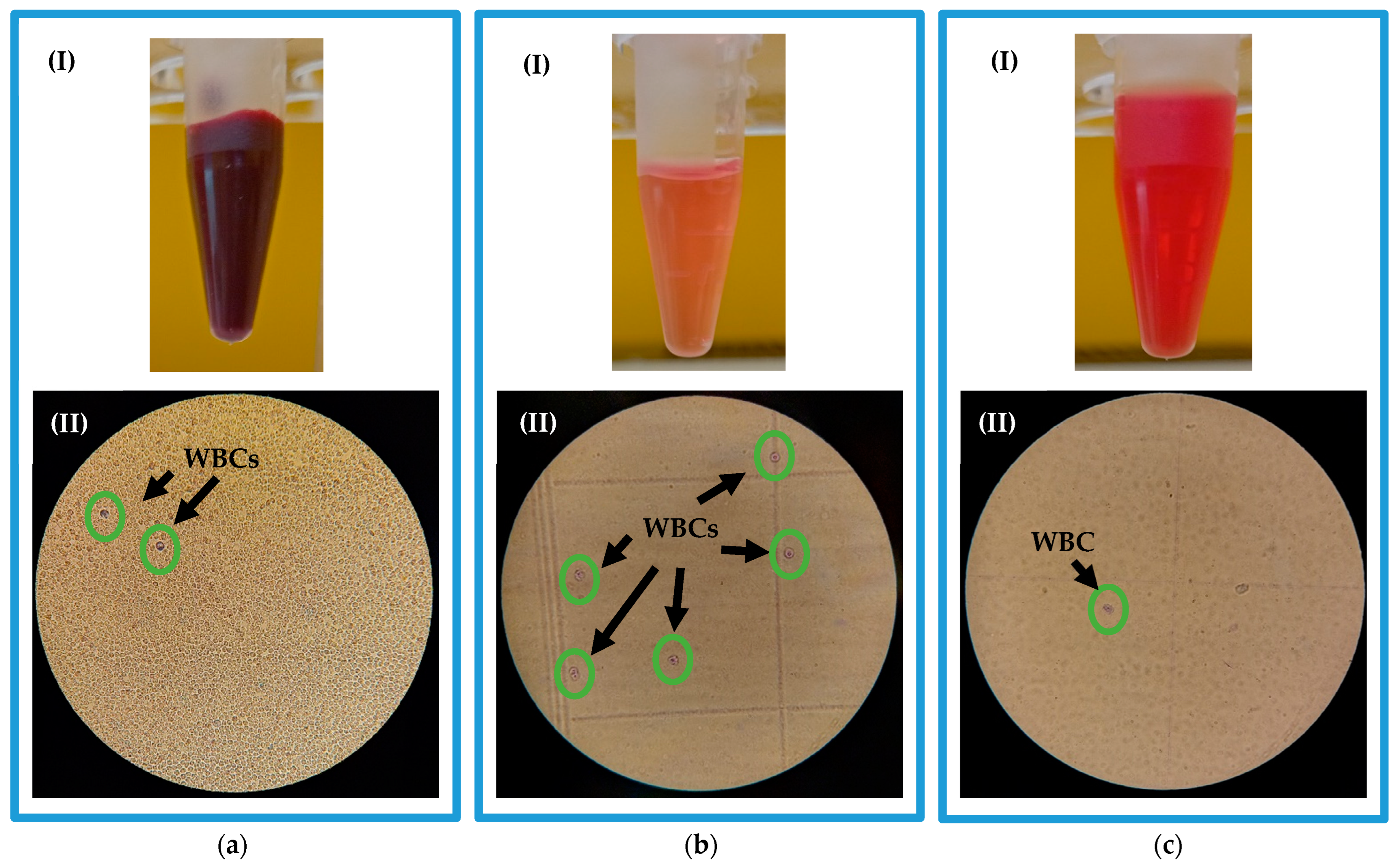
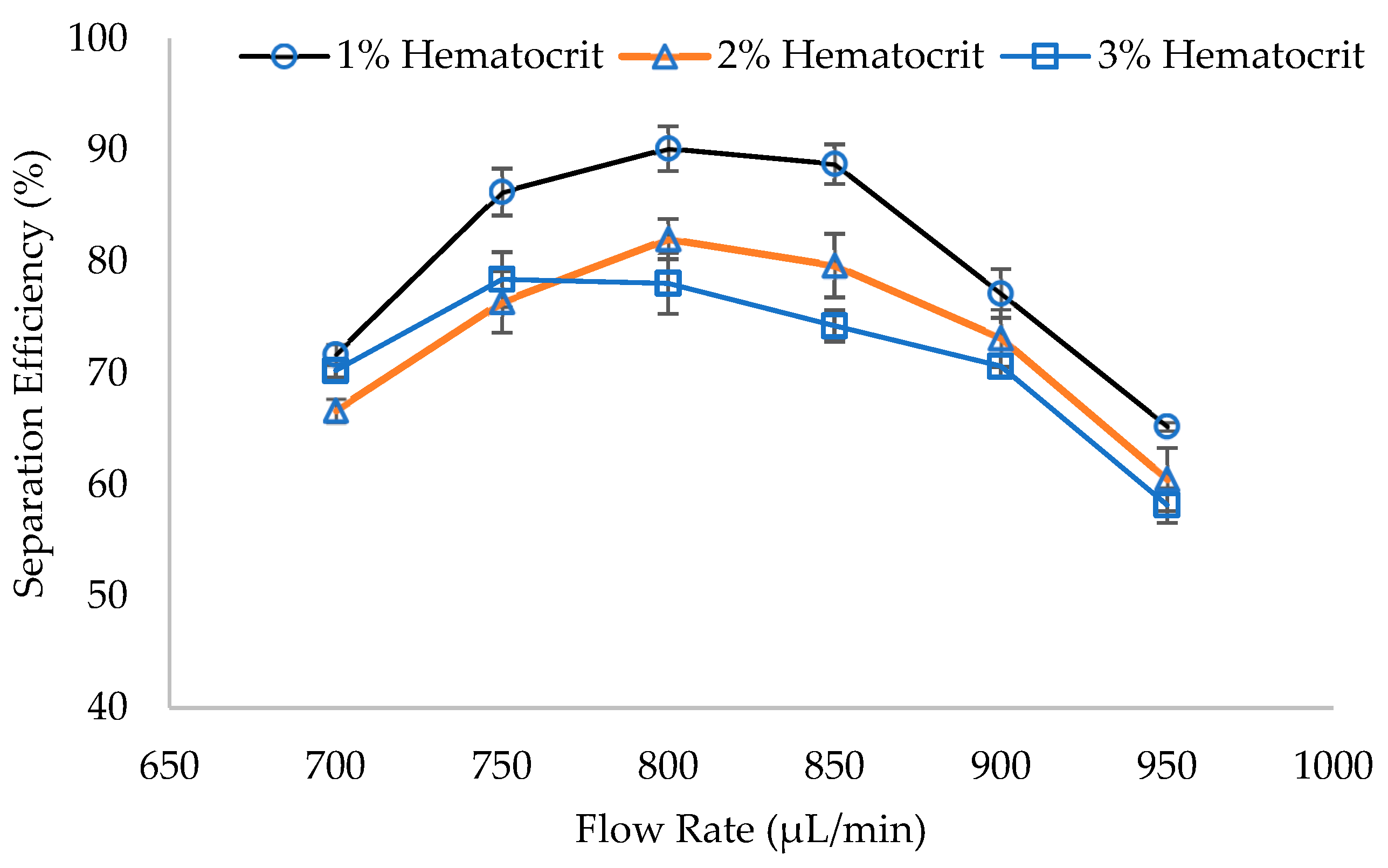
3.2. WBCs Separation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Halhouli, A.; Al-Faqheri, W.; Alhamarneh, B.; Hecht, L.; Dietzel, A. Spiral Microchannels with Trapezoidal Cross Section Fabricated by Femtosecond Laser Ablation in Glass for the Inertial Separation of Microparticles. Micromachines 2018, 9, 171. [Google Scholar] [CrossRef] [Green Version]
- Kinnunen, M.; Kauppila, A.; Karmenyan, A.; Myllylä, R. Effect of the Size and Shape of a Red Blood Cell on Elastic Light Scattering Properties at the Single-Cell Level. Biomed. Opt. Express 2011, 2, 1803–1814. [Google Scholar] [CrossRef] [Green Version]
- Tigner, A.; Ibrahim, S.A.; Murray, I. Histology, White Blood Cell; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Stiff, P.J. Platelets. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; Chapter 154; pp. 728–731. [Google Scholar]
- Harwood, R. Cell Separation by Gradient Centrifugation. Int. Rev. Cytol. 1974, 38, 369–403. [Google Scholar]
- Ibrahim, S.F.; van den Engh, G. Flow Cytometry and Cell Sorting. In Cell Separation, 1st ed.; Kumar, A., Galaev, I.Y., Mattiasson, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 106, pp. 19–39. [Google Scholar]
- Nivedita, N.; Papautsky, I. Continuous Separation of Blood Cells in Spiral Microfluidic Devices. Biomicrofluidics 2013, 7, 054101. [Google Scholar] [CrossRef] [Green Version]
- Al-Faqheri, W.; Thio, T.H.G.; Qasaimeh, M.A.; Dietzel, A.; Madou, M.; Al-Halhouli, A. Particle/Cell Separation on Microfluidic Platforms Based on Centrifugation Effect: A Review. Microfluid Nanofluidics 2017, 21, 102. [Google Scholar] [CrossRef]
- Tajik, P.; Saidi, M.S.; Kashaninejad, N.; Nguyen, N.T. Simple, Cost-Effective, and Continuous 3D Dielectrophoretic Microchip for Concentration and Separation of Bioparticles. Ind. Eng. Chem. Res. 2020, 59, 3772–3783. [Google Scholar] [CrossRef]
- Moon, H.S.; Kwon, K.; Kim, S.I.; Han, H.; Sohn, J.; Lee, S.; Jung, H.L. Continuous Separation of Breast Cancer Cells from Blood Samples Using Multi-Orifice Flow Fractionation (MOFF) and Dielectrophoresis (DEP). Lab Chip 2011, 11, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, M.; Li, W.; Nguyen, N.T. Lab on a Chip for Continuous-Flow Magnetic Cell Separation. Lab Chip 2015, 15, 959–970. [Google Scholar] [CrossRef] [Green Version]
- Evander, M.; Johansson, L.; Lilliehorn, T.; Piskur, J.; Lindvall, M.; Johansson, S.; Almqvist, M.; Laurell, T.; Nilsson, J. Noninvasive Acoustic Cell Trapping in a Microfluidic Perfusion System for Online Bioassays. Anal. Chem. 2007, 79, 2984–2991. [Google Scholar] [CrossRef] [Green Version]
- Mehran, A.; Rostami, P.; Saidi, M.S.; Firoozabadi, B.; Kashaninejad, N. High-Throughput, Label-Free Isolation of White Blood Cells from Whole Blood Using Parallel Spiral Microchannels with U-Shaped Cross-Section. Biosensors 2021, 11, 406. [Google Scholar] [CrossRef]
- Yamada, M.; Nakashima, M.; Seki, M. Pinched Flow Fractionation: Continuous Size Separation of Particles Utilizing a Laminar Flow Profile in a Pinched Microchannel. Anal. Chem. 2004, 76, 5465–5471. [Google Scholar] [CrossRef]
- Yamada, M.; Seki, M. Hydrodynamic Filtration for On-Chip Particle Concentration and Classification Utilizing Microfluidics. Lab Chip 2005, 5, 1233–1239. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Warkiani, M.E.; Li, W. Fundamentals and Applications of Inertial Microfluidics: A Review. Lab Chip 2015, 16, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Nivedita, N.; Ligrani, P.; Papautsky, I. Dean Flow Dynamics in Low-Aspect Ratio Spiral Microchannels. Sci. Rep. 2017, 7, 44072. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X. Inertial Microfluidics for Circulating Tumor Cell Separation and Detection. In Proceedings of the 13th IEEE International Conference on Nanotechnology, Beijing, China, 5–8 August 2013. [Google Scholar]
- Son, J.; Samuel, R.; Gale, B.K.; Carrell, D.T.; Hotaling, J.M. Separation of Sperm Cells from Samples Containing High Concentrations of White Blood Cells Using a Spiral Channel. Biomicrofluidics 2017, 11, 054106. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous Particle Separation in Spiral Microchannels Using Dean Flows and Differential Migration. Lab Chip 2008, 8, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.S.; Kumar, G.; Papautsky, I. Inertial Microfluidics for Continuous Particle Separation in Spiral Microchannels. Lab Chip 2009, 9, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H. A Triplet Parallelizing Spiral Microfluidic Chip for Continuous Separation of Tumor Cells. Sci. Rep. 2018, 8, 4042. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.; Wu, L.; Bhagat, A.A.; Li, Z.; Chen, P.C.Y.; Chao, S.; Ong, C.J.; Han, J. Spiral Microchannel with Rectangular and Trapezoidal Cross-Sections for Size Based Particle Separation. Sci. Rep. 2013, 3, 1475. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Guan, G.; Hou, H.W.; Bhagat, A.A.S.; Han, J. Separation of Leukocytes from Blood Using Spiral Channel with Trapezoid Cross-Section. Anal. Chem. 2012, 84, 9324–9331. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Guan, G.; Luan, K.B.; Lee, W.C.; Bhagat, A.A.S.; Kant Chaudhuri, P.; Tan, D.S.W.; Lim, W.T.; Lee, S.C.; Chen, P.C.Y.; et al. Slanted Spiral Microfluidics for the Ultra-Fast, Label-Free Isolation of Circulating Tumor Cells. Lab Chip 2013, 14, 128–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarnataj, K.; Maleki, S.; Rezaeian, M.; Haki, M.; Shamloo, A. Novel Size-Based Design of Spiral Microfluidic Devices with Elliptic Configurations and Trapezoidal Cross-Section for Ultra-Fast Isolation of Circulating Tumor Cells. Talanta 2023, 254, 124125. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical Microfluidic Devices by Using Low-Cost Fabrication Techniques: A Review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greener, J.; Li, W.; Ren, J.; Voicu, D.; Pakharenko, V.; Tang, T.; Kumacheva, E. Rapid, Cost-Efficient Fabrication of Microfluidic Reactors in Thermoplastic Polymers by Combining Photolithography and Hot Embossing. Lab Chip 2010, 10, 522–524. [Google Scholar] [CrossRef]
- Chiarello, E.; Gupta, A.; Mistura, G.; Sbragaglia, M.; Pierno, M. Droplet Breakup Driven by Shear Thinning Solutions in a Microfluidic T-Junction. Phys. Rev. Fluids 2017, 2, 123602. [Google Scholar] [CrossRef] [Green Version]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers Are from PDMS-Land, Biologists Are from Polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef]
- Gervais, T.; El-Ali, J.; Günther, A.; Jensen, K.F. Flow-Induced Deformation of Shallow Microfluidic Channels. Lab Chip 2006, 6, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Al-Halhouli, A.; Albagdady, A.; Dietzel, A. Sheath-Less High Throughput Inertial Separation of Small Microparticles in Spiral Microchannels with Trapezoidal Cross-Section. RSC Adv. 2019, 9, 41970–41976. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Wu, D.; Li, S.; Han, Y.; Xiang, N.; Wang, C.; Ni, Z. A Polymer-Film Inertial Microfluidic Sorter Fabricated by Jigsaw Puzzle Method for Precise Size-Based Cell Separation. Anal. Chim. Acta 2021, 1143, 306–314. [Google Scholar] [CrossRef]
- Islam, M.M.; Loewen, A.; Allen, P.B. Simple, Low-Cost Fabrication of Acrylic Based Droplet Microfluidics and Its Use to Generate DNA-Coated Particles. Sci. Rep. 2018, 8, 8763. [Google Scholar] [CrossRef] [Green Version]
- Nasser, G.A.; El-Bab, A.M.R.F.; Abdel-Mawgood, A.L.; Mohamed, H.; Saleh, A.M. CO2 Laser Fabrication of PMMA Microfluidic Double T-Junction Device with Modified Inlet-Angle for Cost-Effective PCR Application. Micromachines 2019, 10, 678. [Google Scholar] [CrossRef] [Green Version]
- Mansour, H.; Soliman, E.A.; El-Bab, A.M.F.; Abdel-Mawgood, A.L. Development of Epoxy Resin-Based Microfluidic Devices Using CO2 Laser Ablation for DNA Amplification Point-of-Care (POC) Applications. Int. J. Adv. Manuf. Technol. 2022, 120, 4355–4372. [Google Scholar] [CrossRef]
- Nasser, G.A.; Abdel-Mawgood, A.L.; Abouelsoud, A.A.; Mohamed, H.; Umezu, S.; El-Bab, A.M.R.F. New Cost Effective Design of PCR Heating Cycler System Using Peltier Plate without the Conventional Heating Block. J. Mech. Sci. Technol. 2021, 35, 3259–3268. [Google Scholar] [CrossRef]
- Prakash, S.; Kumar, S. Fabrication of Microchannels on Transparent PMMA Using CO2 Laser (10.6 µm) for Microfluidic Applications: An Experimental Investigation. Int. J. Precis. Eng. Manuf. 2015, 16, 361–366. [Google Scholar] [CrossRef]
- Helmy, M.O.; Fath El-Bab, A.M.; El-Hofy, H. Elimination of Clogging in PMMA Microchannels Using Water Assisted CO2 Laser Micromachining. Appl. Mech. Mater. 2015, 799–800, 407–412. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Shen, J. CO2 Laser Ablation of Microchannel on PMMA Substrate for Effective Fabrication of Microfluidic Chips. Int. Polym. Process. 2016, 31, 233–238. [Google Scholar] [CrossRef]
- Imran, M.; Rahman, R.A.; Ahmad, M.; Akhtar, M.N.; Usman, A.; Sattar, A. Fabrication of Microchannels on PMMA Using a Low Power CO2 Laser. Laser Phys. 2016, 26, 096101. [Google Scholar] [CrossRef]
- Prakash, S.; Kumar, S. Fabrication of Rectangular Cross-Sectional Microchannels on PMMA with a CO2 Laser and Underwater Fabricated Copper Mask. Opt. Laser Technol. 2017, 94, 180–192. [Google Scholar] [CrossRef]
- Aguiam, D.E.; Santos, J.D.; Silva, C.; Gentile, F.; Ferreira, C.; Garcia, I.S.; Cunha, J.; Gaspar, J. Fabrication and Optical Characterization of Large Aperture Diffractive Lenses Using Greyscale Lithography. Micro Nano Eng. 2022, 14, 100111. [Google Scholar] [CrossRef]
- Youn, S.W.; Park, S.C.; Wang, Q.; Suzuki, K.; Hiroshima, H. Study on Quartz Multitier Mold Fabrication Using Gray Scale Laser Beam Lithography. Jpn. J. Appl. Phys. 2011, 50, 06GK03. [Google Scholar] [CrossRef]
- Li, B.; Jiang, L.; Li, X.; Lin, Z.; Huang, L.; Wang, A.; Han, W.; Wang, Z.; Lu, Y. Flexible Gray-Scale Surface Patterning Through Spatiotemporal-Interference-Based Femtosecond Laser Shaping. Adv. Opt. Mater. 2018, 6, 1801021. [Google Scholar] [CrossRef]
- Di Carlo, D. Inertial Microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- White, J.G.; Escolar, G. EDTA-Induced Changes in Platelet Structure and Function: Adhesion and Spreading. Platelets 2009, 11, 56–61. [Google Scholar]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Enhanced Particle Filtration in Straight Microchannels Using Shear-Modulated Inertial Migration. Phys. Fluids 2008, 20, 101702. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous Inertial Focusing, Ordering, and Separation of Particles in Microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Reference | [1] | [24] | The Present Work |
|---|---|---|---|
| Material | Glass | PDMS | PMMA |
| Fabrication technology | Femtosecond laser | Soft lithography | CO2 laser |
| Operating flow rate | High | Low | High |
| Surface Roughness | 0.27 µm | 0.8 µm | 1.07 µm |
| Bonding | Thermally at 620 °C in a muffle furnace | Oxygen plasma treatment | Thermally at 150 °C in a natural ventilation oven |
| Fabrication time | 7 h and 9 min | ~1 day | 20 min |
| Estimated cost/chip | >$5 | >$5 | <30 cents |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adel, M.; Allam, A.; Sayour, A.E.; Ragai, H.F.; Umezu, S.; Fath El-Bab, A.M.R. Fabrication of Spiral Low-Cost Microchannel with Trapezoidal Cross Section for Cell Separation Using a Grayscale Approach. Micromachines 2023, 14, 1340. https://doi.org/10.3390/mi14071340
Adel M, Allam A, Sayour AE, Ragai HF, Umezu S, Fath El-Bab AMR. Fabrication of Spiral Low-Cost Microchannel with Trapezoidal Cross Section for Cell Separation Using a Grayscale Approach. Micromachines. 2023; 14(7):1340. https://doi.org/10.3390/mi14071340
Chicago/Turabian StyleAdel, Mohamed, Ahmed Allam, Ashraf E. Sayour, Hani F. Ragai, Shinjiro Umezu, and Ahmed M. R. Fath El-Bab. 2023. "Fabrication of Spiral Low-Cost Microchannel with Trapezoidal Cross Section for Cell Separation Using a Grayscale Approach" Micromachines 14, no. 7: 1340. https://doi.org/10.3390/mi14071340







